Effects of Siberian Marmot Density in an Anthropogenic Ecosystem on Habitat Vegetation Modification
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
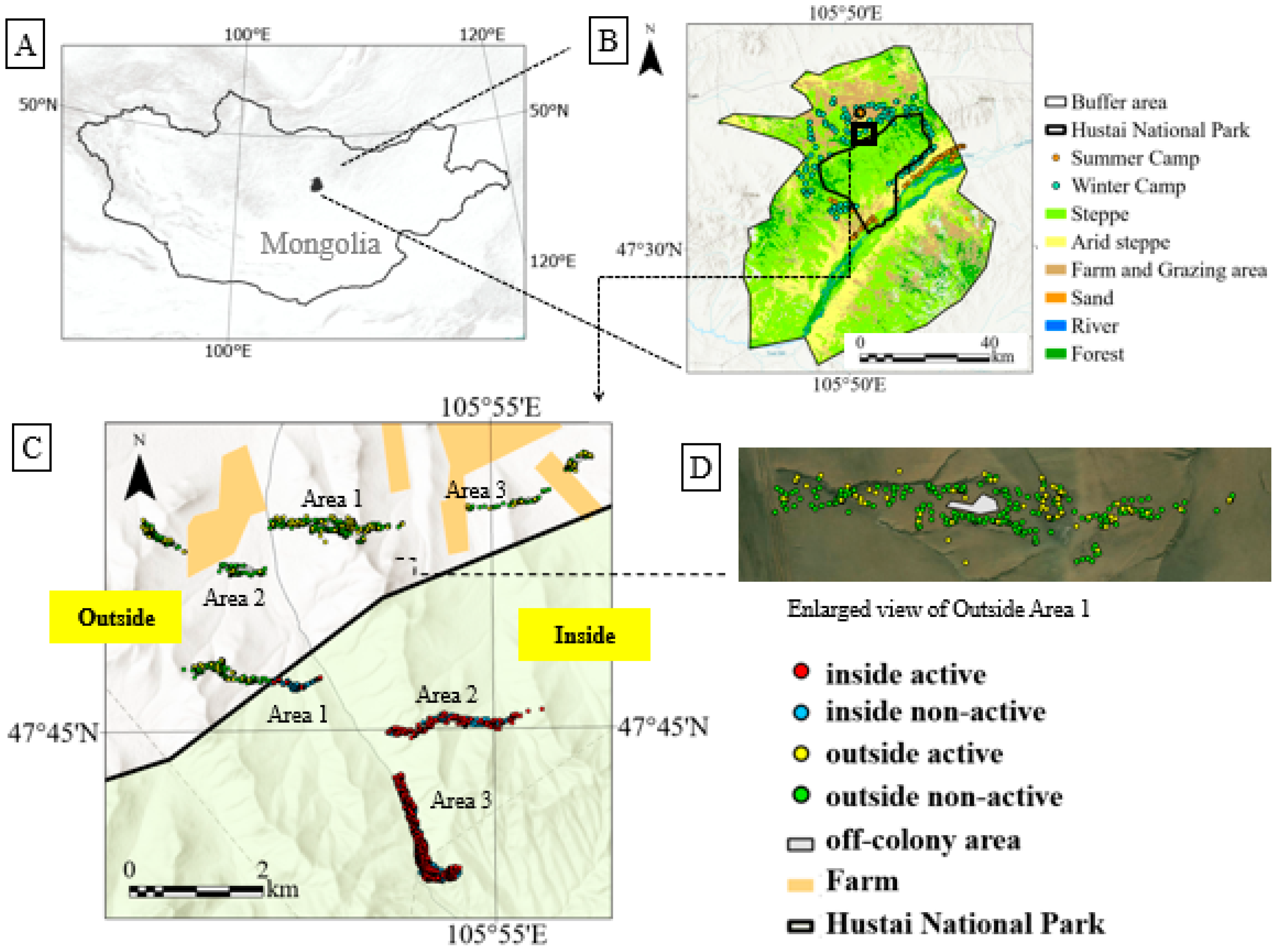
2.1. Survey Area
2.2. Data Used
2.3. Analysis of Habitat Structure
2.4. Vegetation Survey
2.5. Vegetation Index Extraction and Analysis
2.6. Analysis of the Relationship Between Burrow Activity and NDVI
3. Results
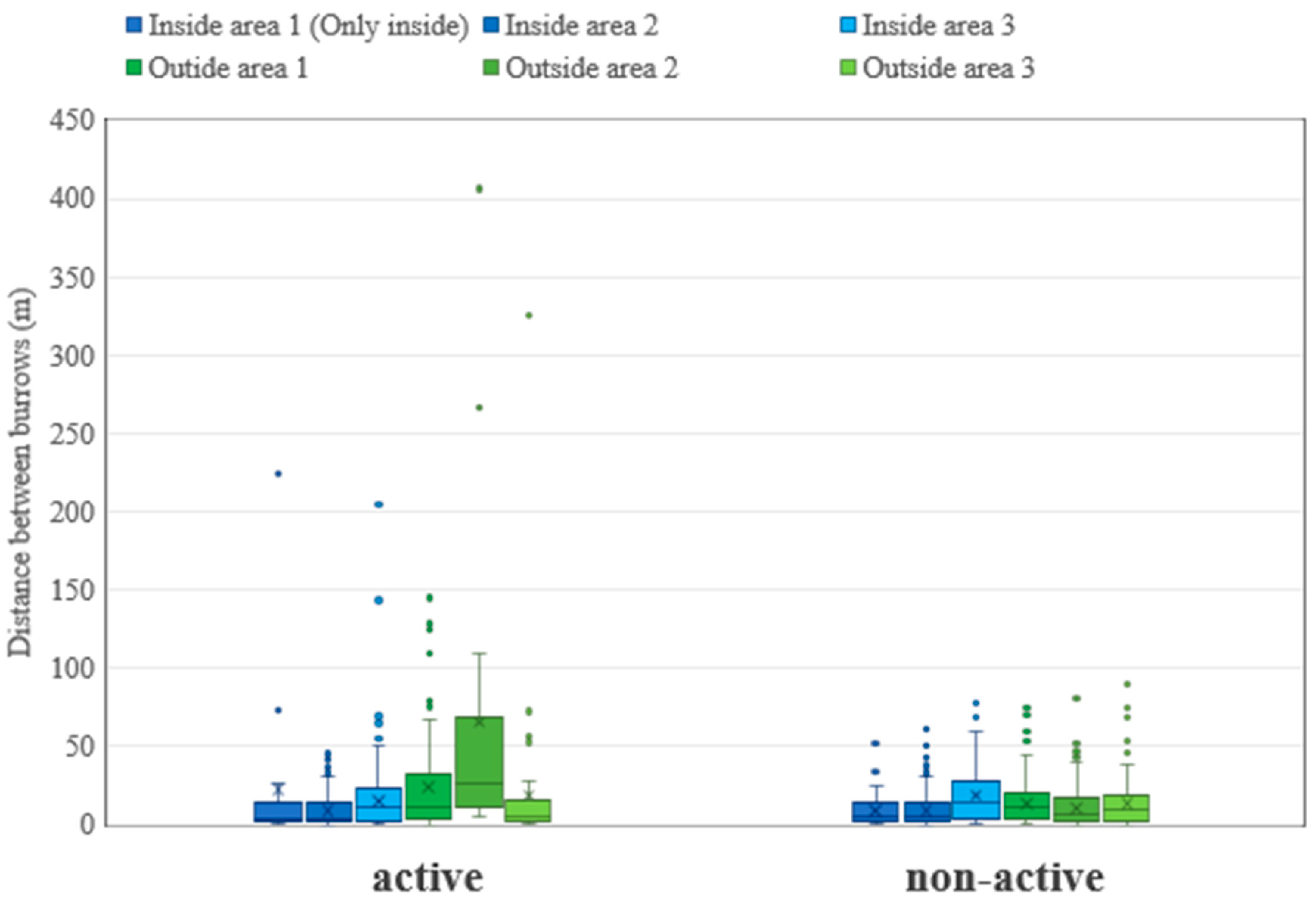
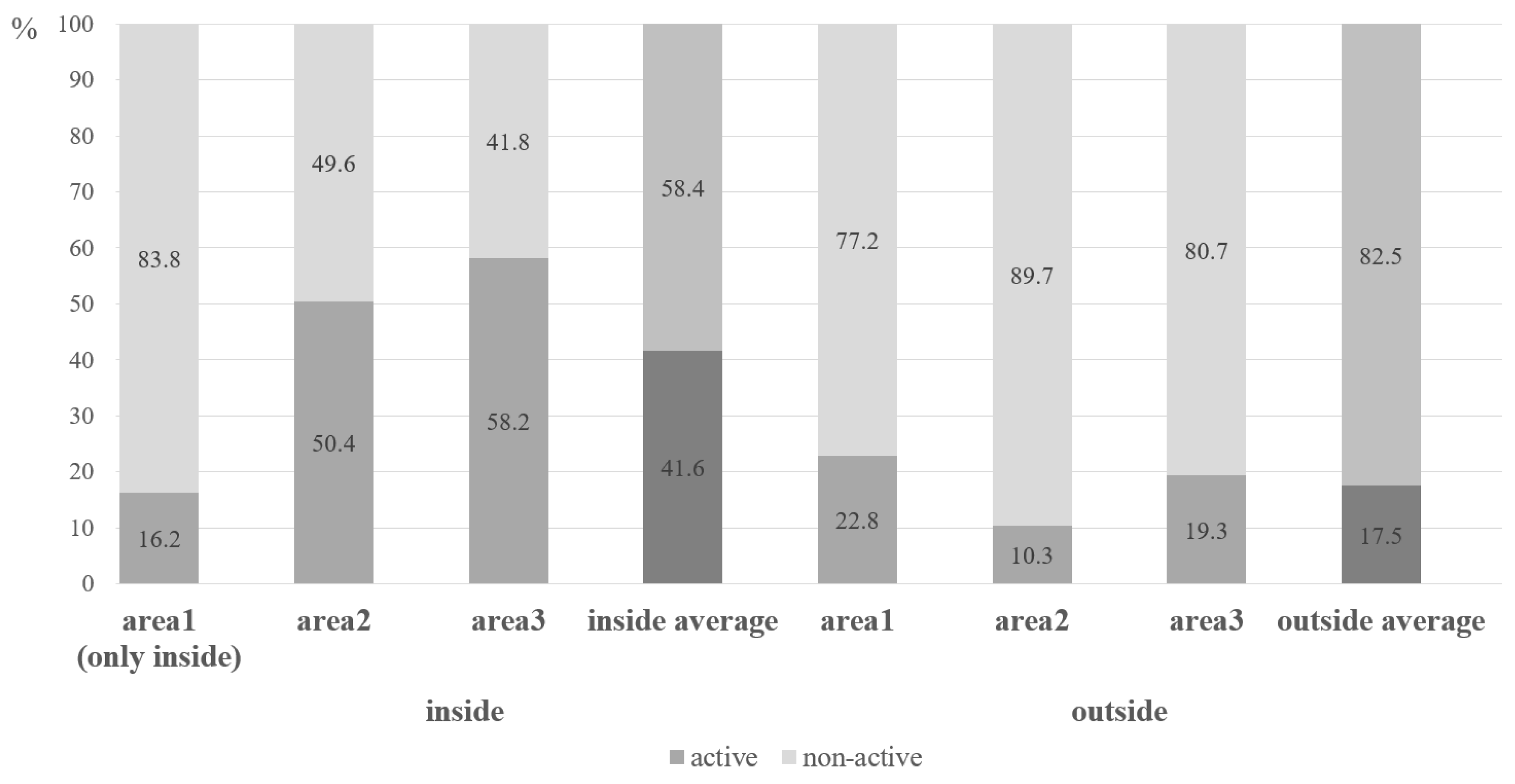
3.1. Habitat Structure and Activity Patterns of Marmots
3.2. Plant Community Structure in Marmot Habitats
3.2.1. Effects of the Protected Area and Marmot Burrows on Plant Community Structure (2023–2024)
(i) Results for 2023
(ii) Results for 2024
3.2.2. Comparison of Vegetation Height
- For Artemisia frigida, the vegetation height in active burrows inside the park (mean: 9.2 cm) was significantly lower than in the off-colony areas (mean: 22.6 cm) (t = −6.04, p = 0.0004), indicating potential suppression of vegetation cover or growth in burrows.
- For Artemisia adamsii, the vegetation height in active burrows outside the park (mean: 20.8 cm) was significantly higher than in the off-colony areas (mean: 9.8 cm) (t = 4.18, p = 0.0041). However, due to a limited sample size, statistical testing could not be conducted for this species inside the park.
- For Leymus chinensis, no significant differences in the vegetation height were observed in either area (inside: p = 0.191; outside: NA), suggesting that the impact of the burrows may be limited for this species.
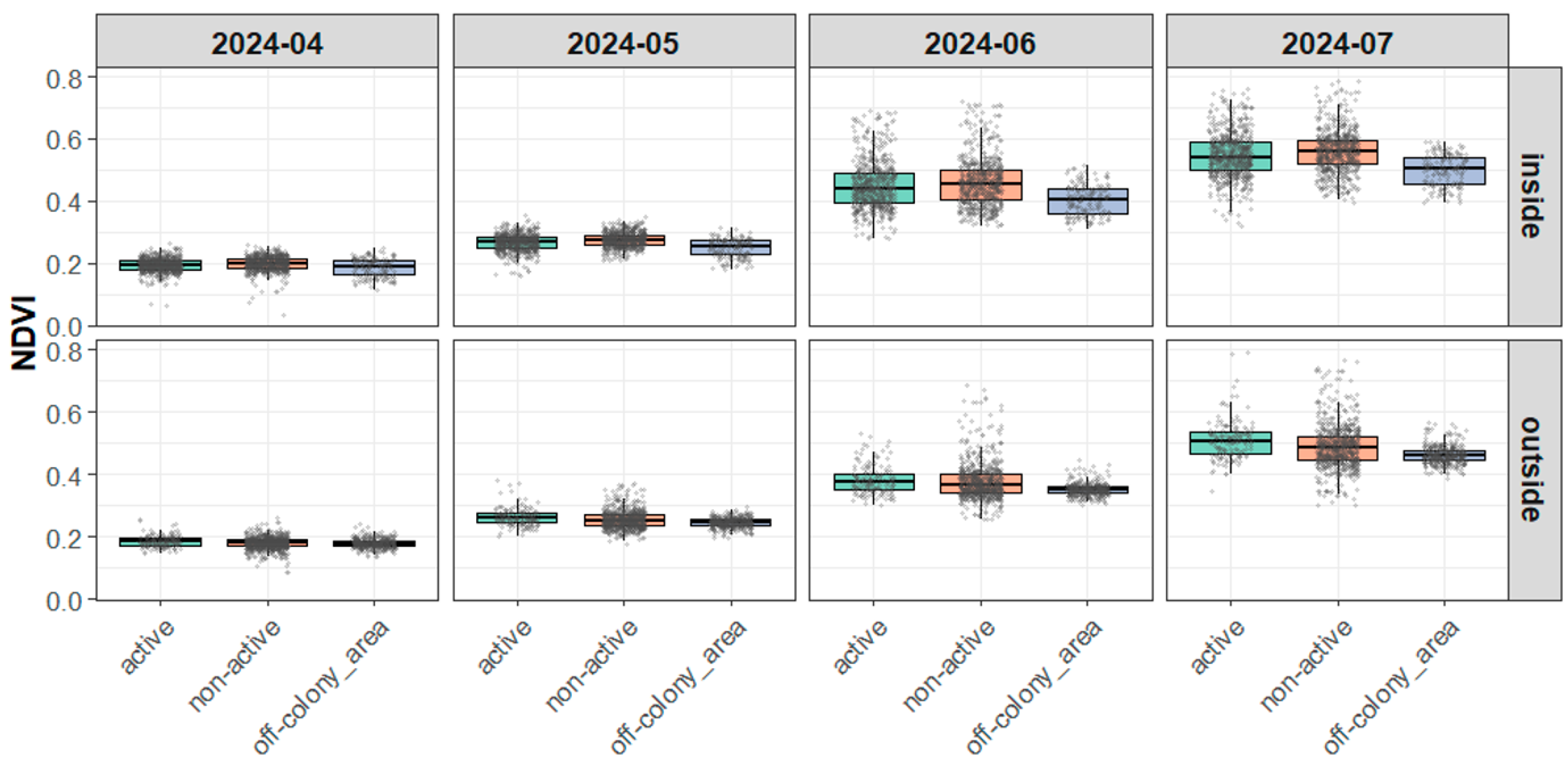
3.3. Results of Burrow Activity and Vegetation Index (NDVI)
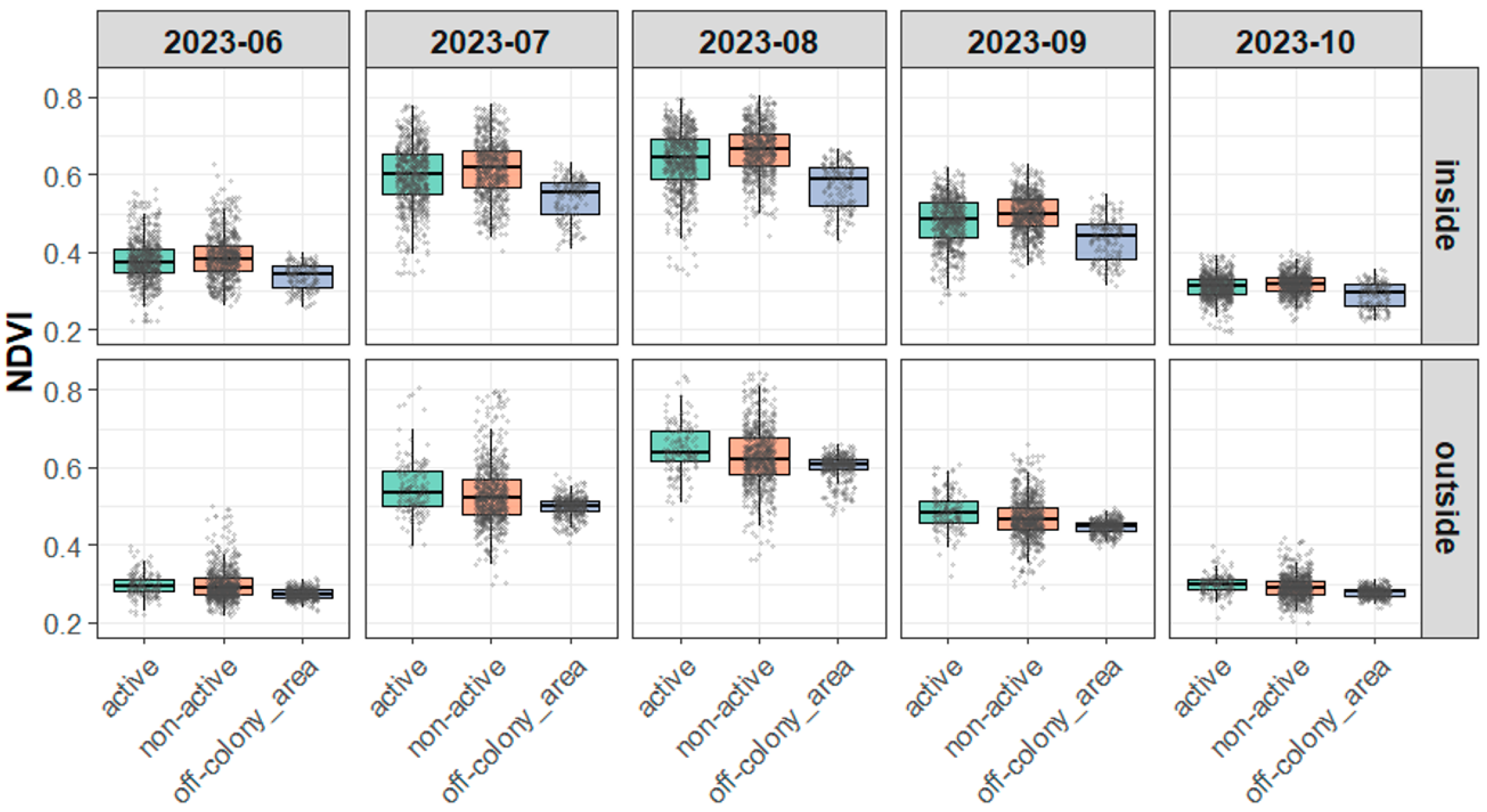
3.3.1. NDVI Distribution Characteristics and Model Fit
3.3.2. Variance in NDVI Across Burrow Types
3.3.3. Visual Supplement
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Year | Month | Area | Comparison | F Value | Pr (>F) | Significance |
|---|---|---|---|---|---|---|
| 2023 | June | Inside | active vs. non-active | 1.6856 | 0.1944 | n.s. |
| active vs. off-colony | 19.41 | 1.19 × 10−5 | *** | |||
| non-active vs. off-colony | 25.757 | 4.77 × 10−7 | *** | |||
| Outside | active vs. non-active | 3.8999 | 0.04859 | * | ||
| active vs. off-colony | 106.06 | <2.2 × 10−16 | *** | |||
| non-active vs. off-colony | 115.81 | <2.2 × 10−16 | *** | |||
| 2023 | July | Inside | active vs. non-active | 2.6713 | 0.1024 | n.s. |
| active vs. off-colony | 25.562 | 5.27 × 10−7 | *** | |||
| non-active vs. off-colony | 19.292 | 1.26 × 10−5 | *** | |||
| Outside | active vs. non-active | 1.0398 | 0.3081 | n.s. | ||
| active vs. off-colony | 133.46 | <2.2 × 10−16 | *** | |||
| non-active vs off-colony | 177.51 | <2.2 × 10−16 | *** | |||
| 2023 | August | Inside | active vs non-active | 23.42 | 1.45 × 10−6 | *** |
| active vs off-colony | 7.2054 | 0.007412 | ** | |||
| non-active vs. off-colony | 0.0665 | 0.7966 | n.s. | |||
| Outside | active vs. non-active | 3.0339 | 0.08188 | . | ||
| active vs. off-colony | 96.862 | <2.2 × 10−16 | *** | |||
| non-active vs. off-colony | 182.43 | <2.2 × 10−16 | *** | |||
| 2023 | September | Inside | active vs. non-active | 23.398 | 1.47 × 10−6 | *** |
| active vs. off-colony | 0.4516 | 0.5018 | n.s. | |||
| non-active vs. off-colony | 7.1883 | 0.007481 | ** | |||
| Outside | active vs. non-active | 0.5464 | 0.46 | n.s. | ||
| active vs. off-colony | 181.59 | <2.2 × 10−16 | *** | |||
| non-active vs. off-colony | 193.47 | <2.2 × 10−16 | *** | |||
| 2023 | October | Inside | active vs. non-active | 9.032 | 0.002702 | ** |
| active vs. off-colony | 5.0711 | 0.02459 | * | |||
| non-active vs. off-colony | 20.634 | 6.37 × 10−6 | *** | |||
| Outside | active vs. non-active | 2.7936 | 0.09499 | . | ||
| active vs off-colony | 70.386 | 4.12 × 10−16 | *** | |||
| non-active vs off-colony | 118.84 | <2.2 × 10−16 | *** | |||
| 2024 | April | Inside | active vs non-active | 0.3341 | 0.5633 | n.s. |
| active vs. off-colony | 39.726 | 4.72 × 10−10 | *** | |||
| non-active vs. off-colony | 30.622 | 4.19 × 10−8 | *** | |||
| Outside | active vs. non-active | 0.3207 | 0.5713 | n.s. | ||
| active vs. off-colony | 4.7067 | 0.03047 | * | |||
| non-active vs. off-colony | 13.22 | 0.000289 | *** | |||
| 2024 | May | Inside | active vs. non-active | 4.8423 | 0.02794 | * |
| active vs. off-colony | 8.6904 | 0.003288 | ** | |||
| non-active vs. off-colony | 24.639 | 8.37 × 10−7 | *** | |||
| Outside | active vs. non-active | 0.444 | 0.5054 | n.s. | ||
| active vs. off-colony | 35.685 | 4.16 × 10−9 | *** | |||
| non-active vs. off-colony | 69.166 | 2.57 × 10−16 | *** | |||
| 2024 | June | Inside | active vs. non-active | 0.0069 | 0.9336 | n.s. |
| active vs. off-colony | 17.613 | 2.99 × 10−5 | *** | |||
| non-active vs. off-colony | 18.283 | 2.12 × 10−5 | *** | |||
| Outside | active vs. non-active | 2.5934 | 0.1077 | n.s. | ||
| active vs. off-colony | 107.89 | <2.2 × 10−16 | *** | |||
| non-active vs. off-colony | 119.88 | <2.2 × 10−16 | *** | |||
| 2024 | July | Inside | active vs. non-active | 2.4171 | 0.1203 | n.s. |
| active vs. off-colony | 13.105 | 0.000312 | *** | |||
| non-active vs. off-colony | 7.4029 | 0.006646 | ** | |||
| Outside | active vs. non-active | 2.7545 | 0.09733 | . | ||
| active vs. off-colony | 73.145 | <2.2 × 10−16 | *** | |||
| non-active vs. off-colony | 142.14 | <2.2 × 10−16 | *** |
References
- Reichman, O.J.; Seabloom, E.W. The role of pocket gophers as subterranean ecosystem engineers. Trends Ecol. Evol. 2002, 17, 44–49. [Google Scholar] [CrossRef]
- Davidson, A.D.; Detling, J.K.; Brown, J.H. Ecological roles and conservation challenges of social, burrowing, herbivorous mammals in the world’s grasslands. Front. Ecol. Environ. 2012, 10, 477–486. [Google Scholar] [CrossRef]
- Sun, S.; Dou, H.; Wei, S.; Fang, Y.; Long, Z.; Wang, J.; An, F.; Xu, J.; Xue, T.; Qiu, H.; et al. A review of the engineering role of burrowing animals: Implication of Chinese pangolin as an ecosystem engineer. J. Zool. Res. 2021, 3. [Google Scholar] [CrossRef]
- Whicker, A.D.; Detling, J.K. Ecological consequences of prairie dog disturbances. BioScience 1988, 38, 778–785. [Google Scholar] [CrossRef]
- Wright, J.P.; Jones, C.G. The concept of organisms as ecosystem engineers ten years on: Progress, limitations, and challenges. BioScience 2006, 56, 203–209. Available online: https://www.researchgate.net/publication/202001805_The_Concept_of_Organisms_as_Ecosystem_Engineers_Ten_Years_On_Progress_Limitations_and_Challenges (accessed on 4 April 2025). [CrossRef]
- Wesche, K.; Nadrowski, K.; Retzer, V. Habitat engineering under dry conditions: The impact of pikas (Ochotona pallasi) on vegetation and site conditions in southern Mongolian steppes. J. Veg. Sci. 2007, 18, 665–674. [Google Scholar] [CrossRef]
- Prugh, L.R.; Brashares, J.S. Partitioning the effects of an ecosystem engineer: Kangaroo rats control community structure via multiple pathways. J. Anim. Ecol. 2012, 81, 667–678. [Google Scholar] [CrossRef]
- Clark, H.O.; Murdoch, J.D.; Newman, D.P.; Sillero-Zubiri, C. Vulpes corsac (Carnivora: Canidae). Mamm. Species 2009, 832, 1–8. [Google Scholar] [CrossRef]
- Andersen, M.L.; Bennett, D.E.; Holbrook, J.D. Burrow webs: Clawing the surface of interactions with burrows excavated by American badgers. Ecol. Evol. 2021, 11, 11559–11568. [Google Scholar] [CrossRef] [PubMed]
- Gharajehdaghipour, T.; Roth, J.D.; Fafard, P.M.; Markham, J.H. Arctic foxes as ecosystem engineers: Increased soil nutrients lead to increased plant productivity on fox dens. Sci. Rep. 2016, 6, 24020. [Google Scholar] [CrossRef]
- Hagenah, N.; Bennett, N.C. Mole rats act as ecosystem engineers within a biodiversity hotspot, the C ape F ynbos. J. Zool. 2013, 289, 19–26. [Google Scholar] [CrossRef]
- Parsons, A.W.; Apollonio, M.; Krausman, P.R.; Rachlow, J.L. Pygmy rabbit burrows increase microhabitat heterogeneity in sagebrush-steppe ecosystems. Ecosphere 2016, 7, e01334. [Google Scholar] [CrossRef]
- Valkó, O.; Tölgyesi, C.; Kelemen, A.; Bátori, Z.; Gallé, R.; Rádai, Z.; Bragina, T.M.; Bragin, Y.A.; Deák, B. Steppe Marmot (Marmota bobak) as ecosystem engineer in arid steppes. J. Arid. Environ. 2021, 184, 104244. [Google Scholar] [CrossRef]
- Murdoch, J.D.; Munkhzul, T.; Buyandelger, S.; Reading, R.P.; Sillero-Zubiri, C. The endangered Siberian marmot Marmota sibirica as a keystone species? Observations and implications of burrow use by corsac foxes Vulpes corsac in Mongolia. Oryx 2009, 43, 431–434. [Google Scholar] [CrossRef]
- Buyandelger, S.; Otgonbayar, B. Mongolian marmot burrow influences an occupancy of Isabelline wheatear. Landsc. Ecol. Eng. 2022, 18, 239–245. [Google Scholar] [CrossRef]
- Becchina, R.A. Recovering Endangered Siberian Marmot (Marmota Sibirica): Status and Distribution in A Steppe Region of Mongolia. 2020. Available online: https://scholarworks.uvm.edu/hcoltheses/331 (accessed on 6 April 2025).
- Buyandelger, S.; Baatargal, O.; Bayartogtokh, B.; Reading, R.P. Ecosystem engineering influence of Mongolian marmots (Marmota sibirica) on small mammal communities in Mongolia. J. Asia-Pac. Biodivers. 2022, 15, 172–179. [Google Scholar] [CrossRef]
- Kolesnikov, V.V.; Brandler, O.V.; Badmaev, B.B.; Zoje, D.; Adiya, Y. Factors that lead to a decline in numbers of Mongolian marmot populations. Ethol. Ecol. Evol. 2009, 21, 371–379. [Google Scholar] [CrossRef]
- Buyandelger, S.; Enkhbayar, T.; Otgonbayar, B.; Zulbayar, M.; Bayartogtokh, B. Ecosystem engineering effects of Mongolian marmots (Marmota Sibirica) on terrestrial arthropod communities. Mong. J. Biol. Sci. 2021, 19, 17–30. Available online: https://www.biotaxa.org/mjbs/article/view/66415 (accessed on 2 May 2025). [CrossRef]
- Townsend, S.E. Estimating Siberian marmot (Marmota sibirica) densities in the Eastern Steppe of Mongolia. Ethol. Ecol. Evol. 2009, 21, 325–338. [Google Scholar] [CrossRef]
- Fijn, N.; Terbish, B. The multiple faces of the marmot: Associations with the plague, hunting, and cosmology in Mongolia. Hum. Ecol. 2021, 49, 539–549. [Google Scholar] [CrossRef]
- Thapaliya, K. Analysis of Factors Related to the Distribution of Red Deer, Cervus Elephus L., in Hustai National Park, Mongolia; ITC: Kolkata, India, 2008; Available online: https://www.researchgate.net/publication/237632048_Analysis_of_factors_related_to_the_distribution_of_Red_deer_Cervus_elephus_L_in_Hustai_National_Park_Mongolia (accessed on 24 April 2025).
- Suzuki, K.; Kamijo, T.; Jamsran, U.; Tamura, K. Evaluation of the effect of protected area designation by comparing steppe vegetation inside and outside Hustai National Park, Mongolia. J. Veg. Sci. 2013, 30, 85–93. (In Japanese) [Google Scholar] [CrossRef]
- Yoshihara, Y.; Okuro, T.; Buuveibaatar, B.; Undarmaa, J.; Takeuchi, K. Clustered animal burrows yield higher spatial heterogeneity. Plant Ecol. 2010, 206, 211–224. [Google Scholar] [CrossRef]
- International Monetary Fund. Mongolia: Overgrazing Problem and Its Impact on Grasslands; 2019. Available online: https://www.elibrary.imf.org/view/journals/002/2019/298/article-A002-en.xml (accessed on 15 April 2025).
- Shen, H.; Dong, S.; DiTommaso, A.; Xiao, J.; Zhi, Y. N deposition may accelerate grassland degradation succession from grasses-and sedges-dominated into forbs-dominated in overgrazed alpine grassland systems on Qinghai-Tibetan Plateau. Ecol. Indic. 2021, 129, 107898. [Google Scholar] [CrossRef]
- Yang, C.; Sun, J. Impact of soil degradation on plant communities in an overgrazed Tibetan alpine meadow. J. Arid. Environ. 2021, 193, 104586. [Google Scholar] [CrossRef]
- Yoshihara, Y.; Ohkuro, T.; Buuveibaatar, B.; Undarmaa, J.; Takeuchi, K. Pollinators are attracted to mounds created by burrowing animals (marmots) in a Mongolian grassland. J. Arid. Environ. 2010, 74, 159–163. [Google Scholar] [CrossRef]
- Ulziibaatar, M.; Matsui, K. Herders’ Perceptions about Rangeland Degradation and Herd Management: A Case among Traditional and Non-Traditional Herders in Khentii Province of Mongolia. Sustainability 2021, 13, 7896. [Google Scholar] [CrossRef]
- Narmandakh, D.; Sakurai, T. Impact of Rangeland Degradation on Farm Performance and Household Welfare in the Case of Mongolia. Jpn. J. Agric. Econ. 2022, 24, 52–57. [Google Scholar] [CrossRef]
- Yoshihara, Y.; Ohkuro, T.; Bayarbaatar, B.; Takeuchi, K. Effects of disturbance by Siberian marmots (Marmota sibirica) on spatial heterogeneity of vegetation at multiple spatial scales. Grassl. Sci. 2009, 55, 89–95. [Google Scholar] [CrossRef]
- King, S.R.; Gurnell, J. Habitat use and spatial dynamics of takhi introduced to Hustai National Park, Mongolia. Biol. Conserv. 2005, 124, 277–290. [Google Scholar] [CrossRef]
- Hustai National Park. Brief History of Hustai National Park Establishment. Available online: https://www.hustai.mn/language/en (accessed on 15 April 2025).
- Van Staalduinen, M.A.; Werger, M.J.A. Marmot disturbances in a Mongolian steppe vegetation. J. Arid. Environ. 2007, 69, 344–351. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y. Study on the degradation mechanism of typical steppe in Inner Mongolia. Chin. J. Appl. Ecol. 1999, 10, 437–441. Available online: https://www.cjae.net/CN/abstract/abstract5017.shtml (accessed on 15 April 2025). (In Chinese).
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring Vegetation Systems in the Great Plains with ERTS; Third ERTS Symp. NASA SP-351 I; NASA: Washington, DC, USA, 1973; pp. 309–317. Available online: https://ntrs.nasa.gov/citations/19740022614 (accessed on 16 April 2025).
- Kizilgeci, F.; Yildirim, M.; Islam, M.S.; Ratnasekera, D.; Iqbal, M.A.; Sabagh, A.E. Normalized Difference Vegetation Index and Chlorophyll Content for Precision Nitrogen Management in Durum Wheat Cultivars under Semi-Arid Conditions. Sustainability 2021, 13, 3725. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. GlmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. Available online: https://journal.r-project.org/archive/2017/RJ-2017-066/index.html (accessed on 16 April 2025). [CrossRef]
- Ballova, Z.; Pekarik, L.; Píš, V.; Šibík, J. How much do ecosystem engineers contribute to landscape evolution? A case study on Tatra marmots. Catena 2019, 182, 104121. [Google Scholar] [CrossRef]
- Buuveibaatar, B.; Yoshihara, Y. Effects of food availability on time budget and home range of Siberian marmots in Mongolia. Mong. J. Biol. Sci. 2012, 10, 25–31. Available online: https://www.biotaxa.org/mjbs/article/view/26596 (accessed on 10 April 2025). [CrossRef]
- Yokohama, M.; Shimada, S.; Sekiyama, A. Vegetation and forage preference of livestock in the Mongolian steppe. Bull. Tokyo Univ. Arts 2011, 56, 1–9. Available online: https://nodai.repo.nii.ac.jp/records/458 (accessed on 10 April 2025). (In Japanese).
- Kawashima, K.; Hoshino, B.; Ganzorig, S.; Sawamuki, M.; Asakawa, M.; Batsaikhan, N. Distribution and expansion of Microtus brandti in overgrazed areas in Mongolia. Grassl. Sci. 2012, 59, 217–224. (In Japanese) [Google Scholar] [CrossRef]
- Zhou, Y.; Batelaan, O.; Guan, H.; Duan, L.; Liu, T.; Wang, Y.; Li, X.; Yang, B. Evaluation of the contributions of climate change and overgrazing to runoff in a typical grassland inland river basin. J. Hydrol. Reg. Stud. 2024, 52, 101725. [Google Scholar] [CrossRef]
- Whitesides, C.J. Olympic marmot burrow densities and their effects on alpine soil properties. Phys. Geogr. 2015, 36, 293–307. [Google Scholar] [CrossRef]
- Miura, N.; Ito, T.Y.; Lhagvasuren, B.; Enkhbileg, D.; Tsunekawa, A.; Takatsuki, S.; Jiang, Z.; Mochizuki, K. Analysis of the seasonal migrations of Mongolian gazelle, using MODIS data. Int. Arch. Photogramm. Remote Sens. Spatial Inform. Sci. 2004, 35, 418–422. Available online: https://www.readkong.com/page/analysis-of-the-seasonal-migrations-of-mongolian-gazelle-5744763 (accessed on 9 April 2025).
- Ito, T.Y.; Sakamoto, Y.; Lhagvasuren, B.; Kinugasa, T.; Shinoda, M. Winter habitat of Mongolian gazelles in areas of southern Mongolia under new railroad construction: An estimation of interannual changes in suitable habitats. Mamm. Biol. 2018, 93, 13–20. [Google Scholar] [CrossRef]
- Harmse, C.J.; Gerber, H.; Van Niekerk, A. Evaluating several vegetation indices derived from Sentinel-2 imagery for quantifying localized overgrazing in a semi-arid region of South Africa. Remote Sens. 2022, 14, 1720. [Google Scholar] [CrossRef]
- Yoshihara, Y.; Okuro, T.; Buuveibaatar, B.; Undarmaa, J.; Takeuchi, K. Complementary effects of disturbance by livestock and marmots on the spatial heterogeneity of vegetation and soil in a Mongolian steppe ecosystem. Agric. Ecosyst. Environ. 2010, 135, 155–159. [Google Scholar] [CrossRef]
- FAO. Mongolian Grasslands and Drylands: Overview of Grazing Lands and Land Use in Mongolia; Food and Agriculture Organization of the United Nations: Rome, Italy, 2005; Available online: https://www.fao.org/4/y8344e/y8344e0e.htm (accessed on 7 April 2025).
- WWF. The Parliament of Mongolia has Approved 22 Areas for National Protected Areas; World Wildlife Fund: Gland, Switzerland, 2021; Available online: https://www.wwf.mg/en/?346744/The-Parliament-of-Mongolia-has-approved-22-areas-for-national-protected-areas (accessed on 7 April 2025).
- Yoshihara, Y. Impact of overgrazing on ecosystems and proposals for grassland restoration in Mongolian steppe (Special Issue: Overuse and underuse of grassland ecosystems). J. Jpn. Soc. Grassl. Sci. 2013, 59, 212–216. (In Japanese) [Google Scholar] [CrossRef]
| Year | Factor | Df | Sum of Squares | R2 | F | p-Value | Significance |
|---|---|---|---|---|---|---|---|
| 2023 | Area (inside/outside) | 1 | 0.6576 | 0.2001 | 4.3645 | 0.001 | *** |
| Status (active burrow/off-colony area) | 1 | 0.1502 | 0.0457 | 0.9969 | 0.417 | n.s | |
| Area × Status | 1 | 0.0678 | 0.0206 | 0.45 | 0.870 | n.s | |
| 2024 | Area | 1 | 0.2775 | 0.2161 | 2.6054 | 0.027 | * |
| Status | 1 | 0.3844 | 0.2993 | 3.6084 | 0.04 | ** | |
| Area × Status | 1 | 0.1961 | 0.1527 | 1.841 | 0.081 | (marginal) |
| 2023 | Inside | Outside | ||||||
|---|---|---|---|---|---|---|---|---|
| Active Burrow | Off-Colony Area | Active Burrow | Off-Colony Area | |||||
| Artemisia adamsii | 13.6% | Artemisia adamsii | 22.8% | Stipa krylovii | 17.0% | Heteropappus hispidus | 19.0% | |
| Heteropappus hispidus | 9.8% | Carex duriuscula | 14.2% | Artemisia adamsii | 16.0% | Allium anisopodium | 12.0% | |
| Stipa krylovii | 9.2% | Leymus chinensis | 13.4% | Leymus chinensis | 9.2% | Stipa krylovii | 11.0% | |
| 2024 | Inside | Outside | ||||||
|---|---|---|---|---|---|---|---|---|
| Active Burrow | Off-Colony Area | Active Burrow | Off-Colony Area | |||||
| Artemisia adamsii | 15.6% | Stipa krylovii | 22.5% | Leymus chinensis | 25.0% | Heteropappus hispidus | 19.5% | |
| Stipa krylovii | 10.0% | Artrmisia frigida | 8.5% | Stipa krylovii | 13.0% | Stipa krylovii | 14.0% | |
| Artemisia dracunculus | 7.2% | Cleistogenes squarrosa | 8.0% | Artemisia glauca | 11.0% | Cleistogenes squarrosa | 10.0% | |
| Species | Area | Active Burrow Mean (n) | Off-Colony Area Mean (n) | Test Statistic | p-Value |
|---|---|---|---|---|---|
| Stipa krylovii | Inside | 59.3 (6) | 57.8 (6) | 0.214 | 0.836 |
| Stipa krylovii | Outside | 55.5 (6) | 35.2 (11) | 3.59 | 0.0027 |
| Leymus chinensis | Inside | 23.0 (6) | 28.0 (5) | −1.45 | 0.191 |
| Leymus chinensis | Outside | – (5) | – (0) | – | – |
| Artemisia frigida | Inside | 9.17 (6) | 22.6 (5) | −6.04 | <0.001 |
| Artemisia adamsii | Inside | – (0) | – (6) | – | – |
| Artemisia adamsii | Outside | 20.8 (6) | 9.83 (6) | 4.18 | 0.0041 |
| Predictor | Estimate (2023) | Std. Error | z-Value | Estimate (2024) | Std. Error | z-Value |
|---|---|---|---|---|---|---|
| (Intercept) | −0.1198 | 0.0322 | −3.72 | −0.6123 | 0.0321 | −19.10 |
| area (outside) | −0.0532 | 0.0457 | −1.16 | −0.0641 | 0.0467 | −1.37 |
| burrow type (non-active) | 0.0827 | 0.0137 | 6.05 *** | 0.0575 | 0.0162 | 3.56 ** |
| burrow type (off-colony area) | −0.1385 | 0.0635 | −2.18 * | −0.0728 | 0.0626 | −1.16 |
| area × burrow type (non-active) | −0.1344 | 0.0257 | −5.23 *** | −0.0950 | 0.0306 | −3.11 ** |
| area × burrow type (off-colony area) | −0.0063 | 0.0887 | −0.07 | −0.0278 | 0.0876 | −0.32 |
| Statistic | 2023 | 2024 | ||||
| AIC (interaction model) | −16,285.7 | −13,792.7 | ||||
| AIC (additive model) | −16,261.2 | −13,734.1 | ||||
| ΔAIC (interaction–additive) | 24.6 | 58.6 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taguchi, H.; Ganbold, U.; Ikeda, M.; Ackermann, K.; Hoshino, B. Effects of Siberian Marmot Density in an Anthropogenic Ecosystem on Habitat Vegetation Modification. Wild 2025, 2, 32. https://doi.org/10.3390/wild2030032
Taguchi H, Ganbold U, Ikeda M, Ackermann K, Hoshino B. Effects of Siberian Marmot Density in an Anthropogenic Ecosystem on Habitat Vegetation Modification. Wild. 2025; 2(3):32. https://doi.org/10.3390/wild2030032
Chicago/Turabian StyleTaguchi, Hiroto, Uuganbayar Ganbold, Mai Ikeda, Kurt Ackermann, and Buho Hoshino. 2025. "Effects of Siberian Marmot Density in an Anthropogenic Ecosystem on Habitat Vegetation Modification" Wild 2, no. 3: 32. https://doi.org/10.3390/wild2030032
APA StyleTaguchi, H., Ganbold, U., Ikeda, M., Ackermann, K., & Hoshino, B. (2025). Effects of Siberian Marmot Density in an Anthropogenic Ecosystem on Habitat Vegetation Modification. Wild, 2(3), 32. https://doi.org/10.3390/wild2030032






