Abstract
Background: College students face significant stress from academic demands and high pressures, which can contribute to long-term physical and mental health issues. Existing stress-relief strategies are not always immediately available to this population, highlighting the need for accessible, low-cost solutions. Methods: This randomised controlled trial examined the effects of nature exposure on stress and well-being in a sample of 29 healthy college students compared to a healthy control group (n = 28). The intervention group engaged in 30 min walks in a natural environment four times per week over a four-week period. Stress levels and general well-being were assessed using validated self-report questionnaires administered before and after the intervention period, allowing for a comparison of changes in mental health outcomes between an intervention and control group. Eye-tracking analysis during a battery of cognitive tests assessed cognitive functioning. Findings: The intervention was associated with a greater reduction in psychological distress over time (β = −2.98, p = 0.007) and showed a trend toward reduced burnout symptoms (β = −0.12, p = 0.08) compared to the control group. These associations are independent of sex, age, BMI, smoking status, COVID-19 history, and previous diagnosis of mental illness. An increase in the number of saccades during the visual working memory task was observed in the intervention group compared to controls (β = 5.01, p = 0.046), while saccadic activity in other tasks remained unchanged. No significant effects were found for the neurocognitive performance measures. Conclusions: These findings suggest that short-term nature exposure may support psychological well-being and mental engagement in young adults. Our research highlights the use of walking in nature as a realistic and accessible strategy to promote mental health and neurocognitive functioning among students.
1. Introduction
Chronic stress is a widespread concern with profound effects on both physical and mental health, contributing to conditions such as cardiovascular disease, hypertension, anxiety, and depression [1,2]. Among vulnerable populations, college students experience particularly high levels of stress due to academic pressures, social transitions, and possibly financial uncertainty [3,4]. These are factors that are consistently linked to declines in academic performance and overall well-being [5,6].
Nature-based interventions have shown promising results in mitigating stress and promoting psychological well-being [7]. Specifically, regular exposure to green spaces, such as parks, forests, and gardens, has been associated with improved mental health, reduced prevalence of certain illnesses, and faster cognitive recovery from mental fatigue [8,9,10]. Emerging evidence emphasises that the time individuals actively spend in nature may be a valuable predictor of health and well-being [11]. While living in a green neighbourhood can contribute to better health outcomes [12,13,14,15,16,17], studies increasingly show that direct and repeated engagement with nature, even brief but regular visits, has strong and immediate effects on reducing stress and improving mental clarity [11,18,19,20]. This suggests that the benefits of exposure to nature are not restricted to those living near parks or forests but are accessible through intentional, repeated contact with natural environments.
Exposure to natural environments has been linked to improvements in cognitive functioning. These effects are often explained by the Attention Restoration Theory (ART), which explains that natural environments can restore mental fatigue and improve concentration [21,22]. Neurocognitive functioning is typically assessed through standard performance-based tasks evaluating specific neurological domains, including short-term memory, deductive reasoning, visual working memory, attention, and visuospatial processing [23,24]. In addition to these cognitive tasks, eye-tracking technology is often used as an objective proxy for cognitive processing, identifying subtle shifts in mental effort [23,25,26].
While the positive impact of green space exposure on stress is well-documented, questions remain about the dose of exposure required to obtain beneficial improvements [18,19,27,28]. In particular, it is unclear whether only brief interventions are sufficient to generate improvements in stress levels and neurocognitive functioning. According to Shanahan et al., the health benefits of nature depend on intensity (quality and quantity of natural exposure), frequency (how often exposure occurs) and duration (length of each exposure) [29]. This dose–response framework provides a theoretical basis for designing and evaluating brief nature-based interventions by identifying the “minimum effective dose” of nature required to produce measurable psychological and physiological benefits. Several studies suggest that even brief but repeated contact may yield significant improvements in stress regulation and attentional recovery, thereby indicating that short, regular exposures may enhance health outcomes [11,18,19,28,29]. However, empirical evidence in student populations remains limited, and the effectiveness of such short, structured interventions for both psychological and neurocognitive outcomes is still unclear. Understanding the efficacy of a brief dose of nature exposure is essential for further developing accessible and evidence-based strategies for stress relief among students.
This study aims to fill these knowledge gaps by investigating the effectiveness of a four-week intervention featuring regular (minimal 4 times a week), 30 min walks in natural settings. Using validated self-reported measures and neurocognitive assessments, changes in both perceived stress and neurocognitive performance and functioning are assessed. The results will provide insight into whether this amount of nature exposure offers a meaningful, low-cost, and scalable approach to enhancing mental health and cognitive resilience in college student populations.
2. Materials and Methods
2.1. Study Population
This randomised controlled trial focused on college and Ph.D. students from Hasselt University (UHasselt, Diepenbeek, Belgium), University College PXL (Diepenbeek, Belgium), and UC-Leuven-Limburg (UCLL, Hasselt, Belgium). Study details were shared via email and social media. Interested individuals (n = 78) were invited to an online information session. The inclusion criteria required participants to be capable of completing a regular 30 min walk over four weeks and to complete questionnaires in Dutch. A total of 61 volunteers were initially recruited to participate in the study. Four participants dropped out during the follow-up period, leaving 57 students in the study. Participants were randomly assigned using a randomisation function in R to either a control group (n = 28) or an intervention group (n = 29). A flowchart describing the included participants can be found in Figure 1. The study adhered to the principles of the Declaration of Helsinki for research involving human participants and received approval from the Medical Ethics Commission of Hasselt University (CME2020/006). All participants provided informed consent prior to inclusion in the study. This study was registered at ClinicalTrials.gov (Trial registration number: NCT05249296, registration date: 2 April 2020).
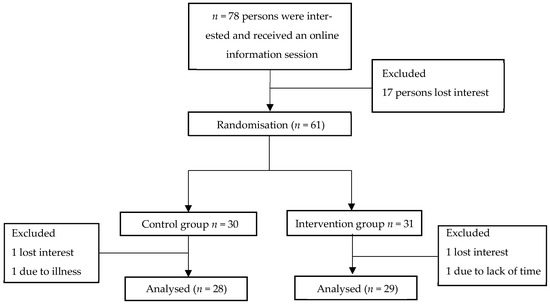
Figure 1.
Flowchart describing participants enrolled in the study.
2.2. Study Design
The study included a four-week intervention period during which participants in the intervention group took a 30 min walk in a natural environment at least four times per week in addition to their regular daily lifestyle. A suggested route near the aforementioned schools was provided that could be used either as a walking route or as an example of the type of green environment intended for the intervention. The suggested route consisted primarily of tree-lined paths and grassy areas. However, to enhance feasibility and accessibility, participants were also able to choose a location that was most suitable for them, using the suggested route as a guideline for the type of natural environment intended for the intervention. Participants in the control group were instructed to maintain their regular daily lifestyle. Baseline physical activity levels were not assessed; therefore, potential differences in prior activity between groups could not be controlled for in the analyses. Evaluations were conducted at the start (day 0) and end (day 28) of the four-week intervention period, and included validated self-reported questionnaires and neurocognitive tests assessing neurocognitive performance and functioning. Compliance with the prescribed walking frequency and duration was monitored primarily through self-report logs (e.g., daily journals). Additionally, participants were given the choice to record their walks using the Strava app to provide an objective verification of their activity. Figure 2 provides an overview of the study design for this randomised controlled trial. Data were collected between 28 March 2021, and 7 May 2021.
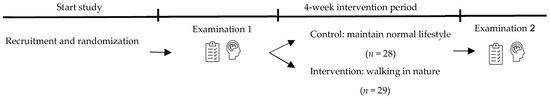
Figure 2.
Overview of study design.
Participants were divided into an intervention group and a control group. The intervention group performed a four-week nature-based intervention period, and the control group maintained their normal lifestyle. Self-reported questionnaires and neurocognitive tests were performed during examination 1 (day 0 or baseline) and examination 2 (day 28). Four participants were excluded during the intervention period due to loss of interest, lack of time, and illness.
2.3. Outcomes
2.3.1. Questionnaires
The study gathered information on the participants through (1) a general research questionnaire, (2) a questionnaire regarding the coronavirus (SARS-CoV-2) pandemic, (3) a 12-item General Health Questionnaire (GHQ-12) and 4) Burnout Assessment Tool (BAT). In the general research questionnaire, data collected included age, sex, weight, height, smoking status (coded as ‘does not smoke currently’ or ‘currently smokes’), and previous medical diagnosis of mental illness (coded as ‘no’ or ‘yes’). The body mass index (BMI) (kg/m2) was calculated as weight divided by squared height and categorised as ‘underweight’ (BMI ≤ 18.5), ‘normal’ (BMI > 18.5 and <25), ‘overweight’ (BMI ≥ 25 and <30), and ‘obese’ (BMI ≥ 30). We collected information on previous infection with SARS-CoV-2, diagnosed by a PCR test (coded as ‘yes’, ‘no’, or ‘presumptive’). The participants completed the 12-item General Health Questionnaire (GHQ-12) questionnaire at baseline (day 0) and at the end of the four-week intervention period (day 28) to estimate general well-being and psychological distress [30,31,32]. The GHQ-12 was selected as a brief and widely validated screening tool for assessing general psychological distress and short-term changes in mental health in non-clinical populations, making it suitable for detecting fluctuations that may result from environmental or lifestyle interventions such as nature exposure. This questionnaire includes six positive items and six negative items. Scoring was performed as determined by Sánches-Lòpez and Dresch [31]. The positive items were scored from 0 (always) to 3 (never), whereas the negative items were scored from 0 (never) to 3 (always). The final sum score ranged from 0 to 36, with higher scores indicating an increased likelihood of psychological distress. Additionally, the Burnout Assessment Tool (BAT) was completed at the beginning and at the end of the intervention period to identify symptoms of burnout [33]. This tool captures stress-related symptoms specifically associated with exhaustion and cognitive-emotional strain, which are known to contribute to higher rates of absenteeism and drop-out rates. The BAT is a self-assessment questionnaire that contains four core dimensions: exhaustion, mental distance, emotional loss of control, and cognitive loss of control, which are categorised from 1 (never) to 5 (always). A mean score was obtained by dividing the total sum score by the number of items, in this case 23.
2.3.2. Neurocognitive Tests
The Tobii Pro Nano eye tracking device (Tobii, Stockholm, Sweden) was used to investigate whether exposure to green spaces improves neurocognitive performance and functioning. Eye-tracking technology can be used as a more sensitive, objective, non-invasive method of identifying cognitive processes and subtle shifts in mental effort, and cognitive functioning as compared to regular behavioural measures [25,26]. Eye-tracking-based neurocognitive performance was assessed during five standard performance-based consecutive tests adapted from Oyama et al. [23], evaluating specific neurological domains, including short-term memory, deductive reasoning, visual working memory, attention and calculation, and visuospatial processing. This test battery has been shown to provide a rapid, reliable, and sensitive assessment of cognitive performance using picture tasks, minimising examiner bias and allowing semi-automated evaluation. Although originally developed for cognitive impairment screening, its high measurement sensitivity and repeatability make it suitable for detecting subtle fluctuations in cognitive performance and cognitive workload in non-impaired populations, such as university students. To eliminate potential experimenter bias, the evaluator’s role was limited to supervision during the tests. The participants received standardised written instructions and visualisations displayed on the monitor immediately before each task, ensuring the evaluator was not involved in explaining the tasks or influencing participant behaviour during the testing process. During the memory task, a set of different types of fruits was shown. The participant was asked to remember which kind of fruit was selected. At the end of the test battery, the different types of fruits were displayed again. The participant was asked to focus on the kind of fruit that was selected at the beginning of the test battery. The second test assessed deductive reasoning (odd one out), in which four different objects were shown. The participant was asked to focus on the object that was different from the others. In the third test (visual working memory), an object was shown for 10 s, and participants were instructed to concentrate on that same image in the following segment. The fourth test (attention and calculation) required participants to calculate the total number of apples and bananas, then focus on the sum of these quantities, and finally focus on the difference between these quantities. During the fifth test (visuospatial function), participants first saw an object and then three distinct objects. They were asked to identify and focus on the same shape they had seen initially. Data collection involved setting an area of interest (AOI) on the correct answer, with the percentage fixation duration on the AOI used as an outcome to measure neurocognitive performance (Tobii Pro Lab, version 1.152). In addition, the number of saccades that occur in a target AOI is also used as an outcome measure to assess neurocognitive functioning. Saccades are defined as rapid movements of the eyes that occur from one point to another to redirect focus [34]. An increase in the size of saccades might be an indication of higher mental effort [35]. Neurocognitive tasks were completed on day 0 and day 28. A visual presentation of the five neurocognitive tasks is provided in Figures S1–S5.
2.4. Statistical Analysis
Statistical analysis was performed using R (version 4.4.0). Continuous data are presented as means and standard deviations (SD), and categorical data are presented as numbers and frequencies (%). Data normality was checked using the Shapiro–Wilk test. Differences between the control and intervention groups were analysed by a Student’s t-test or a Chi-Squared test to indicate whether the randomisation of the participants was successful. Mixed-effects models were used for the intervention-response analysis, which included random effects for each participant. An interaction term between the group (control group and intervention group) and time of examination (baseline and day 28 after baseline) was included to assess the possible effect of the intervention over the time of the study. We adjusted our models for a priori selected potential confounders and covariates that may be associated with the outcome, including sex, age, BMI (‘underweight’ (BMI ≤ 18.5), ‘normal’ (BMI > 18.5 and <25), ‘overweight’ (BMI ≥ 25 and <30), and ‘obese’ (BMI ≥ 30)), date of examination, and smoking status (‘Currently smokes’ or ‘Does not smoke currently’). The model was additionally adjusted for previous diagnoses of mental illness (yes or no), and previous infection with SARS-CoV-2, confirmed by a PCR test (yes, no, or presumptive). To assess the saccadic activity during the neurocognitive tests, additional correction for pupil diameter during the test was performed. The results of the mixed models are expressed as corrected averages (Estimated Marginal Means) for the different outcomes in the intervention and control group at baseline and after the intervention period.
2.5. Population Characteristics
In the present study n = 28 students participated in the control group and n = 29 in the intervention group. No differences in population characteristics were found between the intervention and control group (Table 1). The participants were on average 22.2 ± 2.06 years old. The majority of the participants were females (61.4%), had a normal BMI (75.4%), and did not smoke currently (89.5%).

Table 1.
Characteristics of the participants (n = 57). Mean ± SD or numbers and frequencies (%) for all variables are given. p-value for the difference between groups is given.
3. Results
A significant interaction effect between time and group (intervention vs. control) was observed for psychological distress, indicating a greater reduction in GHQ-12 scores over time in the intervention group compared to the control group (β = −2.98, 95% CI −5.09 to −0.88, p = 0.007, Figure S6) adjusted for sex, date of examination, age, BMI, smoking status, COVID-19 history, and history of mental illness. Additionally, we detected a greater reduction in BAT over time in the intervention group compared to the control group (β = −0.12, 95% CI −0.26 to 0.01, p = 0.08). A significant interaction effect was found for saccadic activity during the visual working memory task, with an increase in the number of saccades over time in the intervention group as compared to the control group (β = 5.01, 95% CI 0.17 to 9.73, p = 0.046). No significant effects were observed for saccadic measures in the remaining eye-tracking tasks. Additionally, no significant improvements were observed for neurocognitive performance measures, including memory recall, deductive reasoning, pattern recognition, attention, and visuospatial processing. An overview of all model estimates, confidence intervals, and p-values is provided in Table 2.

Table 2.
Estimates, confidence intervals, and p-values for the interaction effects in the mixed effects models of the questionnaires, neurocognitive tests, and eye-tracking analysis.
4. Discussion
The findings of this randomised control trial suggest that regular, four times per week, short-duration exposure to natural environments, through a structured walking intervention, may have a beneficial impact on psychological well-being among university students. The significant reduction in GHQ-12 scores in the intervention group indicates a measurable decrease in psychological distress following the four-week period, aligning with previous research highlighting the mental health benefits of green space exposure and physical activity [36]. Although the reduction in the assessment of burnout symptoms did not reach statistical significance (p = 0.08), the observed reduction supports the hypothesis that short engagement with nature may alleviate work- or study-related emotional exhaustion [21,22,37,38], warranting further exploration in larger samples. Interestingly, the significant increase in saccadic activity during the visual working memory task could reflect enhanced engagement or altered mental effort following nature exposure, a finding consistent with the emerging literature on the neurologic effects of natural environments [12,39]. Contrary to our expectations, neurocognitive performance did not significantly improve, which could suggest that mental effort does not imply enhanced task outcomes. The absence of significant changes in other eye-tracking tasks suggests that the effects on neurocognitive function may be task-specific or may only emerge over the long term. In the short term, an optimal workload might not immediately translate into better performance, but maintaining an adequate workload over time could facilitate learning and ultimately lead to improved outcomes.
The current study adds to the growing body of literature suggesting that even brief and repeated interactions with nature can yield meaningful benefits for psychological well-being [28]. Importantly, the intervention required only a moderate commitment, 30 min of walking in nature, four times per week, which aligns with real-world feasibility for students managing demanding academic schedules. These findings indicate that this minimal yet regular exposure is sufficient to reduce psychological distress over a four-week period, supporting earlier findings that emphasise the cumulative value of ‘doses’ of nature for mental health benefits [11,18]. Additionally, our findings align with experimental research by Bratman et al., who demonstrated that a single 90 min walk in a natural environment, compared to an urban setting, significantly reduced self-reported rumination—defined as a maladaptive cognitive process associated with depression—and was accompanied by decreased neural activity in the subgenual prefrontal cortex, a brain region involved in mood regulation [20]. Since both groups engaged in walking, these results suggest that the mental health benefits are attributable to the distinct qualities of the natural environment, rather than physical activity alone. While the results of our study do not directly show that the frequency and consistency of nature contact may be more critical than the length of any single session, they raise a question about the relative importance of the frequency and consistency of nature exposure. Prior research has proposed a weekly threshold of 120 min in nature as a benchmark for improved well-being [11], and this intervention meets this threshold. This reinforces the idea that 30 min sessions, when repeated consistently, can be an effective and low-barrier intervention for stress reduction. Furthermore, this intervention was independent of proximity to nature, showing that intentional exposure, rather than passive or incidental contact, is sufficient to obtain health benefits.
Our findings align with previous observational studies that associate exposure to green space with increased well-being. Numerous systematic reviews and meta-analyses of observational studies have demonstrated that higher amounts of green space in residential settings or more engagement with green environments are linked to better mental health outcomes, including reduced symptoms of depression and anxiety, and improved well-being [36,40,41,42]. For instance, White et al. discovered that spending at least 120 min a week was associated with noticeably higher levels of self-reported health and well-being [11]. Likewise, a systematic review by Gascon et al. shows that increased exposure to residential green is associated with lower psychological distress as measured by the GHQ-12 questionnaire [42].
Saccadic eye movements are quick eye movements that are influenced by neurocognitive processes. These rapid eye movements contribute to complex neurocognitive processes, particularly within memory tasks in healthy populations [43]. Therefore, saccadic parameters serve as a valuable and measurable index of internal neurocognitive effort and ongoing mental processing during memory tasks [44]. Studies reveal that saccade frequency increases when demands on our working memory increase, which helps in information retrieval [25,45]. Despite the absence of improvements in broader neurocognitive performance, the observed increase in saccadic activity during the visual working memory task may suggest a higher level of neurocognitive engagement or alertness, as the participant is more actively scanning and processing information [35,43]. This outcome, while limited to one specific task, suggests that nature exposure could influence certain dimensions of mental functioning, although perhaps not to an extent detectable over a short duration. It remains plausible that more prolonged interventions, or those coupled with more cognitively demanding tasks, might yield stronger cognitive effects. No significant improvements were observed across most neurocognitive domains, which may be attributed to the relatively short intervention duration or ceiling effects in neurocognitive performance among this young and high-functioning population [46,47].
A strength of this study is its randomised design, which minimises bias and enhances the validity of the findings. The use of mixed-effects models allowed us to control for several potential confounders, such as age, sex, BMI, smoking status, COVID-19 history, and prior mental health diagnoses. Additionally, the combination of subjective and objective outcome measures, the questionnaires and eye-tracking, respectively, provides a more comprehensive view of the intervention’s effects. However, the study also has limitations. First, the sample size was relatively small, which may limit statistical power and the generalizability of the results. Second, the duration of the intervention period was short, and it remains uncertain whether the observed benefits would persist over time or increase with longer exposure. Additionally, we could not correct for baseline physical activity as this was not known. Third, although eye-tracking offered valuable insight into cognitive engagement, the neurocognitive tasks may not have been sufficiently challenging to detect subtle improvements in performance, particularly in a high-functioning population like university students. Future studies should aim to include participants representing a broader age range to examine whether the effects of nature exposure on cognitive performance differ across stages of adulthood. Moreover, systematically varying the duration and frequency of exposure would help identify the minimum effective ‘dose’ of nature contact required to produce measurable benefits in psychological and neurocognitive outcomes. Larger and more diverse samples, along with longer follow-up periods, are also needed to refine our understanding of the optimal dose of nature and to assess the sustainability of the observed effects. Further research should clarify which neurocognitive domains are most sensitive to nature-based interventions and elucidate the underlying neurobiological mechanisms involved.
Although not yet completely understood, the underlying biological pathways and mechanisms by which taking a walk in a natural environment may reduce stress are becoming more widely acknowledged. Numerous plausible pathways have been proposed, such as improvements in mood and attention, alterations in brain activity associated with emotion regulation, and decreases in physiological stress markers [7,20,48,49,50]. According to Markevych et al., being in natural settings can have a positive impact on health in a number of interrelated ways, including increased physical activity, decreased air pollution, decreased noise exposure, and direct psychophysiological stress reduction [51]. Our research adds to this body of evidence by indicating that intentional and structured nature-based experiences, even if they are brief, might have a favourable impact on mental health, most likely through these interconnected pathways.
Taken together, these findings advocate for the incorporation of regular, short green-space interactions into student routines as a realistic strategy to support their mental well-being and level of cognitive engagement or alertness. Given the accessibility and low cost of such interventions, educational institutions might consider promoting structured nature exposure through campus green areas, guided outdoor activities, or integration into academic schedules as part of broader mental health promotion efforts. These results also highlight the complex interplay between psychological and neurocognitive outcomes and underscore the potential of nature-based interventions as accessible and low-cost strategies for promoting mental well-being in student populations.
5. Conclusions
We observed a significant negative association between the interaction of the intervention and time of examination on GHQ-12 scores, indicating improved psychological well-being following brief, regular exposure to natural environments. Although no significant improvements were found in neurocognitive performance, a significant increase in saccadic activity suggests enhanced neurocognitive engagement. These findings confirm previous evidence on the mental health benefits of nature exposure while providing new insights by demonstrating that brief, repeated exposure can reduce psychological distress in university students. The study also introduces eye-tracking as an objective and sensitive approach to detect subtle cognitive changes related to nature exposure. Overall, the findings contribute to the growing understanding of the minimum effective dose of nature and highlight the feasibility of walking in nature as a realistic and accessible strategy for stress reduction among university students. Nonetheless, future research should explore the long-term effects of such interventions and their integration into educational settings as a practical strategy for stress reduction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/greenhealth1030018/s1, Figure S1: Memory task (10s); Figure S2: Deductive reasoning (odd one out) (75s); Figure S3: Visual working memory (10s); Figure S4: Attention and calculation (14s); Figure S5: Visuospatial function task (10s); Figure S6: Individual and mean changes in GHQ-12 scores between baseline and post intervention period in the control group (a) and intervention group (b).
Author Contributions
Conceptualization, K.V. and M.P.; methodology, L.V., R.A., K.V. and M.P.; software, K.V.; formal analysis, L.V. and R.A.; investigation, L.V., M.V., H.S., E.R. and K.V.; data curation, L.V., R.A., K.V. and M.P.; writing—original draft preparation, L.V.; writing—review and editing, M.V., R.A., H.S., E.R., T.S.N., K.V. and M.P.; visualisation, L.V.; supervision, R.A., K.V. and M.P.; project administration, K.V. and M.P.; funding acquisition, M.P., K.V., M.V. and L.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Flemish Scientific Research Foundation (FWO; G059219, G026222N, 1296523N), BOF (Lore Verheyen and Maartje Vangeneugden) and Methusalem.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Medical Ethics Commission of Hasselt University (CME2020/006) on 21 February 2020.
Informed Consent Statement
Informed consent for participation was obtained from all subjects involved in the study. Informed consent for publication was obtained from all identifiable human participants.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request. The data are not publicly available due to privacy restrictions.
Acknowledgments
We are thankful to all participating students. We acknowledge several colleagues for their assistance with the research. During the preparation of this work, the author(s) used ChatGPT 5 in order to improve the language and readability of the manuscript. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SARS-CoV-2 | coronavirus pandemic |
| GHQ-12 | 12-item General Health Questionnaire |
| BAT | Burnout Assessment Tool |
| BMI | Body Mass Index |
| AOI | Area Of Interest |
| SD | Standard Deviation |
References
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The impact of stress on body function: A review. EXCLI J. 2017, 16, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Kivimäki, M.; Steptoe, A. Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 2018, 15, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Regehr, C.; Glancy, D.; Pitts, A. Interventions to reduce stress in university students: A review and meta-analysis. J. Affect. Disord. 2013, 148, 1–11. [Google Scholar] [CrossRef] [PubMed]
- American College Health Association. American College Health Association National College Health Assessment: Graduate/Professional Student Reference Group. Executive Summary Fall 2024; American College Health Association: Silver Spring, MD, USA, 2024. [Google Scholar]
- Pascoe, M.C.; Hetrick, S.E.; Parker, A.G. The impact of stress on students in secondary school and higher education. Int. J. Adolesc. Youth 2020, 25, 104–112. [Google Scholar] [CrossRef]
- Ramón-Arbués, E.; Gea-Caballero, V.; Granada-López, J.M.; Juárez-Vela, R.; Pellicer-García, B.; Antón-Solanas, I. The Prevalence of Depression, Anxiety and Stress and Their Associated Factors in College Students. Int. J. Environ. Res. Public Health 2020, 17, 7001. [Google Scholar] [CrossRef]
- Haluza, D.; Schönbauer, R.; Cervinka, R. Green perspectives for public health: A narrative review on the physiological effects of experiencing outdoor nature. Int. J. Environ. Res. Public Health 2014, 11, 5445–5461. [Google Scholar] [CrossRef]
- Maas, J.; Verheij, R.A.; Groenewegen, P.P.; de Vries, S.; Spreeuwenberg, P. Green space, urbanity, and health: How strong is the relation? J. Epidemiol. Community Health 2006, 60, 587–592. [Google Scholar] [CrossRef]
- Takano, T.; Nakamura, K.; Watanabe, M. Urban residential environments and senior citizens’ longevity in megacity areas: The importance of walkable green spaces. J. Epidemiol. Community Health 2002, 56, 913–918. [Google Scholar] [CrossRef]
- van den berg, A.; Maas, J.; Verheij, R.; Groenewegen, P. Green space as a buffer between stressful life and health. Soc. Sci. Med. 2010, 70, 1203–1210. [Google Scholar] [CrossRef]
- White, M.P.; Alcock, I.; Grellier, J.; Wheeler, B.W.; Hartig, T.; Warber, S.L.; Bone, A.; Depledge, M.H.; Fleming, L.E. Spending at least 120 minutes a week in nature is associated with good health and wellbeing. Sci. Rep. 2019, 9, 7730. [Google Scholar] [CrossRef]
- Dadvand, P.; Pujol, J.; Macia, D.; Martinez-Vilavella, G.; Blanco-Hinojo, L.; Mortamais, M.; Alvarez-Pedrerol, M.; Fenoll, R.; Esnaola, M.; Dalmau-Bueno, A.; et al. The Association between Lifelong Greenspace Exposure and 3-Dimensional Brain Magnetic Resonance Imaging in Barcelona Schoolchildren. Environ. Health Perspect. 2018, 126, 027012. [Google Scholar] [CrossRef]
- Nieuwenhuijsen, M.J.; Dadvand, P.; Márquez, S.; Bartoll, X.; Barboza, E.P.; Cirach, M.; Borrell, C.; Zijlema, W.L. The evaluation of the 3-30-300 green space rule and mental health. Environ. Res. 2022, 215, 114387. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.F.; Wilson, J.S.; Liu, G.C. Neighborhood greenness and 2-year changes in body mass index of children and youth. Am. J. Prev. Med. 2008, 35, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Bijnens, E.M.; Derom, C.; Thiery, E.; Weyers, S.; Nawrot, T.S. Residential green space and child intelligence and behavior across urban, suburban, and rural areas in Belgium: A longitudinal birth cohort study of twins. PLoS Med. 2020, 17, e1003213. [Google Scholar] [CrossRef] [PubMed]
- James, P.; Banay, R.F.; Hart, J.E.; Laden, F. A Review of the Health Benefits of Greenness. Curr. Epidemiol. Rep. 2015, 2, 131–142. [Google Scholar] [CrossRef]
- Dadvand, P.; Nieuwenhuijsen, M.J.; Esnaola, M.; Forns, J.; Basagaña, X.; Alvarez-Pedrerol, M.; Rivas, I.; López-Vicente, M.; De Castro Pascual, M.; Su, J.; et al. Green spaces and cognitive development in primary schoolchildren. Proc. Natl. Acad. Sci. USA 2015, 112, 7937–7942. [Google Scholar] [CrossRef]
- Shanahan, D.F.; Bush, R.; Gaston, K.J.; Lin, B.B.; Dean, J.; Barber, E.; Fuller, R.A. Health Benefits from Nature Experiences Depend on Dose. Sci. Rep. 2016, 6, 28551. [Google Scholar] [CrossRef]
- Barton, J.; Pretty, J. What is the Best Dose of Nature and Green Exercise for Improving Mental Health? A Multi-Study Analysis. Environ. Sci. Technol. 2010, 44, 3947–3955. [Google Scholar] [CrossRef]
- Bratman, G.N.; Hamilton, J.P.; Hahn, K.S.; Daily, G.C.; Gross, J.J. Nature experience reduces rumination and subgenual prefrontal cortex activation. Proc. Natl. Acad. Sci. USA 2015, 112, 8567–8572. [Google Scholar] [CrossRef]
- Kaplan, R.; Kaplan, S. The Experience of Nature: A Psychological Perspective; Cambridge University Press: New York, NY, USA, 1989; p. xii-340. [Google Scholar]
- Kaplan, S. The restorative benefits of nature: Toward an integrative framework. J. Environ. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- Oyama, A.; Takeda, S.; Ito, Y.; Nakajima, T.; Takami, Y.; Takeya, Y.; Yamamoto, K.; Sugimoto, K.; Shimizu, H.; Shimamura, M.; et al. Novel Method for Rapid Assessment of Cognitive Impairment Using High-Performance Eye-Tracking Technology. Sci. Rep. 2019, 9, 12932. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.L.; Letz, R.E.; Fidler, A.T.; Shalat, S.; Plantamura, D.; Lyndon, M. A computer-based neurobehavioral evaluation system for occupational and environmental epidemiology: Methodology and validation studies. Neurobehav. Toxicol. Teratol. 1985, 7, 369–377. [Google Scholar] [PubMed]
- Low, S.C.; Verschure, P.; Santos-Pata, D. Saccade rate is associated with recall of items in working memory. Learn. Mem. 2022, 29, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Just, M.A.; Carpenter, P.A. Eye fixations and cognitive processes. Cogn. Psychol. 1976, 8, 441–480. [Google Scholar] [CrossRef]
- Bratman, G.N.; Hamilton, J.P.; Daily, G.C. The impacts of nature experience on human cognitive function and mental health. Ann. NY Acad. Sci. 2012, 1249, 118–136. [Google Scholar] [CrossRef]
- Meredith, G.R.; Rakow, D.A.; Eldermire, E.R.B.; Madsen, C.G.; Shelley, S.P.; Sachs, N.A. Minimum Time Dose in Nature to Positively Impact the Mental Health of College-Aged Students, and How to Measure It: A Scoping Review. Front. Psychol. 2019, 10, 2942. [Google Scholar] [CrossRef]
- Shanahan, D.F.; Fuller, R.A.; Bush, R.; Lin, B.B.; Gaston, K.J. The Health Benefits of Urban Nature: How Much Do We Need? BioScience 2015, 65, 476–485. [Google Scholar] [CrossRef]
- Goldberg, D.P. The Detection of Psychiatric Illness by Questionnaire: A Technique for the Identification and Assessment of Non-Psychotic Psychiatric Illness; Oxford University Press: Oxford, UK, 1972; p. xii-156. [Google Scholar]
- Sánchez-López Mdel, P.; Dresch, V. The 12-Item General Health Questionnaire (GHQ-12): Reliability, external validity and factor structure in the Spanish population. Psicothema 2008, 20, 839–843. [Google Scholar]
- Goldberg, D.P.; Hillier, V.F. A scaled version of the General Health Questionnaire. Psychol. Med. 1979, 9, 139–145. [Google Scholar] [CrossRef]
- Schaufeli, W.B.; Desart, S.; De Witte, H. Burnout Assessment Tool (BAT)—Development, Validity, and Reliability. Int. J. Environ. Res. Public Health 2020, 17, 9495. [Google Scholar] [CrossRef]
- Keller, E.L.; Lee, B.-T.; Lee, K.-M. Chapter 2.4—Frontal eye field signals that may trigger the brainstem saccade generator. In Progress in Brain Research; Kennard, C., Leigh, R.J., Eds.; Elsevier: Oxford, UK, 2008; Volume 171, pp. 107–114. [Google Scholar]
- Chen, S.; Epps, J.; Ruiz, N.; Chen, F. Eye activity as a measure of human mental effort in HCI. In Proceedings of the 16th International Conference on Intelligent user interfaces, Palo Alto, CA, USA, 13–16 February 2011; pp. 315–318. [Google Scholar]
- Twohig-Bennett, C.; Jones, A. The health benefits of the great outdoors: A systematic review and meta-analysis of greenspace exposure and health outcomes. Environ. Res. 2018, 166, 628–637. [Google Scholar] [CrossRef]
- Daniels, S.; Clemente, D.B.P.; Desart, S.; Saenen, N.; Sleurs, H.; Nawrot, T.S.; Malina, R.; Plusquin, M. Introducing nature at the work floor: A nature-based intervention to reduce stress and improve cognitive performance. Int. J. Hyg. Environ. Health 2022, 240, 113884. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, M.; Koskinen, J.; Jokiaho, S.; Vahanne, T.; Pohjola, M.; Kontio, E. A short simulated nature experience as an effective way to promote restoration from work-related stress. Scand. J. Psychol. 2024, 65, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Berman, M.G.; Kross, E.; Krpan, K.M.; Askren, M.K.; Burson, A.; Deldin, P.J.; Kaplan, S.; Sherdell, L.; Gotlib, I.H.; Jonides, J. Interacting with nature improves cognition and affect for individuals with depression. J. Affect. Disord. 2012, 140, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.-Y.; Zhao, T.; Hu, L.-X.; Browning, M.H.E.M.; Heinrich, J.; Dharmage, S.C.; Jalaludin, B.; Knibbs, L.D.; Liu, X.-X.; Luo, Y.-N.; et al. Greenspace and human health: An umbrella review. Innovation 2021, 2, 100164. [Google Scholar] [CrossRef]
- Coventry, P.A.; Brown, J.E.; Pervin, J.; Brabyn, S.; Pateman, R.; Breedvelt, J.; Gilbody, S.; Stancliffe, R.; McEachan, R.; White, P.L. Nature-based outdoor activities for mental and physical health: Systematic review and meta-analysis. SSM Popul. Health 2021, 16, 100934. [Google Scholar] [CrossRef]
- Gascon, M.; Triguero-Mas, M.; Martínez, D.; Dadvand, P.; Forns, J.; Plasència, A.; Nieuwenhuijsen, M.J. Mental health benefits of long-term exposure to residential green and blue spaces: A systematic review. Int. J. Environ. Res. Public Health 2015, 12, 4354–4379. [Google Scholar] [CrossRef]
- Yang, H.W.; Choe, J.Y.; Noh, S.R.; Kim, J.L.; Han, J.W.; Kim, K.W. Exploring age-related changes in saccades during cognitive tasks in healthy adults. Front. Behav. Neurosci. 2024, 17, 1301318. [Google Scholar] [CrossRef]
- Salehi Fadardi, M.; Salehi Fadardi, J.; Mahjoob, M.; Doosti, H. Post-saccadic Eye Movement Indices Under Cognitive Load: A Path Analysis to Determine Visual Performance. J. Ophthalmic Vis. Res. 2022, 17, 397–404. [Google Scholar] [CrossRef]
- Kulkarni, M.; Nickel, A.E.; Minor, G.N.; Hannula, D.E. Control of memory retrieval alters memory-based eye movements. J. Exp. Psychol. Learn. Mem. Cogn. 2024, 50, 1199–1219. [Google Scholar] [CrossRef]
- Bornholt, L.J.; Ajersch, S.; Fisher, I.H.; Markham, R.H.; Ouvrier, R.A. Cognitive screening for children and adolescents: General limits or ceiling effects? J. Child. Neurol. 2010, 25, 567–571. [Google Scholar] [CrossRef]
- Scollard, P.; Choi, S.E.; Lee, M.L.; Mukherjee, S.; Trittschuh, E.H.; Sanders, R.E.; Gibbons, L.E.; Joshi, P.; Devine, S.; Au, R.; et al. Ceiling effects and differential measurement precision across calibrated cognitive scores in the Framingham Study. Neuropsychology 2023, 37, 383–397. [Google Scholar] [CrossRef]
- Khalil, M.H. Green Environments for Sustainable Brains: Parameters Shaping Adaptive Neuroplasticity and Lifespan Neurosustainability—A Systematic Review and Future Directions. Int. J. Environ. Res. Public Health 2025, 22, 690. [Google Scholar] [CrossRef] [PubMed]
- Bijnens, E.M.; Vos, S.; Verheyen, V.V.; Bruckers, L.; Covaci, A.; De Henauw, S.; Den Hond, E.; Loots, I.; Nelen, V.; Plusquin, M.; et al. Higher surrounding green space is associated with better attention in Flemish adolescents. Environ. Int. 2022, 159, 107016. [Google Scholar] [CrossRef] [PubMed]
- Markevych, I.; Schoierer, J.; Hartig, T.; Chudnovsky, A.; Hystad, P.; Dzhambov, A.M.; de Vries, S.; Triguero-Mas, M.; Brauer, M.; Nieuwenhuijsen, M.J.; et al. Exploring pathways linking greenspace to health: Theoretical and methodological guidance. Environ. Res. 2017, 158, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Markevych, I.; Thiering, E.; Fuertes, E.; Sugiri, D.; Berdel, D.; Koletzko, S.; von Berg, A.; Bauer, C.-P.; Heinrich, J. A cross-sectional analysis of the effects of residential greenness on blood pressure in 10-year old children: Results from the GINIplus and LISAplus studies. BMC Public Health 2014, 14, 477. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).