Associations of Prenatal Exposures to Fine Particulate Matter and Its Compositions with Preterm Birth Risk in Twins
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Outcome Measurement
2.3. Exposure Assessment
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Study Subjects
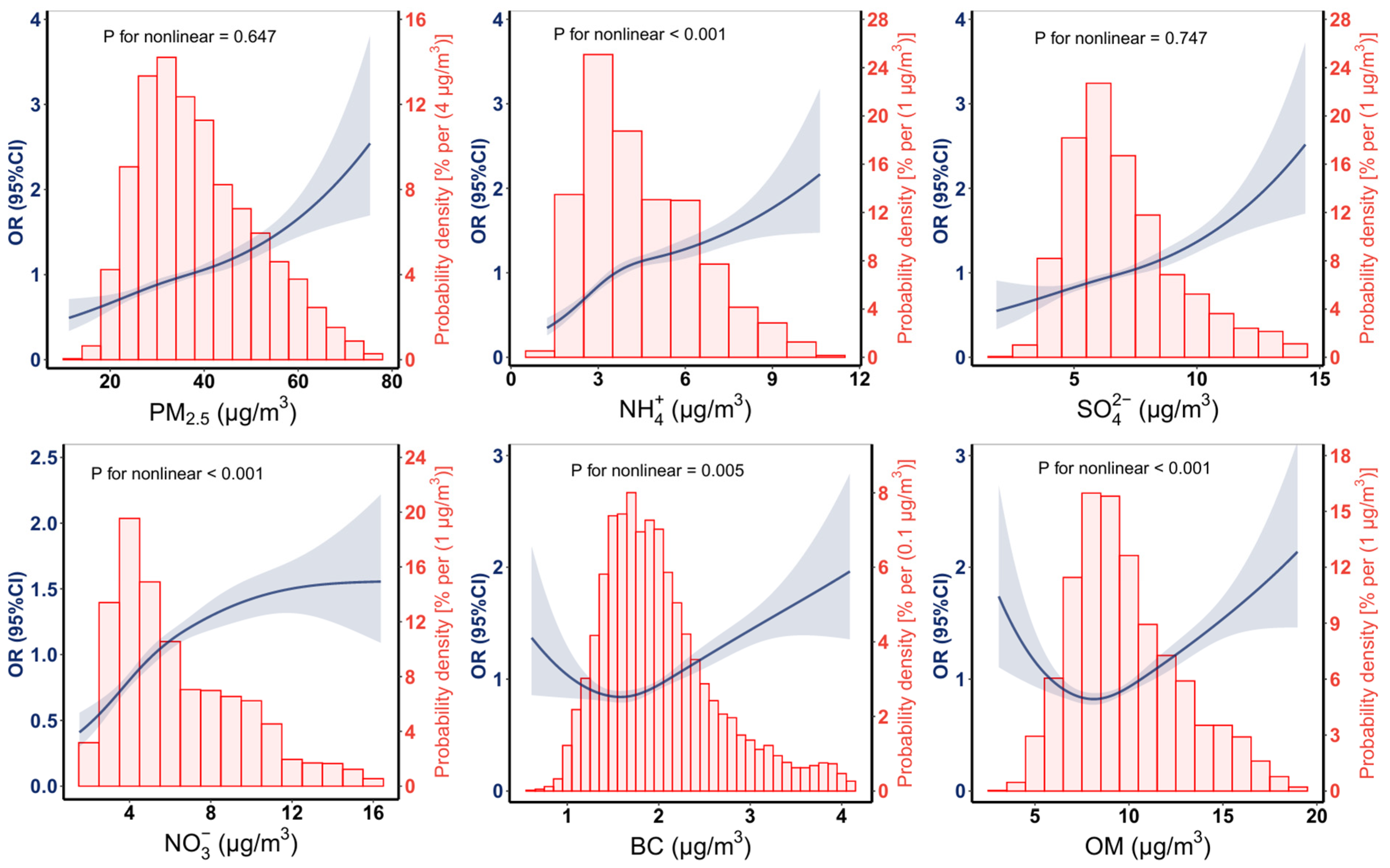
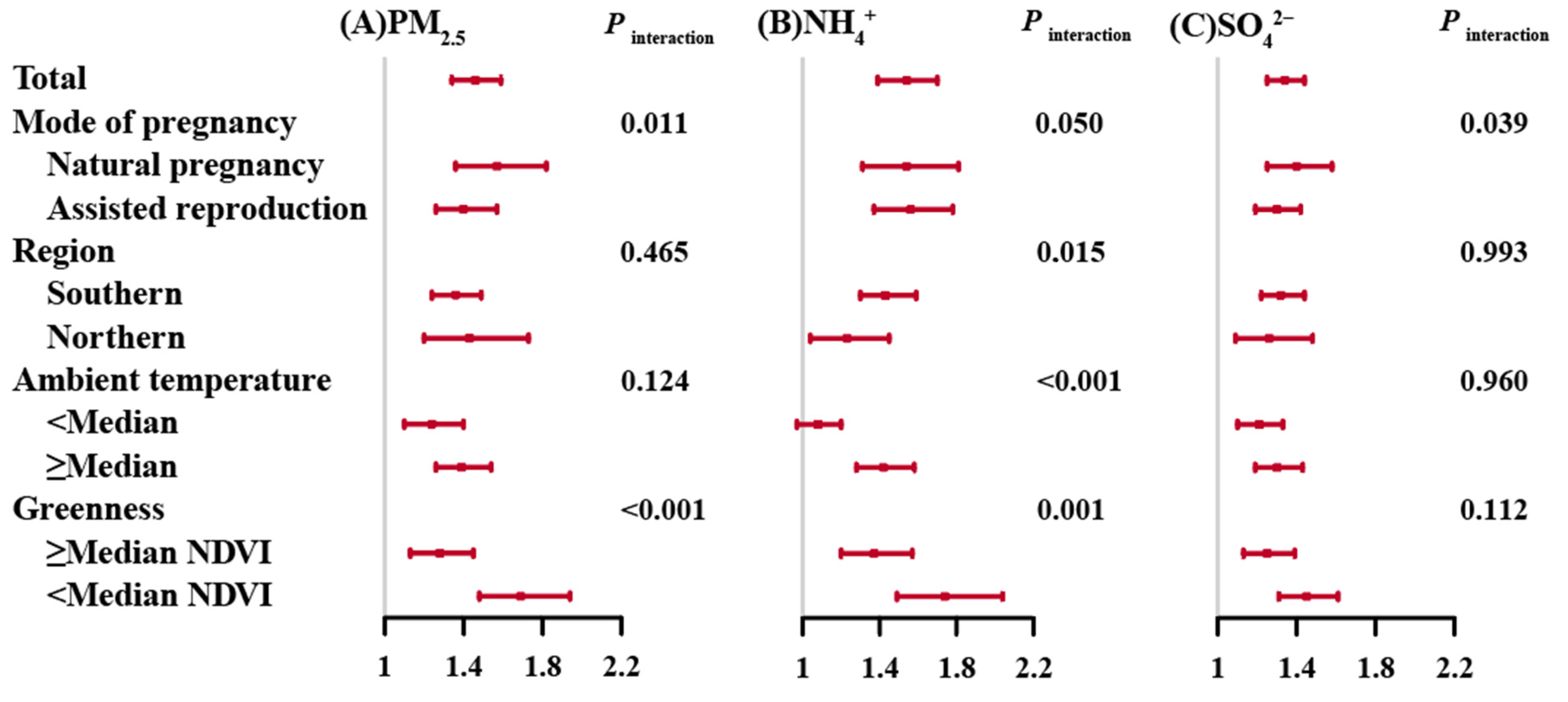
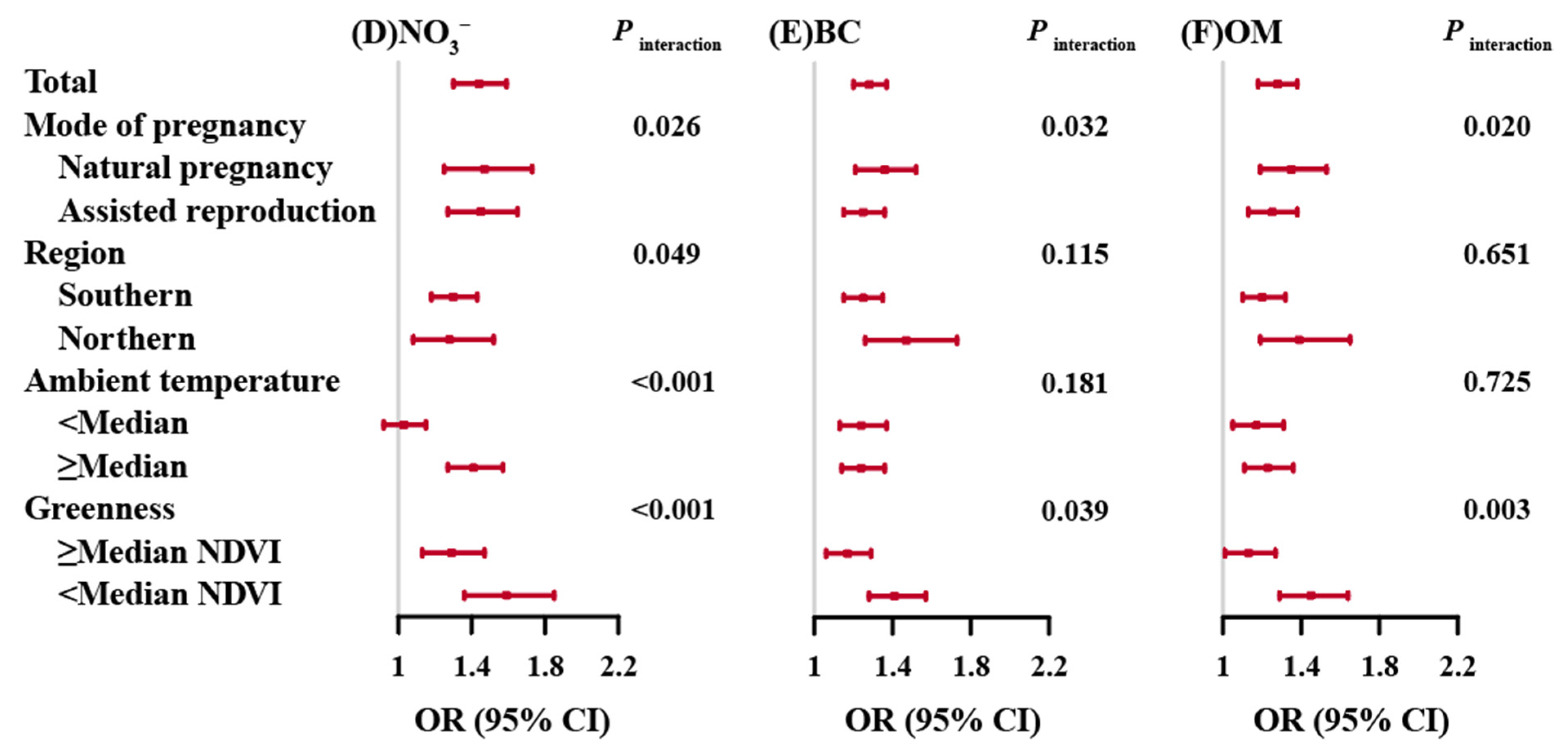
3.2. Associations of Prenatal PM2.5 and Its Composition Exposures with PTB
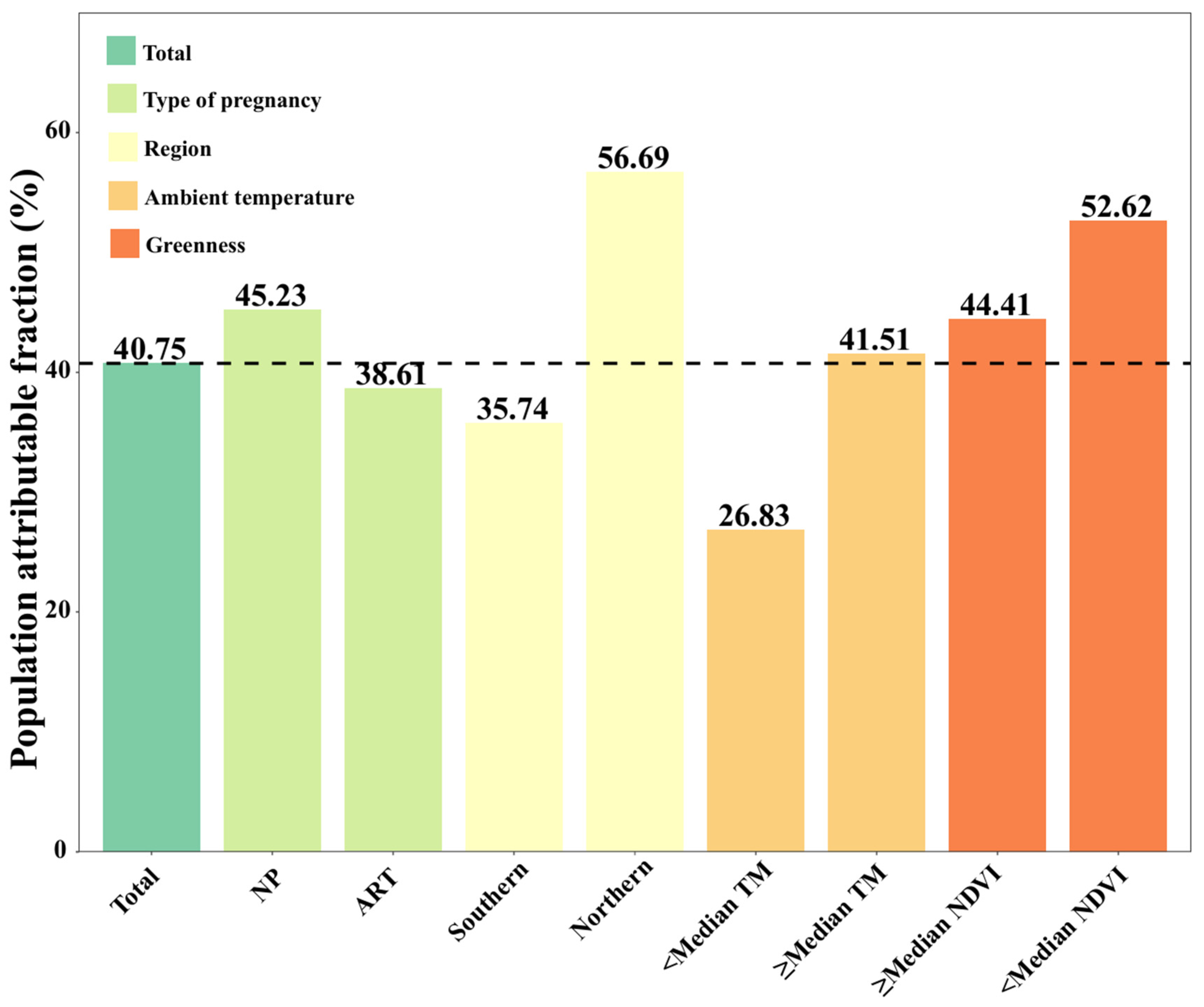
3.3. PAF of PTB Attributable to Prenatal Exposure to PM2.5
3.4. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, C.; Yang, J.; Wei, J.; Liu, Y.; Zhu, H.; Li, X.; Wang, J.; Chen, R. Individual ambient ozone exposure during pregnancy and adverse birth outcomes: Exploration of the potentially vulnerable windows. J. Hazard. Mater. 2024, 464, 132945. [Google Scholar] [CrossRef]
- Picciotto, S.; Huang, S.; Lurmann, F.; Pavlovic, N.; Chang, S.Y.; Mukherjee, A.; Goin, D.E.; Sklar, R.; Noth, E.; Morello-Frosch, R.; et al. Pregnancy exposure to PM(2.5) from wildland fire smoke and preterm birth in California. Environ. Int. 2024, 186, 108583. [Google Scholar] [CrossRef] [PubMed]
- Ohuma, E.O.; Moller, A.B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Remy, J.; Daburon, F. In-vitro growth potential of fibroblasts isolated from pigs with radiation-induced fibrosis. Br. J. Radiol. Suppl. 1986, 19, 99–100. [Google Scholar] [PubMed]
- Sarda, S.P.; Sarri, G.; Siffel, C. Global prevalence of long-term neurodevelopmental impairment following extremely preterm birth: A systematic literature review. The J. Int. Med. Res. 2021, 49, 3000605211028026. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, V.V.; Abiramalatha, T.; Bandyopadhyay, T.; Shaik, N.B.; Bandiya, P.; Nanda, D.; Pullattayil, S.A.; Murki, S.; Roehr, C.C. ELBW and ELGAN outcomes in developing nations-Systematic review and meta-analysis. PLoS ONE 2021, 16, e0255352. [Google Scholar] [CrossRef]
- Chu, C.; Zhu, Y.; Liu, C.; Chen, R.; Yan, Y.; Ren, Y.; Li, X.; Wang, J.; Ge, W.; Kan, H.; et al. Ambient fine particulate matter air pollution and the risk of preterm birth: A multicenter birth cohort study in China. Environ. Pollut. 2021, 287, 117629. [Google Scholar] [CrossRef]
- Quraishi, S.M.; Hazlehurst, M.F.; Loftus, C.T.; Nguyen, R.H.N.; Barrett, E.S.; Kaufman, J.D.; Bush, N.R.; Karr, C.J.; LeWinn, K.Z.; Sathyanarayana, S.; et al. Association of prenatal exposure to ambient air pollution with adverse birth outcomes and effect modification by socioeconomic factors. Environ. Res. 2022, 212, 113571. [Google Scholar] [CrossRef]
- Hyder, A.; Lee, H.J.; Ebisu, K.; Koutrakis, P.; Belanger, K.; Bell, M.L. PM2.5 exposure and birth outcomes: Use of satellite- and monitor-based data. Epidemiology 2014, 25, 58–67. [Google Scholar] [CrossRef]
- Guo, T.; Wang, Y.; Zhang, H.; Zhang, Y.; Zhao, J.; Wang, Q.; Shen, H.; Wang, Y.; Xie, X.; Wang, L.; et al. The association between ambient PM2.5 exposure and the risk of preterm birth in China: A retrospective cohort study. Sci. Total Environ. 2018, 633, 1453–1459. [Google Scholar] [CrossRef]
- van Donkelaar, A.; Martin, R.V.; Li, C.; Burnett, R.T. Regional Estimates of Chemical Composition of Fine Particulate Matter Using a Combined Geoscience-Statistical Method with Information from Satellites, Models, and Monitors. Environ. Sci. Technol. 2019, 53, 2595–2611. [Google Scholar] [CrossRef]
- Bell, M.L.; Dominici, F.; Ebisu, K.; Zeger, S.L.; Samet, J.M. Spatial and temporal variation in PM(2.5) chemical composition in the United States for health effects studies. Environ. Health Perspect. 2007, 115, 989–995. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment, P.s.R.o.C. 2023 China Ecological Environment Status Bulletin. 2024. Available online: https://www.mee.gov.cn/hjzl/sthjzk/zghjzkgb/202406/P020240604551536165161.pdf (accessed on 1 December 2024).
- Ananth, C.V.; Chauhan, S.P. Epidemiology of twinning in developed countries. Semin. Perinatol. 2012, 36, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Adamson, G.D.; Norman, R.J. Why are multiple pregnancy rates and single embryo transfer rates so different globally, and what do we do about it? Fertil. Steril. 2020, 114, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Santana, D.S.; Cecatti, J.G.; Surita, F.G.; Silveira, C.; Costa, M.L.; Souza, J.P.; Mazhar, S.B.; Jayaratne, K.; Qureshi, Z.; Sousa, M.H.; et al. Twin Pregnancy and Severe Maternal Outcomes: The World Health Organization Multicountry Survey on Maternal and Newborn Health. Obstet. Gynecol. 2016, 127, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.K.; Driscoll, A.K. Births: Final Data for 2018. Natl. Vital Stat. Syst. 2019, 68, 1–47. [Google Scholar]
- Li, Y.; Yin, S.; Liu, C. The obstetric prognostic analysis of advanced maternal age women with twin pregnancy in multiple centers in China. Prog. Obstet. Gynecol. 2018, 678–681. [Google Scholar]
- Liu, S.; Geng, G.; Xiao, Q.; Zheng, Y.; Liu, X.; Cheng, J.; Zhang, Q. Tracking Daily Concentrations of PM(2.5) Chemical Composition in China since 2000. Environ. Sci. Technol. 2022, 56, 16517–16527. [Google Scholar] [CrossRef]
- Geng, G.; Xiao, Q.; Liu, S.; Liu, X.; Cheng, J.; Zheng, Y.; Xue, T.; Tong, D.; Zheng, B.; Peng, Y.; et al. Tracking Air Pollution in China: Near Real-Time PM(2.5) Retrievals from Multisource Data Fusion. Environ. Sci. Technol. 2021, 55, 12106–12115. [Google Scholar] [CrossRef]
- Zhou, G.; Wu, J.; Yang, M.; Sun, P.; Gong, Y.; Chai, J.; Zhang, J.; Afrim, F.K.; Dong, W.; Sun, R.; et al. Prenatal exposure to air pollution and the risk of preterm birth in rural population of Henan Province. Chemosphere 2022, 286, 131833. [Google Scholar] [CrossRef]
- Xue, T.; Zheng, Y.; Geng, G.; Xiao, Q.; Meng, X.; Wang, M.; Li, X.; Wu, N.; Zhang, Q.; Zhu, T. Estimating Spatiotemporal Variation in Ambient Ozone Exposure during 2013-2017 Using a Data-Fusion Model. Environ. Sci. Technol. 2020, 54, 14877–14888. [Google Scholar] [CrossRef]
- Hersbach, H.; Bell, W.; Berrisford, P.; Hoŕanyi, A.J.; Muñoz-Sabater, J.J.; Nicolas, J.; Radu, R.; Schepers, D.; Simmons, A.; Soci, C.; et al. Global reanalysis: Goodbye ERA-Interim, hello ERA5. ECMWF Newsl. 2019, 17–24. [Google Scholar] [CrossRef]
- Zhu, Q.; Ye, P.; Wang, Y.; Duan, L.; He, G.; Er, Y.; Jin, Y.; Ji, C.; Hu, J.; Deng, X.; et al. Heatwaves increase road traffic injury morbidity risk and burden in China and its provinces. Environ. Int. 2024, 188, 108760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Feng, D.; Gao, Z.; Gong, Y.; Zhang, J.; Song, S.; Yu, Z.; Huang, C. Interaction effects of night-time temperature and PM(2.5) on preterm birth in Huai River Basin, China. Environ. Int. 2023, 171, 107729. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jiang, Y.; Yang, Y.; Xu, J.; Zhang, Y.; Wang, Q.; Shen, H.; Zhang, Y.; Yan, D.; Peng, Z.; et al. Composition of fine particulate matter and risk of preterm birth: A nationwide birth cohort study in 336 Chinese cities. J. Hazard. Mater. 2022, 425, 127645. [Google Scholar] [CrossRef]
- Fu, L.; Guo, Y.; Zhu, Q.; Chen, Z.; Yu, S.; Xu, J.; Tang, W.; Wu, C.; He, G.; Hu, J.; et al. Effects of long-term exposure to ambient fine particulate matter and its specific components on blood pressure and hypertension incidence. Environ. Int. 2024, 184, 108464. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, Y.; Wang, W.; Chen, R.; Liu, Y.; Liu, C.; Kan, H.; Gao, Y.; Tian, Y. Critical windows for maternal fine particulate matter exposure and adverse birth outcomes: The Shanghai birth cohort study. Chemosphere 2020, 240, 124904. [Google Scholar] [CrossRef]
- Cai, J.; Zhao, Y.; Kan, J.; Chen, R.; Martin, R.; van Donkelaar, A.; Ao, J.; Zhang, J.; Kan, H.; Hua, J. Prenatal Exposure to Specific PM(2.5) Chemical Constituents and Preterm Birth in China: A Nationwide Cohort Study. Environ. Sci. Technol. 2020, 54, 14494–14501. [Google Scholar] [CrossRef]
- Smith, R.B.; Beevers, S.D.; Gulliver, J.; Dajnak, D.; Fecht, D.; Blangiardo, M.; Douglass, M.; Hansell, A.L.; Anderson, H.R.; Kelly, F.J.; et al. Impacts of air pollution and noise on risk of preterm birth and stillbirth in London. Environ. Int. 2020, 134, 105290. [Google Scholar] [CrossRef]
- Donaldson, K.; MacNee, W. Potential mechanisms of adverse pulmonary and cardiovascular effects of particulate air pollution (PM10). Int. J. Hyg. Environ. Health 2001, 203, 411–415. [Google Scholar] [CrossRef]
- Mohorovic, L. First two months of pregnancy—Critical time for preterm delivery and low birthweight caused by adverse effects of coal combustion toxics. Early Hum. Dev. 2004, 80, 115–123. [Google Scholar] [CrossRef]
- Vadillo-Ortega, F.; Osornio-Vargas, A.; Buxton, M.A.; Sánchez, B.N.; Rojas-Bracho, L.; Viveros-Alcaráz, M.; Castillo-Castrejón, M.; Beltrán-Montoya, J.; Brown, D.G.; O’Neill, M.S. Air pollution, inflammation and preterm birth: A potential mechanistic link. Med. Hypotheses 2014, 82, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Saenen, N.D.; Vrijens, K.; Janssen, B.G.; Madhloum, N.; Peusens, M.; Gyselaers, W.; Vanpoucke, C.; Lefebvre, W.; Roels, H.A.; Nawrot, T.S. Placental Nitrosative Stress and Exposure to Ambient Air Pollution During Gestation: A Population Study. Am. J. Epidemiol. 2016, 184, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.H.; Nelin, T.; Gorr, M.W.; Wold, L.E. Early life exposure to air pollution: How bad is it? Toxicol. Lett. 2013, 216, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Gehring, U.; Beelen, R.; Wang, M.; Giorgis-Allemand, L.; Andersen, A.M.; Basagaña, X.; Bernard, C.; Cirach, M.; Forastiere, F.; et al. Elemental Constituents of Particulate Matter and Newborn’s Size in Eight European Cohorts. Environ. Health Perspect. 2016, 124, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Ritz, B.; Yu, F.; Chapa, G.; Fruin, S. Effect of air pollution on preterm birth among children born in Southern California between 1989 and 1993. Epidemiology 2000, 11, 502–511. [Google Scholar] [CrossRef]
- Chang, H.H.; Reich, B.J.; Miranda, M.L. Time-to-event analysis of fine particle air pollution and preterm birth: Results from North Carolina, 2001–2005. Am. J. Epidemiol. 2012, 175, 91–98. [Google Scholar] [CrossRef]
- Mayer, C.; Joseph, K.S. Fetal growth: A review of terms, concepts and issues relevant to obstetrics. Ultrasound Obstet. Gynecol. 2013, 41, 136–145. [Google Scholar] [CrossRef]
- Sun, X.; Luo, X.; Zhao, C.; Chung Ng, R.W.; Lim, C.E.; Zhang, B.; Liu, T. The association between fine particulate matter exposure during pregnancy and preterm birth: A meta-analysis. BMC Pregnancy Childbirth 2015, 15, 300. [Google Scholar] [CrossRef]
- Sun, X.; Luo, X.; Zhao, C.; Zhang, B.; Tao, J.; Yang, Z.; Ma, W.; Liu, T. The associations between birth weight and exposure to fine particulate matter (PM2.5) and its chemical constituents during pregnancy: A meta-analysis. Environ. Pollut. 2016, 211, 38–47. [Google Scholar] [CrossRef]
- Grantz, K.L.; Kim, S.; Grobman, W.A.; Newman, R.; Owen, J.; Skupski, D.; Grewal, J.; Chien, E.K.; Wing, D.A.; Wapner, R.J.; et al. Fetal growth velocity: The NICHD fetal growth studies. Am. J. Obstet. Gynecol. 2018, 219, 285.e281–285.e236. [Google Scholar] [CrossRef]
- Laurent, O.; Hu, J.; Li, L.; Kleeman, M.J.; Bartell, S.M.; Cockburn, M.; Escobedo, L.; Wu, J. A Statewide Nested Case-Control Study of Preterm Birth and Air Pollution by Source and Composition: California, 2001–2008. Environ. Health Perspect. 2016, 124, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Darrow, L.A.; Klein, M.; Flanders, W.D.; Waller, L.A.; Correa, A.; Marcus, M.; Mulholland, J.A.; Russell, A.G.; Tolbert, P.E. Ambient air pollution and preterm birth: A time-series analysis. Epidemiology 2009, 20, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, D.; Yao, L.; Fu, H.; Fu, Q.; Wang, H.; Li, Q.; Wang, L.; Yang, X.; Xian, A.; et al. Chemistry-triggered events of PM(2.5) explosive growth during late autumn and winter in Shanghai, China. Environ. Pollut. 2019, 254, 112864. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, G.; Guo, S.; Zamora, M.L.; Ying, Q.; Lin, Y.; Wang, W.; Hu, M.; Wang, Y. Formation of urban fine particulate matter. Chem. Rev. 2015, 115, 3803–3855. [Google Scholar] [CrossRef] [PubMed]
- Ottone, M.; Broccoli, S.; Parmagnani, F.; Giannini, S.; Scotto, F.; Bonvicini, L.; Luberto, F.; Bacco, D.; Trentini, A.; Poluzzi, V.; et al. Source-related components of fine particulate matter and risk of adverse birth outcomes in Northern Italy. Environ. Res. 2020, 186, 109564. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Dai, J.; Liu, R.; Zhai, Y.; Yue, D.; Hu, Q. Integrated assessment of health risk and climate effects of black carbon in the Pearl River Delta region, China. Environ. Res. 2019, 176, 108522. [Google Scholar] [CrossRef] [PubMed]
- Ebisu, K.; Malig, B.; Hasheminassab, S.; Sioutas, C.; Basu, R. Cause-specific stillbirth and exposure to chemical constituents and sources of fine particulate matter. Environ. Res. 2018, 160, 358–364. [Google Scholar] [CrossRef]
- Liu, C.; Cai, J.; Qiao, L.; Wang, H.; Xu, W.; Li, H.; Zhao, Z.; Chen, R.; Kan, H. The Acute Effects of Fine Particulate Matter Constituents on Blood Inflammation and Coagulation. Environ. Sci. Technol. 2017, 51, 8128–8137. [Google Scholar] [CrossRef]
- Weckman, A.M.; Ngai, M.; Wright, J.; McDonald, C.R.; Kain, K.C. The Impact of Infection in Pregnancy on Placental Vascular Development and Adverse Birth Outcomes. Front. Microbiol. 2019, 10, 1924. [Google Scholar] [CrossRef]
- Kannan, S.; Misra, D.P.; Dvonch, J.T.; Krishnakumar, A. Exposures to airborne particulate matter and adverse perinatal outcomes: A biologically plausible mechanistic framework for exploring potential effect modification by nutrition. Environ. Health Perspect. 2006, 114, 1636–1642. [Google Scholar] [CrossRef]
- Basu, R.; Pearson, D.; Ebisu, K.; Malig, B. Association between PM(2.5) and PM(2.5) Constituents and Preterm Delivery in California, 2000–2006. Paediatr. Perinat. Epidemiol. 2017, 31, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Laurent, O.; Hu, J.; Li, L.; Cockburn, M.; Escobedo, L.; Kleeman, M.J.; Wu, J. Sources and contents of air pollution affecting term low birth weight in Los Angeles County, California, 2001-2008. Environ. Res. 2014, 134, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J.; Johnstone, A.F.; Aydin, C. Thermal stress and toxicity. Compr. Physiol. 2014, 4, 995–1016. [Google Scholar] [CrossRef] [PubMed]
- Alahmad, B.; Khraishah, H.; Shakarchi, A.F.; Albaghdadi, M.; Rajagopalan, S.; Koutrakis, P.; Jaffer, F.A. Cardiovascular Mortality and Exposure to Heat in an Inherently Hot Region: Implications for Climate Change. Circulation 2020, 141, 1271–1273. [Google Scholar] [CrossRef] [PubMed]
- Kelly, F.J.; Fussell, J.C. Linking ambient particulate matter pollution effects with oxidative biology and immune responses. Ann. N. Y. Acad. Sci. 2015, 1340, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, L.; Fan, P.; Tian, L.; Lafortezza, R. Do green spaces affect the spatiotemporal changes of PM(2.5) in Nanjing? Ecol. Process. 2016, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, P.J.; Jerrett, M.; Su, J.G.; Weichenthal, S.; Sandler, D.P. Association of residential greenness with obesity and physical activity in a US cohort of women. Environ. Res. 2018, 160, 372–384. [Google Scholar] [CrossRef]
- Wei, J.; Liu, C.; Cui, H.; Zhang, L.; Chen, J.; Liu, S.; Chang, J.; Zheng, D. Diagnosis, treatment and health care guidelines for preterm birth in twin pregnancy (2020 edition). Chin. J. Prac. Gynecol. Obstet. 2020, 36, 949–956. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Arduino, S.; Attini, R.; Parisi, S.; Fassio, F.; Biolcati, M.; Pagano, A.; Bossotti, C.; Vasario, E.; Borgarello, V.; et al. Multiple pregnancies in CKD patients: An explosive mix. Clin. J. Am. Soc. Nephrol. 2013, 8, 41–50. [Google Scholar] [CrossRef]
| Characteristics n (%)/Mean ± SD | Total n = 8458 | PTB n = 5592 | Term n = 2866 | p Value a |
|---|---|---|---|---|
| Maternal age (Years) | 31.1 ± 4.56 | 31.1 ± 4.72 | 31.3 ± 4.25 | 0.038 |
| Maternal age (Years) | ||||
| <30 | 3075 (36.4) | 2094 (37.5) | 981 (34.3) | 0.004 |
| ≥30 | 5370 (63.6) | 3492 (62.5) | 1878 (65.7) | |
| Gestational age (Weeks) | 35.4 ± 2.56 | 34.4 ± 2.55 | 37.5 ± 0.53 | <0.001 |
| Residential area | 0.001 | |||
| Southern | 7031 (83.1) | 4596 (82.2) | 2435 (85.0) | |
| Northern | 1427 (16.9) | 996 (17.8) | 431 (15.0) | |
| Residential area | <0.001 | |||
| Rural | 2870 (33.9) | 2024 (36.2) | 846 (29.5) | |
| Urban | 5588 (66.1) | 3568 (63.8) | 2020 (70.5) | |
| Parity | 1.000 | |||
| Nulliparous | 5510 (65.3) | 3644 (65.2) | 1866 (65.3) | |
| Multiparous | 2934 (34.7) | 1941 (34.8) | 993 (34.7) | |
| Type of conception | 0.001 | |||
| Natural pregnancy | 3372 (40.1) | 2297 (41.3) | 1075 (37.6) | |
| Assisted reproduction | 5047 (59.9) | 3266 (58.7) | 1781 (62.4) | |
| Mode of delivery | <0.001 | |||
| Vaginal | 948 (11.2) | 797 (14.3) | 151 (5.29) | |
| Cesarean | 7483 (88.8) | 4777 (85.7) | 2706 (94.7) | |
| Gestational diabetes | 0.449 | |||
| No | 6511 (78.3) | 4281 (78.0) | 2230 (78.8) | |
| Yes | 1807 (21.7) | 1206 (22.0) | 601 (21.2) | |
| Gestational hypertension | <0.001 | |||
| No | 7027 (84.6) | 4458 (81.3) | 2569 (91.1) | |
| Yes | 1278 (15.4) | 1028 (18.7) | 250 (8.87) | |
| Birth season | 0.731 | |||
| Spring | 2171 (25.7) | 1432 (25.6) | 739 (25.8) | |
| Summer | 2281 (27.0) | 1510 (27.0) | 771 (26.9) | |
| Autumn | 1915 (22.6) | 1250 (22.4) | 665 (23.2) | |
| Winter | 2091 (24.7) | 1400 (25.0) | 691 (24.1) | |
| Sex | <0.001 | |||
| Male-female | 3023 (35.7) | 1901 (34.0) | 1122 (39.1) | |
| Male-male | 3053 (36.1) | 2107 (37.7) | 946 (33.0) | |
| Female-female | 2382 (28.2) | 1584 (28.3) | 798 (27.8) | |
| Zygosity chorionicity | <0.001 | |||
| Monochorionic monoamniotic | 96 (1.20) | 84 (1.61) | 12 (0.43) | |
| Monochorionic diamniotic | 1736 (21.7) | 1338 (25.7) | 398 (14.3) | |
| Dichorionic diamniotic | 6173 (77.1) | 3794 (72.7) | 2379 (85.3) | |
| PM2.5 (μg/m3) | 37.5 (12.8) | 38.2 (13.1) | 36.0 (11.8) | <0.001 |
| NH4+ (μg/m3) | 4.52 (2.04) | 4.63 (2.07) | 4.30 (1.96) | <0.001 |
| SO42− (μg/m3) | 7.11 (2.44) | 7.25 (2.55) | 6.82 (2.19) | <0.001 |
| NO3− (μg/m3) | 6.47 (3.31) | 6.60 (3.36) | 6.20 (3.18) | <0.001 |
| BC (μg/m3) | 2.05 (0.69) | 2.08 (0.73) | 1.97 (0.61) | <0.001 |
| OM (μg/m3) | 9.92 (3.04) | 10.0 (3.19) | 9.69 (2.72) | <0.001 |
| Pollutants | Entire Pregnancy | Trimester 1 | Trimester 2 | Trimester 3 | ||||
|---|---|---|---|---|---|---|---|---|
| IQR | OR (95% CI) | IQR | OR (95% CI) | IQR | OR (95% CI) | IQR | OR (95% CI) | |
| (μg/m3) | (μg/m3) | (μg/m3) | (μg/m3) | |||||
| PM2.5 | 17.57 | 1.46 (1.34, 1.59) | 20.58 | 1.33 (1.22, 1.46) | 19.78 | 1.26 (1.16, 1.37) | 20.48 | 1.40 (1.28, 1.52) |
| NH4+ | 2.98 | 1.54 (1.39, 1.70) | 3.17 | 1.29 (1.18, 1.41) | 3.18 | 1.24 (1.13, 1.35) | 3.15 | 1.37 (1.26, 1.49) |
| SO42− | 2.81 | 1.34 (1.25, 1.44) | 3.19 | 1.24 (1.16, 1.32) | 3.14 | 1.20 (1.13, 1.29) | 3.32 | 1.34 (1.25, 1.43) |
| NO3− | 4.55 | 1.44 (1.30, 1.59) | 5.25 | 1.23 (1.13, 1.35) | 5.27 | 1.19 (1.09, 1.31) | 5.09 | 1.34 (1.23, 1.47) |
| BC | 0.78 | 1.28 (1.20, 1.37) | 0.94 | 1.24 (1.16, 1.32) | 0.93 | 1.17 (1.09, 1.25) | 0.99 | 1.25 (1.17, 1.34) |
| OM | 3.84 | 1.28 (1.18, 1.38) | 4.81 | 1.20 (1.11, 1.30) | 4.69 | 1.14 (1.05, 1.23) | 5.03 | 1.25 (1.16, 1.35) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Zhong, X.; Peng, W.; Chen, Z.; Wang, L.; Xia, C.; Huang, Y.; Zhu, Q.; Fan, Y.; Lai, Y.; et al. Associations of Prenatal Exposures to Fine Particulate Matter and Its Compositions with Preterm Birth Risk in Twins. Green Health 2025, 1, 11. https://doi.org/10.3390/greenhealth1020011
Zheng Y, Zhong X, Peng W, Chen Z, Wang L, Xia C, Huang Y, Zhu Q, Fan Y, Lai Y, et al. Associations of Prenatal Exposures to Fine Particulate Matter and Its Compositions with Preterm Birth Risk in Twins. Green Health. 2025; 1(2):11. https://doi.org/10.3390/greenhealth1020011
Chicago/Turabian StyleZheng, Yuan, Xinqi Zhong, Wan Peng, Zhiqing Chen, Lv Wang, Changshun Xia, Yixiang Huang, Qijiong Zhu, Yuwei Fan, Yiyu Lai, and et al. 2025. "Associations of Prenatal Exposures to Fine Particulate Matter and Its Compositions with Preterm Birth Risk in Twins" Green Health 1, no. 2: 11. https://doi.org/10.3390/greenhealth1020011
APA StyleZheng, Y., Zhong, X., Peng, W., Chen, Z., Wang, L., Xia, C., Huang, Y., Zhu, Q., Fan, Y., Lai, Y., Cui, Q., & Liu, T. (2025). Associations of Prenatal Exposures to Fine Particulate Matter and Its Compositions with Preterm Birth Risk in Twins. Green Health, 1(2), 11. https://doi.org/10.3390/greenhealth1020011





