2. Methodology
This study was carried out at the Nursery of the Institute of Forestry and Forest Management of the Faculty of Forestry Sciences, El Zanjón headquarters of the University of Santiago del Estero, Argentina.
In the first instance, activities related to the sterilization of the containers (250 cm
3 tubes with their respective trays) where the seedlings were going to be developed were carried out. As a second activity, the substrate was prepared (
Figure 1a) with composted pine bark and agricultural perlite in a 50:50 (
v:
v) proportion and slow-release granulated fertilizer. Lastly, a fungicide was incorporated and allowed to rest for one day.
Subsequently, 243 tubes (four-and-a-half trays) were filled to begin the planting stage. The germplasm used came from a mass selection of stands located in the Robles department, province of Santiago del Estero (2021 harvest). Sowing began on 22 February 2024.
Regarding cultural activities, irrigation was automated; in addition, preventive chemical treatments were applied with a backpack sprayer (
Figure 1b) (fungicide and insecticide) on a weekly basis.
The monitoring of the germination percentage per tray was carried out for 8 days (
Table 1) (
Figure 2).
On 10 March 2024 (
Table 2), the rapid growth phase began. It was estimated to last approximately 45 days. The vast majority of individuals had to reach the established DAC and stem height to be able to subsequently move on to the rustification stage.
During this phase, irrigation was reinforced. The plants were watered for 15 min at 7 am and 1 pm. The variations that occurred in the course of the process with respect to automatic irrigation were caused by the meteorological conditions present during the development of the test. In the case of persistent rain, irrigation was canceled because it was not necessary. The optimal fertilizer for this stage was applied once a week.
On 8 May 2024, it was determined that the rustification stage began due to the data collected from the DAC and stem height. As a first measure, the plants were relocated outside the greenhouse, in a space where there was direct light. In addition, the number of waterings per day was reduced to only one 15 min watering at 1 pm.
The collection of the species
Larrea divaricata (
Figure 3a) was carried out in the rural area of Santiago del Estero Capital, specifically, in the town of Santa María on the side of Route 9.
Branches containing leaves, flowers, etc., were collected. They were dried by natural drying, which consisted of leaving them in full sun for 3 days (summer time).
Once the drying process was completed, they were taken to INTA La María, and the jarilla powder which was going to be used for the extract was obtained using a grinding mill (
Figure 3b).
In the FAyA laboratory, the phytoextract began to be prepared, for which the following procedure was carried out:
In the first instance, the mass used for the preparation of a Jarilla concentration of 3% and 4% was calculated, the appropriate concentration and application method are decisive to maximize the benefits of biostimulants [
4]. A volume of 15 mL/plant was determined for the application of the biostimulant; we worked with 81 individuals per treatment.
Therefore, for Jarilla 3% (3 g of jarilla powder per 100 mL), a mass of 75 g of powder was worked, while for Jarilla 4% (4 g of jarilla powder per 100 mL), the mass was 100 g to obtain the phytoextract. To calculate the volume of biostimulant used, per seedling, the base volume of the backpack was taken into account. It was decided to round up to a final volume of 2500 mL.
It should be noted that, in the laboratory, the concentrations were prepared at a volume of 250 mL (concentrated volume); at the time of application, the final volume (2500 mL) was reached with the incorporation of distilled water.
Subsequently, in 2 microwave-safe containers, the masses corresponding to each preparation were weighed (precision balance) and distilled water was added until a volume between 200 and 250 mL was reached (it is important to respect the final volume, and in any case, it is recommended in the first instance to work with a lower volume such as 240 mL and at the end of the process to add the necessary amount of distilled water).
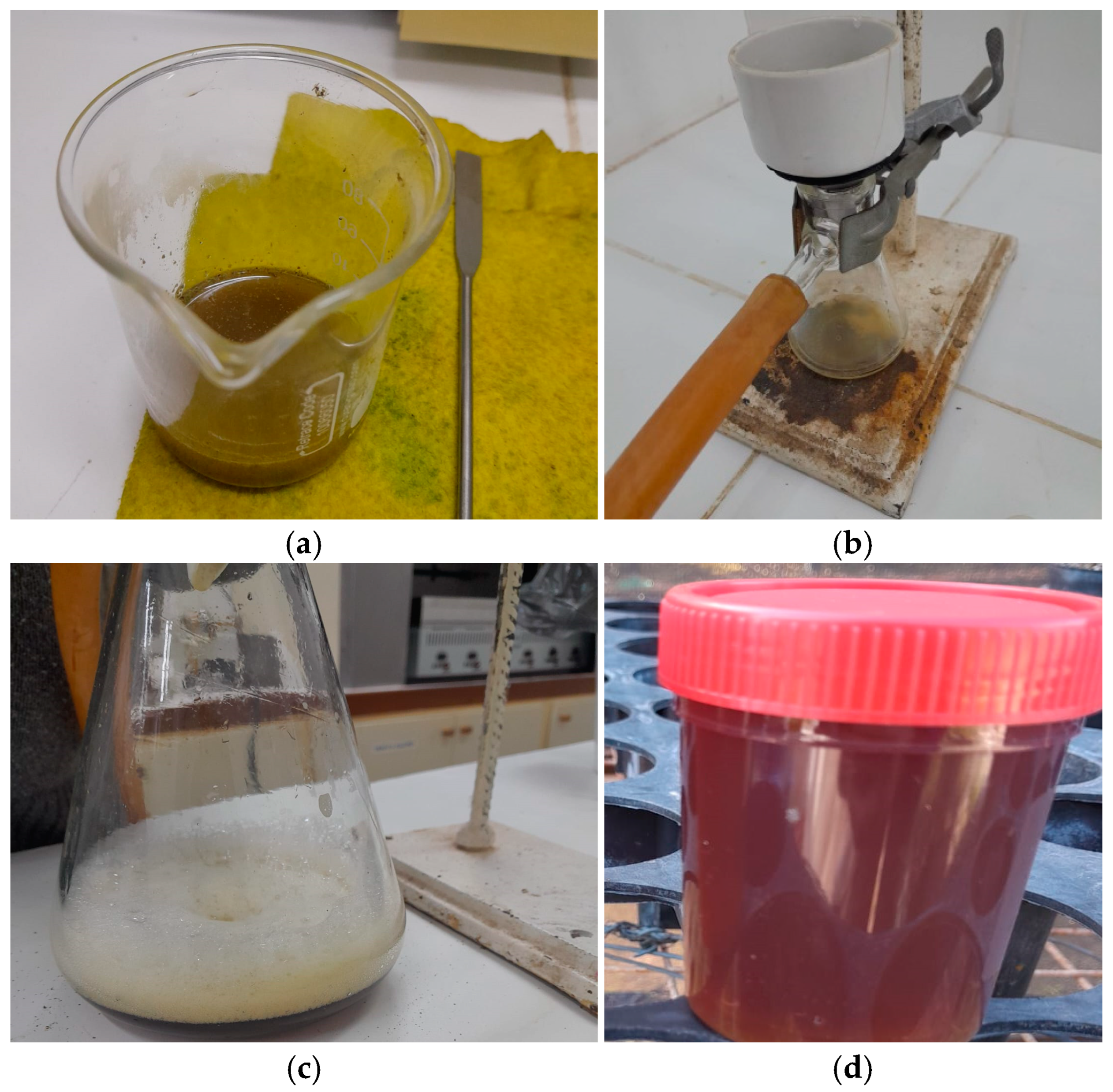
Then, the preparation was placed in the microwave, using the “heat” function for 4 min. This process was conducted at intervals to prevent the mixture from starting to boil (
Figure 4a). It is recommended to remove the preparation approximately every 30 s and cool it with gentle surrounding movements. Alternatively, a damp cloth can be used to reduce the temperature.
Once the microwave heating process was completed, vacuum extraction was carried out with the help of a vacuum pump (
Figure 4b). A paper filter cut to size was placed inside the ceramic funnel, on which the preparation was deposited. The filtered product was transferred to its respective containers, where the initially proposed preparation volume was completed(
Figure 4c).
The biostimulant must be applied in two doses (
Table 3), with a difference of 7 days between them. It is recommended to store the second dose in the freezer until its next application(
Figure 4d).
The morphological parameters total dry weight (PST), aerial dry weight (PSA) and root dry weight (PSR) were recorded 207 days after sowing (16 September 2024). The recording of these parameters was made towards the end of the vegetative dormancy period when the plants began to grow again. Plant biomass was determined by the destructive method, using nine seedlings per treatment (
Figure 5a,b). The aerial and radical parts were separated for subsequent drying in an oven with forced air circulation at 72 °C for 48 h. The dry weight was recorded on an electronic scale with a resolution of 0.001 g (
Figure 5c).
Based on the morphological attributes mentioned above, the following indices were calculated: the aerial biomass and root biomass ratio (PSA/PSR), which represents the weight ratio between the tissue that evapotranspirates and photosynthesizes (aerial part) and the tissue that absorbs water and nutrients and at the same time consumes carbohydrates through respiration (roots); the slenderness index (IE) (Thompson, 1985) [
5], which is the quotient or ratio between the total height of the plant (cm) and its DAC (mm), and is an indicator of crop density; and the plant quality index (Dickson, 1960) [
6] that represents morphological quality; the higher the value of this index, the greater the plant quality.
The treatment with Jarilla 4% shows a tendency to have a greater DAC (mm) (1.96 ± 0.14), which suggests that this concentration could be favoring a greater development of the neck diameter compared to the control (1.71 ± 0.14) and Jarilla 3% (1.61 ± 0.14) (
Table 4). Higher DAC is generally an indicator of a more robust plant, which could be beneficial to transplant success.
Regarding height (cm), the treatment with Jarilla 3% has a slightly higher value (15.71 ± 0.71) compared to Jarilla 4% (14.74 ± 0.71) and the control (15.22 ± 0.71). Although the difference is not significant, there could be a tendency for 3% jarilla to promote more favorable growth in height than the 4% concentration. Taking this information into account, it must be emphasized that a shorter plant in arid climates could be better adapted to reduce water loss through transpiration and minimize water stress caused by high solar radiation and dry wind.
The PSR (g) values are very similar between the three treatments, with a slight tendency for the Jarilla 3% treatment to have a slightly higher value (0.18 ± 0.1) compared to the control (0.17 ± 0.1) and Jarilla 4% (0.17 ± 0.1).
Finally, the Dickson index, an indicator of the overall quality of the plant, is very similar between the treatments, but with a small tendency towards improvement in the treatment with Jarilla 4% (0.03 ± 8 × 10−3). This suggests that the use of 4% jarilla could be contributing to a slightly better distribution of growth between the aerial part and the roots.
3. Conclusions
Although the differences are not significant, a tendency can be observed that the treatment with Jarilla 4% favors the diameter of the neck and the Dickson index, which are important indicators of robustness and plant quality.
DAC is a crucial parameter in the evaluation of plant quality, since a larger diameter indicates greater vigor and robustness, which are important factors for success in the field, especially in stress situations such as transplanting. Although the difference is not significant, the trend towards a higher DAC could reflect that the 4% jarilla concentration promotes greater structural strengthening in the plant, better preparing it to face adverse conditions.
Regarding the Dickson index (CI), which is considered an integral quality parameter because it weighs the aerial and root biomass in relation to height and diameter, it also shows a slight favorable trend in the treatment with Jarilla 4% (0.03 ± 8 × 10−3). This index is essential to evaluate the balance between the development of the aerial part and the roots, and a higher value suggests that the plant has a more balanced growth, with an optimal distribution of biomass to maximize its survival and future growth capacity.
This trend indicates that the 4% jarilla could be helping to optimize the balance between the growth of the aerial part and the roots, improving the efficiency of resource use and favoring the general quality of the plant.
Although the root dry weight (PSR) does not show significant differences, the Jarilla 4% treatment maintains similar values to the control (0.17 ± 0.1). This suggests that, although the 4% jarilla does not promote more pronounced root development than the control, it does not inhibit it either.
These trends do not reach statistical significance (p > 0.05), but they could be indications that the use of 4% jarilla can improve certain structural aspects of the plants, which should be considered in future studies with a greater number of repetitions, different experimental conditions or even higher doses (higher concentration).