Sugar Industry Wastewater Treatment Through Photosynthetic Microbial Desalination Cells: A Sustainable Approach †
Abstract
1. Introduction
2. Materials and Methods
2.1. Reactor Configuration
2.2. Reactor Inoculation
2.3. Reactor Operation
2.4. Reactor Analysis
3. Results and Discussion
3.1. Wastewater Treatment at Anode and Cathode
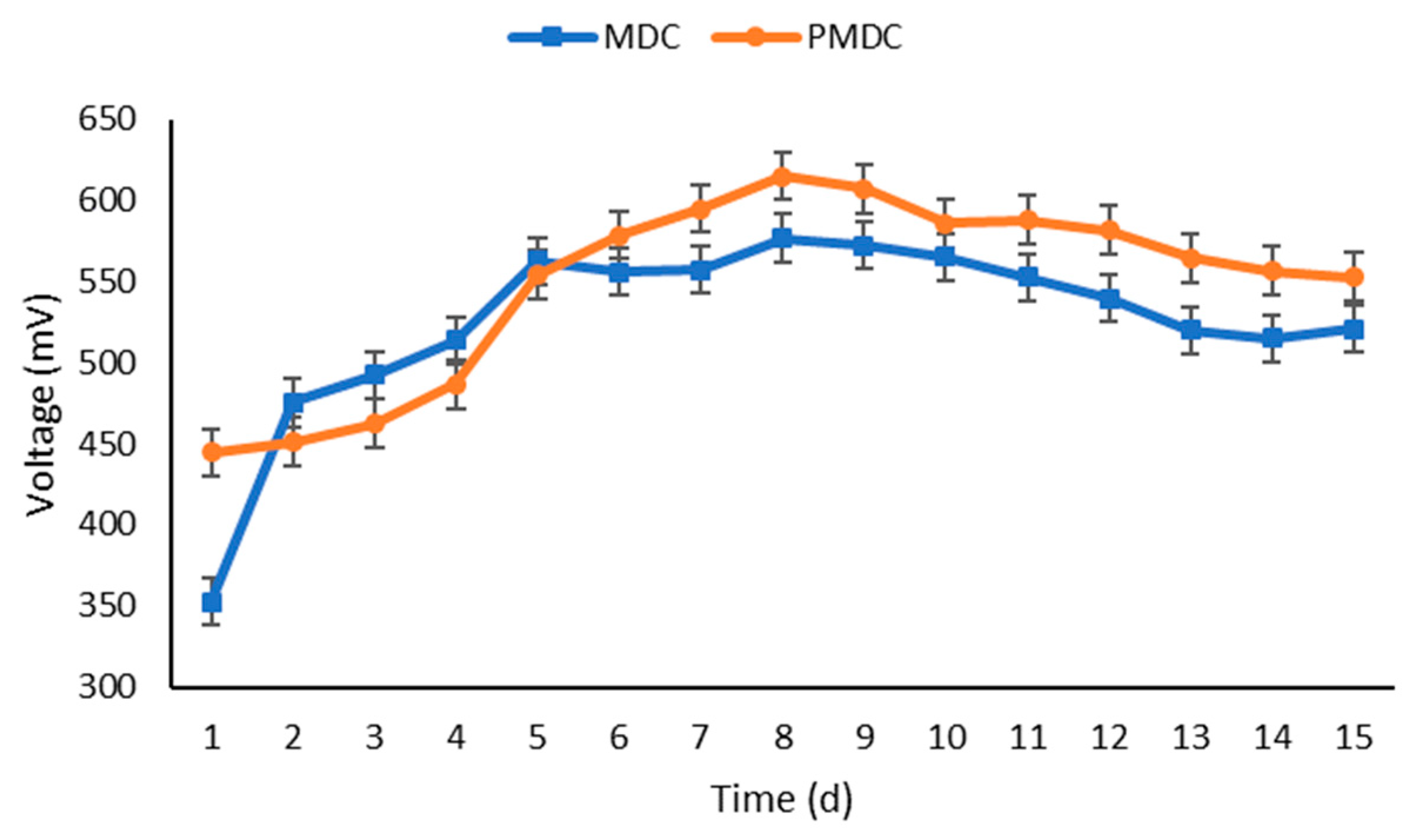
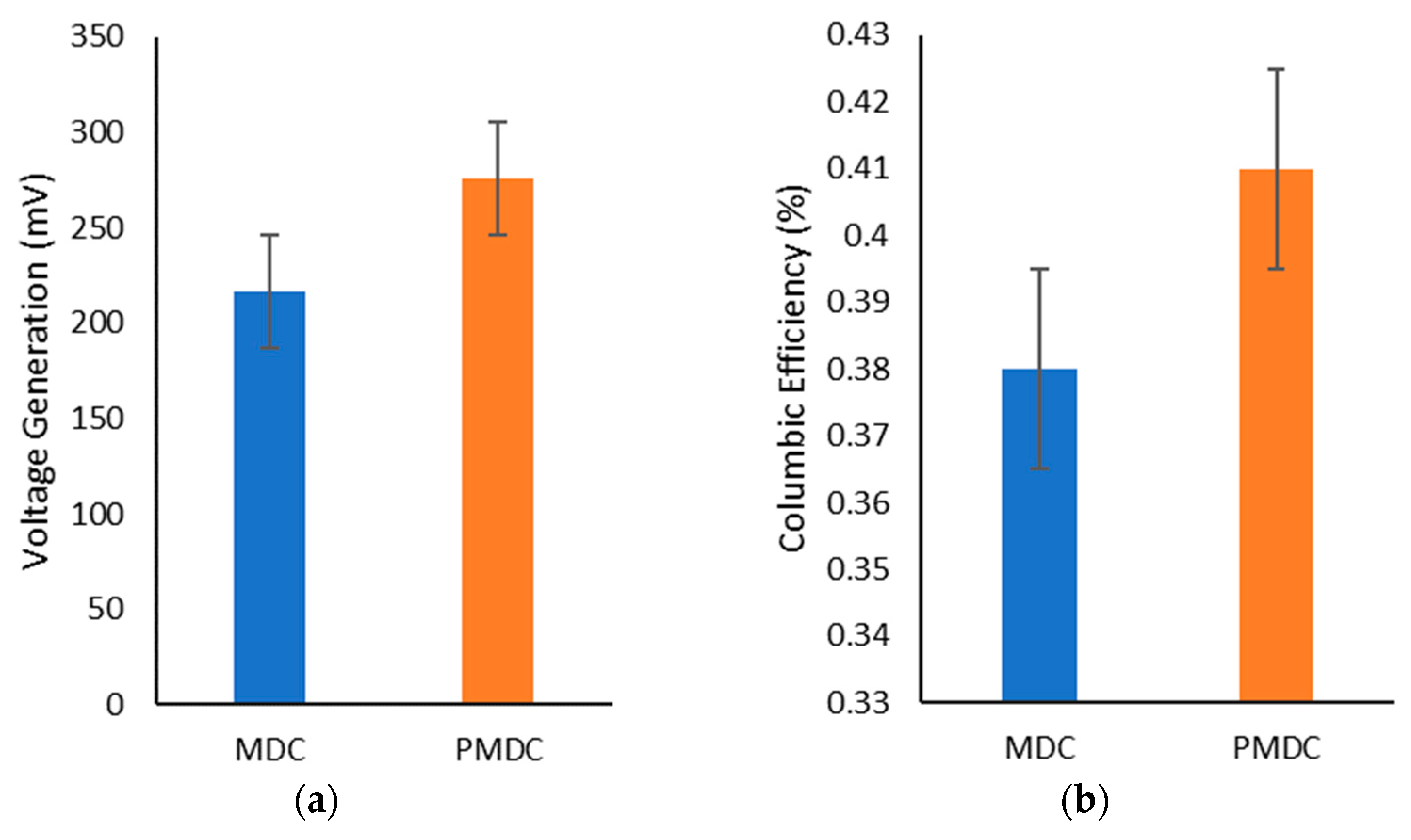
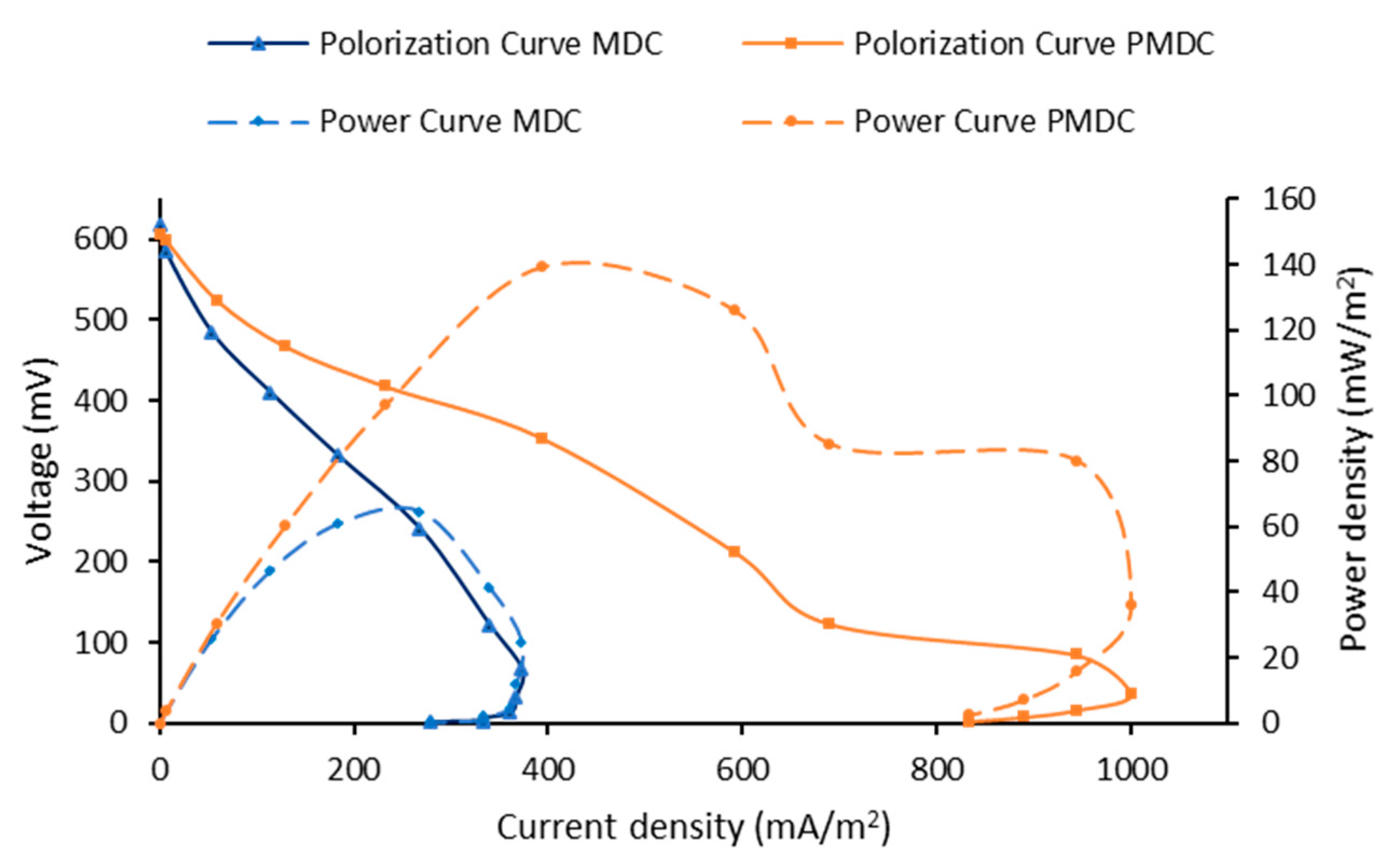
3.2. Power Generation
3.3. Desalination and Conductivity
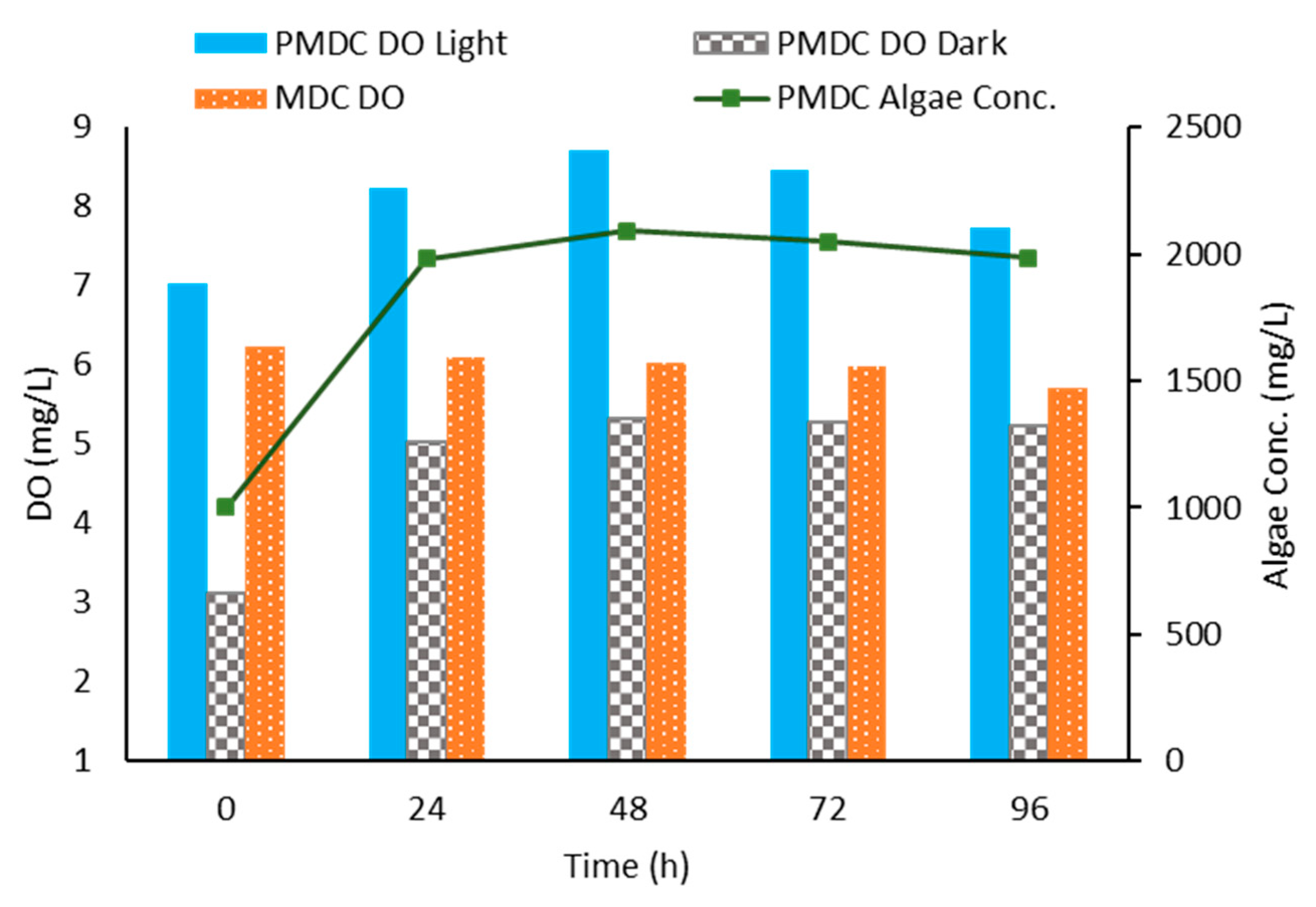
3.4. Dissolved Oxygen Levels at the Cathode
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fito, J.; Tefera, N.; Van Hulle, S.W.H. Sugarcane Biorefineries Wastewater: Bioremediation Technologies for Environmental Sustainability. Chem. Biol. Technol. Agric. 2019, 6, 6. [Google Scholar] [CrossRef]
- Barbosa, F.D.S.; Coelho, R.D.; Barros, T.H.D.S.; Lizcano, J.V.; Fraga Júnior, E.F.; Santos, L.D.C.; Leal, D.P.V.; Ribeiro, N.L.; Costa, J.D.O. Sugarcane Water Productivity for Bioethanol, Sugar and Biomass under Deficit Irrigation. AgriEngineering 2024, 6, 1117–1132. [Google Scholar] [CrossRef]
- Sahu, O.P.; Chaudhari, P.K. Electrochemical Treatment of Sugar Industry Wastewater: COD and Color Removal. J. Electroanal. Chem. 2015, 739, 122–129. [Google Scholar] [CrossRef]
- Sahu, O. Electro-Oxidation and Chemical Oxidation Treatment of Sugar Industry Wastewater with Ferrous Material: An Investigation of Physicochemical Characteristic of Sludge. S. Afr. J. Chem. Eng. 2019, 28, 26–38. [Google Scholar] [CrossRef]
- Sun, Q.; Tang, M.; Hendriksen, P.V.; Chen, B. Biotemplated Fabrication of a 3D Hierarchical Structure of Magnetic ZnFe2O4/MgAl-LDH for Efficient Elimination of Dye from Water. J. Alloys Compd. 2020, 829, 154552. [Google Scholar] [CrossRef]
- Imoro, A.Z.; Mensah, M.; Buamah, R. Developments in the Microbial Desalination Cell Technology: A Review. Water-Energy Nexus 2021, 4, 76–87. [Google Scholar] [CrossRef]
- Sevda, S.; Yuan, H.; He, Z.; Abu-Reesh, I.M. Microbial Desalination Cells as a Versatile Technology: Functions, Optimization and Prospective. Desalination 2015, 371, 9–17. [Google Scholar] [CrossRef]
- Zahid, M.; Savla, N.; Pandit, S.; Thakur, V.K.; Jung, S.P.; Gupta, P.K.; Prasad, R.; Marsili, E. Microbial Desalination Cell: Desalination through Conserving Energy. Desalination 2022, 521, 115381. [Google Scholar] [CrossRef]
- Aber, S.; Shi, Z.; Xing, K.; Rameezdeen, R.; Chow, C.W.K.; Hagare, D.; Jindal, T. Microbial Desalination Cell for Sustainable Water Treatment: A Critical Review. Glob. Chall. 2023, 7, 2300138. [Google Scholar] [CrossRef]
- Prakash, S.; Ponnusamy, K.; Naina Mohamed, S. An Insight on Biocathode Microbial Desalination Cell: Current Challenges and Prospects. Int. J. Energy Res. 2022, 46, 8546–8559. [Google Scholar] [CrossRef]
- Kokabian, B.; Gude, V.G. Photosynthetic Microbial Desalination Cells (PMDCs) for Clean Energy, Water and Biomass Production. Environ. Sci. Process. Impacts 2013, 15, 2178. [Google Scholar] [CrossRef] [PubMed]
- Nadzri, N.A.A.; Mohd Yasin, N.H.; Abu Bakar, M.H.; Thanakodi, S.; Salehmin, M.N.I.; Takriff, M.S.; Ni Aznan, M.F.; Maeda, T. Photosynthetic Microbial Desalination Cell (PhMDC) Using Chlamydomonas Sp. (UKM6) and Scenedesmus Sp. (UKM9) as Biocatalysts for Electricity Production and Water Treatment. Int. J. Hydrog. Energy 2023, 48, 11860–11873. [Google Scholar] [CrossRef]
- Jaroo, S.S.; Jumaah, G.F.; Abbas, T.R. Photosynthetic Microbial Desalination Cell to Treat Oily Wastewater Using Microalgae Chlorella Vulgaris. Civ. Eng. J. 2019, 5, 2686–2699. [Google Scholar] [CrossRef]
- Ashwaniy, V.R.V.; Perumalsamy, M. Reduction of Organic Compounds in Petro-Chemical Industry Effluent and Desalination Using Scenedesmus Abundans Algal Microbial Desalination Cell. J. Environ. Chem. Eng. 2017, 5, 5961–5967. [Google Scholar] [CrossRef]
- Zamanpour, M.K.; Kariminia, H.R.; Vosoughi, M. Electricity Generation, Desalination and Microalgae Cultivation in a Biocathode-Microbial Desalination Cell. J. Environ. Chem. Eng. 2017, 5, 843–848. [Google Scholar] [CrossRef]
- Bejjanki, D.; Muthukumar, K.; Radhakrishnan, T.K.; Alagarsamy, A.; Pugazhendhi, A.; Mohamed, S.N. Simultaneous Bioelectricity Generation and Water Desalination Using Oscillatoria Sp. as Biocatalyst in Photosynthetic Microbial Desalination Cell. Sci. Total Environ. 2021, 754, 142215. [Google Scholar] [CrossRef] [PubMed]
- Tanksali, A.S. Treatment of Sugar Industry Wastewater by Upflow Anaerobic Sludge Blanket Reactor. Int. J. ChemTech Res. 2013, 5, 1246–1253. [Google Scholar]
- Zhou, Q.; Zhang, P.; Zhang, G. Biomass and Pigments Production in Photosynthetic Bacteria Wastewater Treatment: Effects of Light Sources. Bioresour. Technol. 2015, 179, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Z.; Su, X.; Zhao, P.; Zhou, J.; He, Q.; Ai, H. Cost-Effective Domestic Wastewater Treatment and Bioenergy Recovery in an Immobilized Microalgal-Based Photoautotrophic Microbial Fuel Cell (PMFC). Chem. Eng. J. 2019, 372, 956–965. [Google Scholar] [CrossRef]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. (Eds.) Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 2017; ISBN 978-0-87553-287-5. [Google Scholar]
- Zafar, A.M.; Javed, M.A.; Aly Hassan, A. Unprecedented Biodesalination Rates–Shortcomings of Electrical Conductivity Measurements in Determining Salt Removal by Algae and Cyanobacteria. J. Environ. Manag. 2022, 302, 113947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Angelidaki, I. Submersible Microbial Desalination Cell for Simultaneous Ammonia Recovery and Electricity Production from Anaerobic Reactors Containing High Levels of Ammonia. Bioresour. Technol. 2015, 177, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Yang, Z.; Chen, S.; Pei, H. Using an Anaerobic Digestion Tank as the Anodic Chamber of an Algae-Assisted Microbial Fuel Cell to Improve Energy Production from Food Waste. Water Res. 2020, 170, 115305. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Chen, X.; Lei, Z.; Yuan, T.; Shimizu, K.; Zhang, Z.; Lee, D.-J. Insight into Aerobic Phosphorus Removal from Wastewater in Algal-Bacterial Aerobic Granular Sludge System. Bioresour. Technol. 2022, 352, 127104. [Google Scholar] [CrossRef]
- Kokabian, B.; Ghimire, U.; Gude, V.G. Water Deionization with Renewable Energy Production in Microalgae—Microbial Desalination Process. Renew. Energy 2018, 122, 354–361. [Google Scholar] [CrossRef]
- Abadikhah, M.; Rodriguez, M.D.C.; Persson, F.; Wilén, B.-M.; Farewell, A.; Modin, O. Evidence of Competition between Electrogens Shaping Electroactive Microbial Communities in Microbial Electrolysis Cells. Front. Microbiol. 2022, 13, 959211. [Google Scholar] [CrossRef]
- Rizo, R.; Feliu, J.M.; Herrero, E. New Insights into the Hydrogen Peroxide Reduction Reaction and Its Comparison with the Oxygen Reduction Reaction in Alkaline Media on Well-Defined Platinum Surfaces. J. Catal. 2021, 398, 123–132. [Google Scholar] [CrossRef]


| Materials | Specifications |
|---|---|
| Reactor’s working volume (A/D/C) | 900:450:900 mL (1:0.5:1) |
| Surface Area of Graphite Rods | 32.28 cm2 |
| Surface Area of Carbon Fiber Brushes | 183.13 cm2 |
| Surface Area of Ion Exchange Membranes | 128 cm2 |
| Light Intensity for PMDC | 150 μmol/m/S |
| Mixing for PMDC | 145 rpm |
| Aeration for MDC | 100 mL/min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, S.S.; Sheikh, Z. Sugar Industry Wastewater Treatment Through Photosynthetic Microbial Desalination Cells: A Sustainable Approach. Environ. Earth Sci. Proc. 2025, 32, 9. https://doi.org/10.3390/eesp2025032009
Ali SS, Sheikh Z. Sugar Industry Wastewater Treatment Through Photosynthetic Microbial Desalination Cells: A Sustainable Approach. Environmental and Earth Sciences Proceedings. 2025; 32(1):9. https://doi.org/10.3390/eesp2025032009
Chicago/Turabian StyleAli, Syeda Safina, and Zeshan Sheikh. 2025. "Sugar Industry Wastewater Treatment Through Photosynthetic Microbial Desalination Cells: A Sustainable Approach" Environmental and Earth Sciences Proceedings 32, no. 1: 9. https://doi.org/10.3390/eesp2025032009
APA StyleAli, S. S., & Sheikh, Z. (2025). Sugar Industry Wastewater Treatment Through Photosynthetic Microbial Desalination Cells: A Sustainable Approach. Environmental and Earth Sciences Proceedings, 32(1), 9. https://doi.org/10.3390/eesp2025032009







