Simple Summary
If a bird’s nest fails, they lose the opportunity to raise chicks that season unless they nest again and lay additional eggs. Small populations of long-lived birds that typically raise only one chick each year, like cranes, can be especially impacted by failed nests. In this study, we evaluated how many times per year Whooping Crane pairs nested in a reintroduced population during 2005–2024, to determine what affects a pair’s likelihood to have a second nest and more opportunities to raise chicks. Overall, we found that 37.3% of pairs whose first nests failed laid another set of eggs. Older female cranes with more experience, that laid their eggs outside of typical reintroduction areas, or had their nests fail earlier in the breeding season were more likely to nest again. We also collected eggs from first nests when black flies were bothering cranes, and those pairs were more likely to nest again than pairs who had their nests fail naturally. If we understand the conditions in which cranes are more likely to nest again after their first nest fails, we can adjust our management of a population of endangered Whooping Cranes and share our knowledge with other conservation efforts.
Abstract
Laying additional clutches of eggs, or renesting, can provide birds with more opportunities to breed each season, ultimately increasing reproductive potential. This is important for long-lived species like cranes, that produce relatively few offspring annually. The reintroduced Eastern Migratory Population (EMP) of endangered Whooping Cranes relies on releases of captive-reared juveniles for population growth. Using long-term nest monitoring data, we assessed the renesting propensity of 105 unique pairs of Whooping Cranes during 2005–2024 (n = 359). We used a two-tiered analysis of binomial generalized linear mixed-effects models to evaluate the effects of 15 individual covariates, related to parental age or experience, nest management geography, or chronology. Overall, 37.3% of pairs renested following failed first nesting attempts. We documented higher renesting rates from breeding females that were older or had more years of nesting experience (37.1% increase for each unit increase in female age), pairs outside of the regions in which captive-reared cranes were released (264.0% increase in “other” region compared to Necedah), or that had nests fail earlier in the season (10.1% decline for each day later in the season a first nest failed). Additionally, when eggs were collected from first nests as a part of nest management, pairs were more likely to renest (69.4% renested) than if their nest failed naturally (27.3% renested). Low rates of natural reproduction limit growth rates in the EMP, so understanding effects of management actions and limitations on breeding are important conservation tools which can be applied to other endangered species.
Keywords:
Grus americana; nest management; nesting; reintroduction; reproduction; Whooping Crane; Wisconsin 1. Introduction
Whooping Cranes (Grus americana) are long-lived birds, and an endangered species native to North America. The one remaining wild population was reduced to around 16 cranes in 1942 [1], and the species was first protected by the Endangered Species Preservation Act of 1966, and later by the Endangered Species Act of 1973 [2,3]. One of the down-listing criteria in the International Recovery Plan for Whooping Cranes requires the establishment of one or two additional reintroduced self-sustaining populations, separate from the extant population [1]. As of 2024, there are two ongoing reintroduction efforts, the Eastern Migratory Population of Whooping Cranes (EMP), that migrates between breeding areas in Wisconsin and wintering areas in the southeastern US [3,4,5], and the Louisiana Nonmigratory Population (LNMP), which is resident in southwestern Louisiana [6,7].
The EMP of Whooping Cranes began with releases of captive-reared Whooping Cranes in 2001, and since then has had cranes form strong pair bonds, build nests, lay fertile eggs, and hatch chicks in the wild [3]. However, there have been high rates of nest failure caused by avian-feeding blackflies (Simulium spp.) that disturb incubating Whooping Cranes at the core breeding area at Necedah National Wildlife Refuge (NWR) [8,9]. This has contributed to low levels of recruitment and a lack of population growth without the continued releases of captive-reared individuals [3]. To reduce the effects of blackflies at crane nests, beginning in 2011, Whooping Cranes were released into areas in eastern Wisconsin where there were wetland habitats with fewer blackflies [10,11]. Additionally, the first clutches of eggs in nests at Necedah NWR were collected when blackflies emerged, prior to nest abandonment, to encourage cranes to lay a second clutch of eggs (hereafter, renest) after blackfly populations subsided, which is now known as “forced renesting”. A previous study found that during 2014–2016, cranes whose first nests were a part of forced renesting were more likely to renest (79% renested) than cranes who abandoned their nests due to blackfly disturbance at Necedah NWR (42% renested [12]).
In addition to disturbance by blackflies and management actions like forced renesting, other factors may affect the renesting propensity of cranes, including characteristics of previous nests during the season, age of nesting female, and nest location or habitat. Many bird species are less likely to renest when their first clutches are laid or fail later in the season (Kentish Plovers, Charadrius alexandrinu [13]; Mallard, Anas platyrhynchos [14]; Greater Prairie-Chicken, Tympanuchus cupido [15]; Dunlin, Calidris alpina arcticola [16]; Piping Plover, Charadrius melodus [17,18]), or if they fail later in the incubation period (Willow Ptarmigan, Lagopus lagopus [19]; Dunlin [16]). For Piping Plovers, the cause of failure for first nests also affected renesting propensity; pairs with non-viable eggs or that had nests that were depredated were less likely to renest than pairs that had nests fail due to flooding [17,18]. Another factor affecting renesting propensity in birds is the age of the breeding females. Older females were more likely to renest than younger birds for both Willow Ptarmigan [19] and Mallards [14]. Lastly, location or habitat can affect renesting propensity, for example, Piping Plovers nesting on reservoirs were less likely to renest compared to other habitats [18].
Presently, there has been only one evaluation of renesting propensity in reintroduced Whooping Cranes, and it has only been in relation to the effect of forced renesting during blackfly emergence at one site, Necedah NWR, during three breeding seasons, 2014–2016 [12]. This study aims to investigate additional potential factors affecting renesting propensity across the entire breeding area of the population during 2005–2024. Here, we assess the effects of management actions (forced renesting, partial clutch collection where one egg was collected from a two-egg nest), characteristics of the breeding season (year, initiation date, duration of incubation, conclusion date when the nest hatched or failed, and fate of first nest if the nest was not a part of active management), experience (age, years nesting experience), and location (region where nesting occurred) on the likelihood of a Whooping Crane to lay a second clutch of eggs after the first clutch has failed. Understanding the factors affecting renesting rates will inform managers who may be conducting nest management or egg collection, and ultimately will help increase renesting rates, number of chicks hatched in the wild, and reproductive success of Whooping Cranes in the reintroduced EMP.
2. Materials and Methods
2.1. Study Area
This study includes all known nesting areas of Whooping Cranes in the EMP, all of which have been in Wisconsin, USA (Figure 1). The majority of first nesting attempts (66%) have been at Necedah NWR or the surrounding area, which was the first release site for captive-reared cranes in the population. Necedah NWR is a 17,683 ha area, composed of grassland, oak-pine savanna, emergent and forested wetland, and mixed forest [20]. There also have been nesting attempts in the release area in eastern Wisconsin, known as the Eastern Rectangle (11%), which includes White River Marsh State Wildlife Area (SWA, 7%) and Horicon NWR (1%). White River Marsh SWA is a 4856 ha area, containing emergent wetland, tamarack swamp, and oak savanna [21]. Horicon NWR is an 8660 ha area, dominated by cattail marsh, willow woodlands, and uplands [22]. The remaining nests occurred in areas throughout Wisconsin that have included small marshes on private property surrounded by agriculture, wetlands used as reservoirs for cranberry farms, and additional state or county-protected areas (Figure 1).
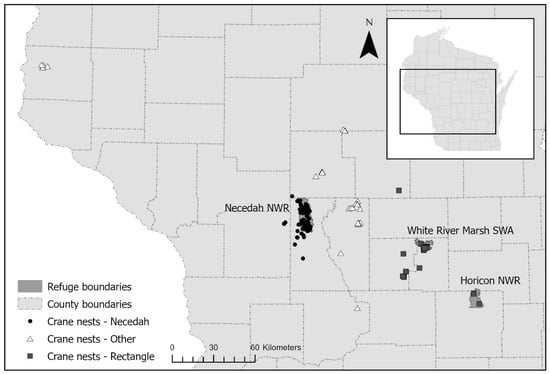
Figure 1.
Map of the known Whooping Crane nests in the Eastern Migratory Population during 2005–2024. Whooping Cranes have been released in two regions of Wisconsin, at Necedah National Wildlife Refuge (NWR) in central Wisconsin, and sites in eastern Wisconsin in an area known as the Eastern Rectangle, which includes White River Marsh State Wildlife Area (SWA) and Horicon NWR. Nests outside of these two regions (Necedah and Rectangle) are classified in the ‘Other’ region. In the inset map, the rectangle shows the extent of the larger map within the state of Wisconsin.
2.2. Reintroduction Techniques and Long-Term Monitoring
Most Whooping Cranes in the EMP (90% of the individuals represented in this study) were raised in captivity prior to release into the wild, either by adult cranes (parent-rearing) or by costumed caretakers (costume-rearing), using techniques described by Wellington et al. [23] and Hartup [24]. Prior to release, the sex of each crane was determined using genetic techniques [25,26], and each crane was banded with a unique combination of colored leg-bands and transmitters (either VHF [Very High Frequency] or remote GPS [27]). Wild-hatched cranes were captured for banding, and blood samples were collected to determine their sex. Whooping Cranes were released and later established nesting territories at Necedah NWR (including the surrounding areas), in the Eastern Rectangle, or in areas outside of those two regions, which we will refer to as “other” (Figure 1) [3,10]. After banding and release, biologists closely monitored the survival, reproduction, and behavior of all Whooping Cranes in the EMP throughout their lifetime.
Biologists located and monitored Whooping Crane nests and renests either by aerial or ground surveys of previously known nesting areas, by GPS or VHF telemetry, or by reports from staff, volunteers, partners, landowners, or the public. In this study, 75.6% of pairs had at least one member with a working GPS or VHF transmitter (n = 359, some pairs represented in multiple years). After a failed first nest, we closely monitored pairs on their breeding territories, which are approximately 3.68 ± 0.95 km2, to determine if they laid an additional clutch of eggs [10]. We did not detect pairs switching mates between first nests and subsequent renests. We defined the nest initiation date as the first date a pair was confirmed incubating eggs. If the eggs were collected and hatched in captivity, we could also back date the initiation date from the hatch date based on the average incubation period of 30 days.
On a subset of nests, biologists deployed trail cameras (Trophy Cam model 119466, Bushnell, Overland Park, KS; or HyperFire HC600, Reconyx, Holmen, WI, USA) at least 5 days after a nest was found or the eggs were laid, to monitor incubation and nest fate (as in previous studies) [12,28,29,30]. We did not deploy nest cameras on all nests for a variety of reasons including limited access, early nest failure, or lack of landowner permissions. At Necedah NWR during 2006–2024, managers also monitored the emergence of blackflies, and their disturbance of incubating Whooping Cranes [3,8]. During 2014–2024, when blackflies emerged and disturbed nesting cranes, biologists conducted forced renesting by preemptively collecting their first clutch of eggs before abandonment, which were brought into captivity for captive-rearing and release into the reintroduced populations [3,12]. If cranes abandoned their nests, biologists attempted to recover their eggs. Lastly, during 2018–2024, biologists collected one egg from two-egg clutches in a subset of nests to increase the number of eggs for captive rearing and supplementation of reintroduced populations, which is known as partial clutch collection. All field work for this study was conducted under ESA 10a1a Recovery Permit #TE048806 and all handling or banding of birds was performed under the United States Bird Banding Lab permit #24022. This research was conducted in compliance with the “Guidelines to the use of wild birds in research” [31].
2.3. Data Analysis
To evaluate factors affecting renesting propensity (dependent variable) of Whooping Cranes in the EMP, we undertook a two-tiered analysis. All analyses were performed using R 4.2.3 [32]. In the first tier of analysis, we used bivariate binomial family Generalized Linear Models with a “logit” or “cloglog” link function to examine the influence of 15 individual parental, nest management, geographical, and chronological covariates (i.e., predictor variables) on renesting probability (Table 1) [33]. Link functions were determined considering the marginal and conditional distributions of the outcome variable and model performance [34,35]. The 15 bivariate models were compared to a basic null model using Akaike Information Criterion corrected for smaller sample sizes using the “MuMIn” package (AICc) [36,37]. All covariates within bivariate models that outperformed the null model were advanced to the second tier of analysis. In the second tier of analyses, we used binomial family Generalized Linear Mixed-Effects Models (GLMMs) to examine 63 uncorrelated combinations of the 14 covariates that were advanced using the “lme4” package [38,39,40]. We also integrated observed nest infestation by blackflies as a predictor variable into tier-2 analysis. We again compared models using AICc and reported the results for all models with an AICc Delta < 2.0 [36].

Table 1.
Covariates used to predict pair renesting after their first nest was deemed failed. We included the covariate’s name, the variable’s general theme, the variable’s type, and a short description.
We employed Pearson Product-Moment Correlation Coefficients to evaluate associations between numeric fixed effects predictor variables with the “Hmisc” package [41]. Variables with >|0.6| correlation were not included as fixed effects covariates in the same model [42]. We employed the Cramer’s V statistic, which also ranges in value from 0 to 1, to examine associations between two categorical variables using the “effectsize” package and similarly employed an inclusion threshold of <|0.6| [43,44]. We used the “ltm” package to conduct Point-Biserial Correlations that likewise range in value between 0 and 1 to examine strength of association between dichotomous categorical and numeric predictor variables and also employed a <|0.6| inclusion threshold for models [45]. Finally, we used Cohen’s f statistics to examine the strength of the relationship between multi-level categorical and numeric predictor variables using the “effectsize” package [44,46]. No predictor variables with more than a >|0.4| association (i.e., effect size) per Cohen’s f were included in the same model [46]. Finally, to ensure multicollinearity did not impact model parameter estimates and performance, we conducted Variance Inflation Tests (VIF) on all models using the “car” package [47]. We excluded models with >5.0 VIF score for any predictor variable from our final analyses [47].
We employed a “logit” link function for GLMMs and a non-linear optimizer using bound optimization by quadratic approximation (BOBYQA) to control the fitting process [48]. To improve model convergence, all numeric data were scaled to modified z-scores representing standard deviations above the minimum value for each variable [49]. Each of the 63 multivariate models included 2–4 fixed effects predictor variables and a single random effect. We included calendar “year” as a random effect in all models considering that EMP nest management strategies fluctuated annually per expert directives. We assessed model fit using conditional Pseudo-R2 values [50]. We used the “effects” package to display the predicted influence of independent variables on renesting probability per top models holding all other covariates constant [51]. The original data presented in the study are openly available in FigShare at https://doi.org/10.6084/m9.figshare.28509062.v1.
3. Results
We assessed the renesting propensity of 105 unique pairs of Whooping Cranes during 2005–2024 (n = 359). First nests were initiated on day of year 104 on average (~14 April; 25th percentile = 96; 75th percentile = 108) and were active for a mean of 14.0 days prior to failure (25th percentile = 6.0; 75th percentile = 20.0), including natural failures or when eggs were collected prior to potential abandonment related to black fly disturbance. We documented renesting in 37.3% of pairs following failed first nesting attempts. Renesting occurred an average of 18.4 days after nest failure was confirmed (25th percentile = 14.0; 75th percentile = 22.0). Forced renesting was applied in 23.8% of nest failures, and for these nests, renesting occurred an average of 17.6 days after egg collection (25th percentile = 14.0; 75th percentile = 20.0). Preliminary analyses suggested Whooping Cranes renested more than expected when forced renesting was applied (69.4% renested; residual = +5.98; χ² = 33.97, p < 0.001) and less than expected when it was not (27.3% renested; residual = −5.98; χ² = 33.97, p < 0.001). However, this finding does not account for other potentially impactful influences on renesting propensity.
In tier-1 analyses, 14 of 15 bivariate GLMs outperformed the null model and thus were advanced to the second round of modeling efforts. Only specific breeding location did not outperform the null model (Appendix A). The top performing parental age covariate was female age, which demonstrated a positive association with renesting probability. The top parental experience variable was similarly the number of years the female had nested, which was also positively associated with the probability of renesting. Forced renesting was the top performing nest management covariate and it was also positively associated with renesting probability. Region was the top performing geographical covariate in the first round of modeling efforts. Finally, nest failure date was the top chronology covariate in tier-1 modeling efforts per AICc and was negatively associated with renesting propensity (Appendix A).
The top performing multivariate model from tier-2 analyses included female age, forced renesting, region, and nest failure date as fixed effects and calendar year as a random effect (Figure 2; Table 2; Appendix B). The probability of renesting increased by 37.1% for each one unit increase in female age (95% CI = 21.1–55.3%, p < 0.001; Figure 3; Table 2). Holding all else at median levels, the model predicted that a 7-year-old female Whooping Crane would have a ~33% probability of renesting after initial nest failure and a 15-year-old would have a ~86% probability. The probability of renesting increased by 99% when collecting a full clutch to stimulate renesting (i.e., “forced renesting”). However, the confidence intervals of the forced renesting impacts overlapped zero (95% CI = −17.7–382.0%; p = 0.127; Figure 4; Table 2). Region as “other” was associated with a 264.0% increase in the probability of renesting relative to Necedah NWR (95% CI = 8.0–1126.1%; p = 0.037; Figure 5; Table 2). Whooping Cranes may have a marginally reduced probability of renesting in the Eastern Rectangle compared to Necedah NWR, but the results were not statistically significant (95% CI = −97.6–102.6%; p = 0.182; Figure 5; Table 2). Finally, our analyses indicated a 10.1% decline in the probability of renesting for each day later in the breeding season a pair’s first nest failed (95% CI = −6.6–−13.4%; p < 0.001; Figure 6; Table 2). Holding all other covariates at their median, our top model suggests that the probability of renesting would be ~64% on day of year 100 (~10 April) but just 17% on day of year 120 (~30 April). Our top model demonstrated a strong fit to the data with a conditional Pseudo-R2 value of 0.642.
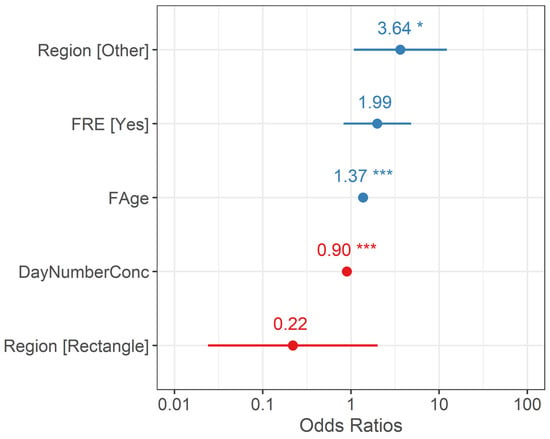
Figure 2.
Odds ratios for covariates from the top performing Generalized Linear Mixed Model predicting renesting probability include the region (Necedah NWR, Eastern Rectangle, Other), if a nest was subject to forced renesting management (FRE), the age of the breeding female crane (FAge), and the day the nest concluded (DayNumberConc). We defined nest conclusion as the day the nest either hatched or failed. Significance is indicated by p < 0.05 * and p < 0.001 ***. Variables positively associated with renesting probability are displayed in blue, while those negatively associated with renesting are displayed in red.

Table 2.
Parameter estimates and corresponding lower and upper 95% confidence limits for variables included in the top model predicting renesting propensity in the Eastern Migratory Population of Whooping Cranes. Parameter estimates are unscaled and exponentiated to facilitate easy interpretation.
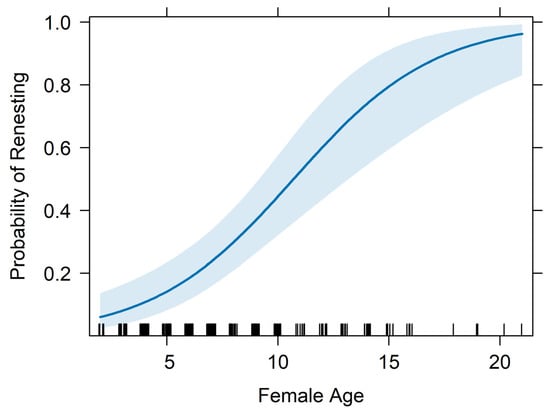
Figure 3.
Renesting probability by breeding female crane’s age. The point estimate is displayed in dark blue, the 95% confidence intervals are in light blue, and black vertical marks on the x-axis indicate observed values of female age in the database.
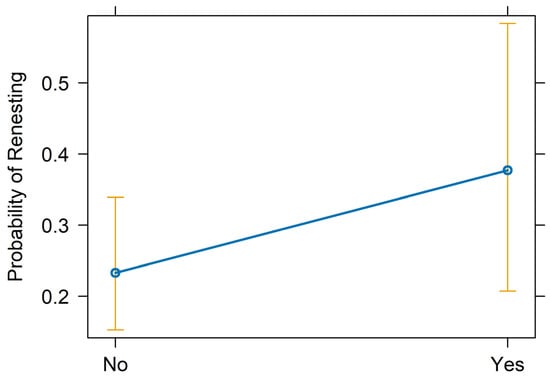
Figure 4.
Renesting probability by complete clutch collection to stimulate renesting (No, Yes), also known as “forced renesting”. The point estimate is indicated by dark blue circles connected by a line of the same color, and the 95% confidence intervals are represented by gold bars.
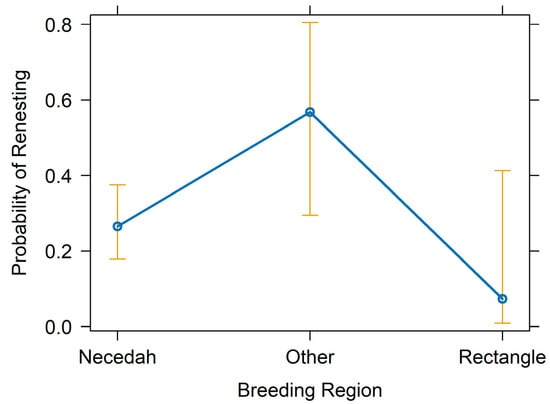
Figure 5.
Renesting probability by breeding region (Necedah NWR, Eastern Rectangle, Other). The point estimate is indicated by dark blue circles connected by a line of the same color, and the 95% confidence intervals are represented by gold bars.
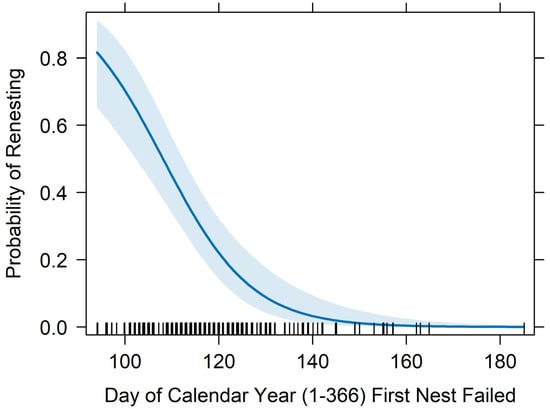
Figure 6.
Renesting probability by the day of the calendar year that a pair’s first nest failed. The point estimate is displayed in dark blue, the 95% confidence intervals are in light blue, and black vertical marks on the x-axis indicate observed values of nest failure dates in the database.
4. Discussion
The age and nesting experience of female cranes influenced the renesting propensity of a pair more than that of the male or the pair as a whole, which has been documented in other bird species [14,19,52]. There may be multiple direct and indirect ways age of a female could influence renesting propensity or reproduction more broadly [53]. Older females may be more experienced in foraging or other behaviors that would increase overall fitness or body condition compared to younger females, which could result in more resources to produce a second clutch of eggs [19,54]. Additionally, poor-quality breeders may disappear from the breeding population over time, resulting in a higher proportion of high-quality breeders at older ages, that may be more likely to renest [52].
Interestingly, birds in alternative breeding habitats (“other” region) outside of the major refuges that have been the target of reintroduction efforts (Necedah NWR or the Eastern Rectangle) may have a higher propensity to renest. Variations in breeding sites may contribute to a difference in renesting propensity through a variety of mechanisms, including constraints on temporal or environmental factors, resource availability, or possible survival–fecundity trade-offs [19]. Swift et al. [18] found that Piping Plovers nesting on reservoirs were less likely to renest than plovers in other types of habitats, unless the availability of nesting habitat increased during the breeding season. Habitat management actions or other fluctuating environmental conditions could affect the availability or quality of breeding habitat later in the season. Additionally, if a breeding site has reduced food availability, females may not have the resources or energy to produce another clutch of eggs [55]. Similar to the previous evaluation of renesting propensity in this population during 2014–2016, pairs of cranes whose nests failed due to egg collection for forced renesting as a way to ameliorate effects of blackflies were more likely to renest than pairs with nests that failed naturally (This study: 69.4% of pairs renested with forced renesting, 27.3% of naturally-failed pairs renested; Jaworski [12]: 79% of pairs renested with forced renesting, 42% of naturally failed pairs renested). Further evaluation of site- or region-specific factors affecting reproduction of Whooping Cranes in Wisconsin is important to inform release site decisions and other population or habitat management actions.
The timing of nest failure also affected the likelihood of a pair of cranes renesting. The probability of renesting declines markedly throughout the month of April, with the probability being >60% during the first two weeks of April while being <20% for the last week of the month. Initiating nests later in the season may result in reduced nest or chick survival [17,18] and, for migratory species like cranes, limits the amount of time a chick can spend in the breeding area prior to migration. Additionally, Whooping Cranes undergo a simultaneous remigial molt every 2–3 years, which typically coincides with the end of nesting season [56]. Assuming both egg production and molting are energetically expensive, cranes may be focusing energy on feather production later in the nesting season as they molt, which could affect renesting propensity.
5. Conclusions
Until the EMP becomes a self-sustainable population, it is important to understand the effects of management actions, like egg collection, and how to employ them the most successfully. Our study assessed the renesting propensity of 105 unique pairs of Whooping Cranes during 2005–2024 (n = 359) and found that older females, pairs nesting outside of traditional release sites, those whose eggs were collected, or that had nests fail earlier in the season were more likely to lay an additional clutch of eggs. Therefore, if eggs are to be collected for nest management, such as forced renesting, it is better to collect eggs from more experienced females and earlier in the season, as it will lead to a higher probability of renesting. Laying additional clutches of eggs can provide birds with more opportunities to breed each season, ultimately increasing reproductive potential [52]. For endangered species, particularly those with active nest management like the Whooping Crane, understanding the drivers of renesting propensity helps balance the risk of affecting natural reproduction or opportunity to nest, with the potential gains in eggs available for captive-rearing [57]. Additionally, we can investigate the fine-scale effects of habitat on renesting propensity and target locations with high renesting rates for releases of captive-reared cranes. For small, reintroduced populations, demonstrating limited negative effects of management actions like egg collection on the population, can also increase buy-in from important stakeholders for conservation actions. For the EMP, additional eggs in breeding facilities results in more captive-reared chicks released into the population, which helps buffer against natural mortality or limited natural recruitment to support population growth. These methods and findings can be applied to other threatened or endangered species, particularly other reintroduction efforts or populations with nest management, and will inform ongoing reintroduction efforts for Whooping Cranes and other crane species around the world.
Author Contributions
Conceptualization, H.L.T.; methodology, H.L.T. and A.J.C.; validation, N.M.G.; formal analysis, A.J.C.; investigation, H.L.T. and N.M.G.; data curation, H.L.T. and N.M.G.; writing—original draft preparation, H.L.T., A.J.C. and N.M.G.; writing—review and editing, H.L.T. and N.M.G.; visualization, H.L.T. and A.J.C.; supervision, H.L.T.; project administration, H.L.T. and N.M.G.; funding acquisition, A.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
ICF receives financial support from private individuals, foundations, members, investment income, government sources, special events, gift shop sales, and tour income. No specific grants were awarded for this work and none of the funders had any influence on the content of the submitted or published manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data presented in the study are openly available in FigShare at https://doi.org/10.6084/m9.figshare.28509062.v1. The README file describing the dataset is available in FigShare at https://doi.org/10.6084/m9.figshare.28509290.
Acknowledgments
Whooping Crane recovery has been a long-term effort by many individuals across a multitude of organizations. We would like to thank everyone who has been involved in the recovery and reintroduction of Whooping Cranes, especially in the Eastern Migratory Population, and those who have contributed to nest monitoring. Thank you to the staff of the Necedah and Horicon National Wildlife Refuges and B. Paulan for conducting aerial and ground surveys of nesting Whooping Cranes. Thank you to G. Whitten and E. Laack for helping with data processing. Thank you also to the reviewers of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Bivariate predictor variables of renesting propensity (binomial) ranked using Akaike Information Criterion corrected for small sample sizes (AICc). Individual models are presented with model intercept, parameter estimate, link function, degrees of freedom (df), Log-likelihood (Log. Lik), AIC Delta, and AIC Model Weight values.
Table A1.
Bivariate predictor variables of renesting propensity (binomial) ranked using Akaike Information Criterion corrected for small sample sizes (AICc). Individual models are presented with model intercept, parameter estimate, link function, degrees of freedom (df), Log-likelihood (Log. Lik), AIC Delta, and AIC Model Weight values.
| Bivariate Predictor | Theme | Int. | B | Link | df | Log Lik. | AICc | Delta | Weight |
|---|---|---|---|---|---|---|---|---|---|
| Nest Fail Dt. | Chronology | 7.89 | −9.01 | cloglog | 2 | −138.5 | 281.1 | 0.0 | 0.996 |
| Female Age | Parental Age | −2.66 | 2.34 | logit | 2 | −144.1 | 292.3 | 11.2 | 0.004 |
| Yrs. Nest Female | Parental Exp. | −1.58 | 1.43 | logit | 2 | −145.9 | 295.8 | 14.7 | 0.001 |
| Years Nesting Total | Parental Exp. | −1.67 | 1.45 | logit | 2 | −148.3 | 300.7 | 19.6 | 0.000 |
| Total Age | Parental Age | −2.71 | 2.33 | logit | 2 | −149.1 | 302.3 | 21.2 | 0.000 |
| Forced Renest-Yes | Nest Mgmt. | −0.98 | 2.34 | logit | 2 | −154.2 | 312.5 | 31.4 | 0.000 |
| Nest Initiate Dt. | Chronology | 6.09 | −7.01 | cloglog | 2 | −155.8 | 315.7 | 34.6 | 0.000 |
| Yrs. Nest Together | Parental Exp. | −1.20 | 0.99 | logit | 2 | −156.8 | 317.5 | 36.4 | 0.000 |
| Duration Nested | Chronology | 0.04 | −1.81 | cloglog | 2 | −156.8 | 317.7 | 36.6 | 0.000 |
| Yrs. Nest Male | Parental Exp. | −1.26 | 0.98 | logit | 2 | −157.9 | 319.9 | 38.8 | 0.000 |
| Male Age | Parental Age | −1.79 | 1.38 | logit | 2 | −161.2 | 326.4 | 45.3 | 0.000 |
| Region | Geography | −0.37 | + | logit | 3 | −164.9 | 335.8 | 54.7 | 0.000 |
| Year | Chronology | −125.70 | 0.06 | logit | 2 | −169.1 | 342.2 | 61.7 | 0.000 |
| Part. Clutch Col.-Yes | Nest Mgmt. | −0.49 | −1.46 | logit | 2 | −170.5 | 345.0 | 63.9 | 0.000 |
| null | - | −0.52 | - | logit | 1 | −171.7 | 345.5 | 64.4 | 0.000 |
| Location | Geography | −0.41 | + | logit | 15 | −158.5 | 349.0 | 67.9 | 0.000 |
Notes: Beta estimates reflect covariates scaled to a modified z-score (standard deviations above the minimum observed value).
Appendix B

Table A2.
Tier-2 model selection results ranked using Akaike Information Criterion corrected for small sample sizes (AICc). Individual models are presented with a unique identifier (Model ID), scaled parameter estimates, model class (GLM or GLMM), degrees of freedom (df), Log-likelihood (Log. Lik), AIC Delta, and AIC Model Weight values.
Table A2.
Tier-2 model selection results ranked using Akaike Information Criterion corrected for small sample sizes (AICc). Individual models are presented with a unique identifier (Model ID), scaled parameter estimates, model class (GLM or GLMM), degrees of freedom (df), Log-likelihood (Log. Lik), AIC Delta, and AIC Model Weight values.
| Model ID | Int. | Black Flies | Female Age | Forced Renest-Y | Region | Male Age | Total Age | Nest Fail Dt. | Part. Clutch Col.-Y | Yrs Nest Tog. | Nest Initiate Dt. | Duration Nested | Yrs. Nest Female | Yrs. Nest Tot. | Yrs. Nest Male | Class | df | Log Lik. | AICc | Delta | Weight |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g33 | 8.96 | 2.65 | 0.69 | + | −12.62 | GLMM | 7 | −108.0 | 230.5 | 0.00 | 0.504 | ||||||||||
| g60 | 9.52 | 0.99 | + | −12.13 | 1.67 | GLMM | 7 | −109.1 | 232.7 | 2.17 | 0.170 | ||||||||||
| g23 | 9.06 | 2.60 | 0.52 | −12.58 | GLMM | 5 | −111.7 | 233.7 | 3.24 | 0.100 | |||||||||||
| g58 | 9.66 | 0.86 | + | −11.95 | 1.45 | GLMM | 7 | −110.0 | 234.5 | 4.04 | 0.067 | ||||||||||
| g28 | 8.07 | 0.93 | + | 2.86 | −12.05 | GLMM | 7 | −110.2 | 234.7 | 4.26 | 0.060 | ||||||||||
| g61 | 9.54 | 0.83 | −12.07 | 1.67 | GLMM | 5 | −112.5 | 235.3 | 4.82 | 0.045 | |||||||||||
| g59 | 9.80 | 0.73 | −12.08 | 1.47 | GLMM | 5 | −113.5 | 237.2 | 6.76 | 0.017 | |||||||||||
| g10 | 8.36 | 2.67 | 0.47 | −12.24 | GLMM | 7 | −111.4 | 237.3 | 6.77 | 0.017 | |||||||||||
| g29 | 8.09 | 0.74 | 2.79 | −11.88 | GLMM | 5 | −113.7 | 237.7 | 7.19 | 0.014 | |||||||||||
| g27 | 7.21 | + | 0.65 | 2.92 | −11.54 | GLMM | 7 | −113.0 | 240.5 | 10.02 | 0.003 | ||||||||||
| g62 | 9.58 | 1.31 | + | −11.83 | 1.19 | GLMM | 7 | −113.3 | 241.1 | 10.66 | 0.002 | ||||||||||
| g63 | 9.60 | 1.15 | −11.80 | 1.20 | GLMM | 5 | −117.0 | 244.2 | 13.77 | 0.001 | |||||||||||
| g31 | 9.17 | 1.28 | + | −11.09 | 1.00 | GLMM | 7 | −115.6 | 245.7 | 15.18 | 0.000 | ||||||||||
| g32 | 9.45 | 1.19 | −11.40 | 0.99 | GLMM | 5 | −119.8 | 249.9 | 19.37 | 0.000 | |||||||||||
| g25 | 8.71 | 1.23 | + | 1.53 | −11.41 | GLMM | 7 | −118.1 | 250.7 | 20.25 | 0.000 | ||||||||||
| g26 | 8.78 | 1.09 | 1.56 | −11.46 | GLMM | 5 | −121.7 | 253.5 | 23.07 | 0.000 | |||||||||||
| g30 | 9.07 | + | 1.16 | −11.28 | 1.01 | GLMM | 7 | −119.7 | 253.8 | 23.32 | 0.000 | ||||||||||
| g35 | 5.58 | 2.35 | 1.01 | + | −8.70 | GLMM | 7 | −120.2 | 254.8 | 24.27 | 0.000 | ||||||||||
| g41 | 5.45 | 1.06 | + | 2.77 | −9.10 | GLMM | 7 | −120.4 | 255.2 | 24.74 | 0.000 | ||||||||||
| g47 | −2.04 | 2.41 | 1.16 | + | −2.45 | GLMM | 7 | −120.6 | 255.6 | 25.14 | 0.000 | ||||||||||
| g24 | 8.34 | + | 1.05 | 1.64 | −11.27 | GLMM | 7 | −121.4 | 257.2 | 26.73 | 0.000 | ||||||||||
| g42 | 5.25 | 1.06 | 2.94 | −9.04 | GLMM | 5 | −123.5 | 257.2 | 26.77 | 0.000 | |||||||||||
| g36 | 5.31 | 2.45 | 0.91 | −8.54 | GLMM | 5 | −124.0 | 258.3 | 27.82 | 0.000 | |||||||||||
| g48 | −1.99 | 2.36 | 1.01 | −2.28 | GLMM | 5 | −124.6 | 259.4 | 28.91 | 0.000 | |||||||||||
| g40 | 4.38 | + | 0.94 | 3.08 | −8.90 | GLMM | 7 | −122.8 | 260.0 | 29.54 | 0.000 | ||||||||||
| g53 | −2.61 | 1.46 | + | 2.70 | −2.19 | GLMM | 7 | −123.2 | 260.8 | 30.28 | 0.000 | ||||||||||
| g34 | 4.75 | + | 2.53 | 0.84 | −8.40 | GLMM | 7 | −123.7 | 261.8 | 31.36 | 0.000 | ||||||||||
| g46 | −2.31 | + | 2.44 | 0.94 | −2.32 | GLMM | 7 | −123.8 | 262.0 | 31.49 | 0.000 | ||||||||||
| g54 | −2.52 | 1.27 | 2.62 | −1.98 | GLMM | 5 | −126.9 | 264.0 | 33.51 | 0.000 | |||||||||||
| g52 | −3.02 | + | 1.18 | 2.74 | −2.02 | GLMM | 7 | −125.9 | 266.2 | 35.73 | 0.000 | ||||||||||
| g56 | −0.65 | 1.64 | + | 1.00 | −2.21 | GLMM | 7 | −126.1 | 266.7 | 36.20 | 0.000 | ||||||||||
| g44 | 6.01 | 1.50 | + | 0.96 | −7.65 | GLMM | 7 | −126.2 | 266.8 | 36.30 | 0.000 | ||||||||||
| g38 | 6.42 | 1.44 | + | 1.60 | −8.94 | GLMM | 7 | −127.8 | 270.1 | 39.64 | 0.000 | ||||||||||
| g57 | −0.68 | 1.57 | 1.00 | −2.19 | GLMM | 5 | −130.4 | 271.1 | 40.58 | 0.000 | |||||||||||
| g2 | −2.95 | 2.21 | 1.34 | + | GLMM | 6 | −130.3 | 272.9 | 42.42 | 0.000 | |||||||||||
| g39 | 6.10 | 1.41 | 1.80 | −8.86 | GLMM | 5 | −131.6 | 273.4 | 42.95 | 0.000 | |||||||||||
| g45 | 5.50 | 1.54 | 0.97 | −7.27 | GLMM | 5 | −131.9 | 274.0 | 43.56 | 0.000 | |||||||||||
| g55 | −0.90 | + | 1.54 | 1.04 | −2.23 | GLMM | 7 | −129.9 | 274.3 | 43.78 | 0.000 | ||||||||||
| g12 | −3.06 | 2.81 | + | −3.09 | GLMM | 6 | −131.3 | 274.9 | 44.38 | 0.000 | |||||||||||
| g8 | −3.54 | 1.59 | + | 2.66 | GLMM | 6 | −131.3 | 274.9 | 44.45 | 0.000 | |||||||||||
| g3 | −3.03 | 2.29 | 1.24 | GLMM | 4 | −133.7 | 275.5 | 44.98 | 0.000 | ||||||||||||
| g9 | −3.59 | 1.47 | 2.75 | GLMM | 4 | −134.1 | 276.3 | 45.81 | 0.000 | ||||||||||||
| g13 | −3.19 | 2.90 | −3.01 | GLMM | 4 | −134.2 | 276.6 | 46.14 | 0.000 | ||||||||||||
| g37 | 5.66 | + | 1.35 | 1.86 | −8.78 | GLMM | 7 | −131.4 | 277.2 | 46.73 | 0.000 | ||||||||||
| g50 | −1.33 | 1.70 | + | 1.30 | −1.99 | GLMM | 7 | −131.6 | 277.6 | 47.12 | 0.000 | ||||||||||
| g43 | 5.18 | + | 1.51 | 1.00 | −7.22 | GLMM | 7 | −131.8 | 278.0 | 47.54 | 0.000 | ||||||||||
| g1 | −3.41 | + | 2.36 | 1.18 | GLMM | 6 | −133.2 | 278.8 | 48.28 | 0.000 | |||||||||||
| g7 | −4.26 | + | 1.36 | 2.86 | GLMM | 6 | −133.3 | 278.9 | 48.44 | 0.000 | |||||||||||
| g11 | −3.39 | + | 2.96 | −2.98 | GLMM | 6 | −133.8 | 279.9 | 49.45 | 0.000 | |||||||||||
| g51 | −1.41 | 1.58 | 1.37 | −1.88 | GLMM | 5 | −135.3 | 280.8 | 50.34 | 0.000 | |||||||||||
| g21 | −1.61 | 1.78 | + | 0.97 | GLMM | 6 | −134.6 | 281.6 | 51.15 | 0.000 | |||||||||||
| g19 | −3.65 | 3.27 | −2.65 | GLMM | 4 | −137.4 | 282.9 | 52.42 | 0.000 | ||||||||||||
| g18 | −3.46 | + | 3.10 | −2.69 | GLMM | 6 | −135.3 | 283.0 | 52.47 | 0.000 | |||||||||||
| g49 | −1.66 | + | 1.54 | 1.44 | −1.91 | GLMM | 7 | −134.8 | 284.1 | 53.57 | 0.000 | ||||||||||
| g17 | −4.27 | + | 3.35 | −2.41 | GLMM | 6 | −136.5 | 285.4 | 54.92 | 0.000 | |||||||||||
| g22 | −1.73 | 1.80 | 0.99 | GLMM | 4 | −139.6 | 287.4 | 56.95 | 0.000 | ||||||||||||
| g5 | −2.27 | 1.82 | + | 1.35 | GLMM | 6 | −139.3 | 290.9 | 60.38 | 0.000 | |||||||||||
| g20 | −2.07 | + | 1.76 | 1.02 | GLMM | 6 | −139.4 | 291.1 | 60.60 | 0.000 | |||||||||||
| g6 | −2.49 | 1.79 | 1.54 | GLMM | 4 | −142.6 | 293.4 | 62.96 | 0.000 | ||||||||||||
| g4 | −2.89 | + | 1.73 | 1.61 | GLMM | 6 | −142.3 | 297.0 | 66.51 | 0.000 | |||||||||||
| g15 | −1.85 | + | 1.47 | −2.07 | GLMM | 6 | −148.4 | 309.0 | 78.56 | 0.000 | |||||||||||
| g16 | −2.23 | 1.78 | −2.06 | GLMM | 4 | −152.0 | 312.1 | 81.66 | 0.000 | ||||||||||||
| g14 | −2.76 | + | 1.85 | −1.79 | GLMM | 6 | −151.6 | 315.5 | 85.03 | 0.000 | |||||||||||
| nullMM | −0.67 | GLMM | 2 | −165.0 | 334.0 1 | 03.48 | 0.000 | ||||||||||||||
| null | −0.52 | GLM | 1 | −171.7 | 345.5 1 | 15.03 | 0.000 |
Notes: Beta estimates reflect covariates scaled to a modified z-score (standard deviations above the minimum observed value). “+” represents a placeholder to indicate the presence of multi-level factors within the model.
References
- Canadian Wildlife Service [CWS]; U.S. Fish and Wildlife Service [USFWS]. International Recovery Plan for the Whooping Crane; Recovery of Nationally Endangered Wildlife (RENEW): Ottawa, ON, Canada; U.S. Fish and Wildlife Service: Albuquerque, NM, USA, 2007. [Google Scholar]
- U.S. Fish and Wildlife Service [USFWS]. Endangered Species List. Fed. Regist. 1967, 22, 4001–4002. [Google Scholar]
- U.S. Fish and Wildlife Service [USFWS]. Endangered Species Act of 1973, as Amended Through the 108th Congress; U.S. Department of the Interior: Washington, DC, USA; U.S. Fish and Wildlife Service: Washington, DC, USA, 1973; 44p. [Google Scholar]
- Thompson, H.L.; Gordon, N.M.; Bolt, D.P.; Lee, J.R.; Szyszkoski, E.K. Twenty-year status of the eastern migratory whooping crane reintroduction. Proc. N. Am. Crane Workshop 2022, 15, 34–52. [Google Scholar]
- Thompson, H.L. EMP Field Team Annual Report 2023; International Crane Foundation: Baraboo, WI, USA, 2024; Available online: https://savingcranes.org/whooping-crane-reintroduction-annual-reports/ (accessed on 6 April 2024).
- U.S. Fish and Wildlife Service [USFWS]. Whooping Crane Recovery Activities: 2022 Breeding Season to 2023 Spring Migration. 2024. Available online: https://www.fws.gov/media/whooping-crane-recovery-activities-2022-breeding-season-2023-spring-migration (accessed on 6 April 2024).
- Szyszkoski, E.K.; Zimorski, S.E.; Thompson, H.L. Thirteen-year status of the Louisiana nonmigratory whooping crane reintroduction. Proc. N. Am. Crane Workshop 2025, 16, 133–145. [Google Scholar]
- Urbanek, R.P.; Zimorski, S.E.; Fasoli, A.M.; Szyszkoski, E.K. Nest desertion in a reintroduced population of migratory whooping cranes. Proc. N. Am. Crane Workshop 2010, 11, 133–141. [Google Scholar]
- Barzen, J.A.; Converse, S.J.; Adler, P.H.; Lacy, A.; Gray, E.; Gossens, A. Examination of multiple working hypotheses to address reproductive failure in reintroduced whooping cranes. Condor Ornithol. Appl. 2018, 120, 632–649. [Google Scholar] [CrossRef]
- Van Schmidt, N.D.; Barzen, J.A.; Engels, M.J.; Lacy, A.E. Refining reintroduction of whooping cranes with habitat use and suitability analysis. J. Wildl. Manag. 2014, 78, 1404–1414. [Google Scholar] [CrossRef]
- Adler, P.H.; Barzen, J.; Gray, E.; Lacy, A.; Urbanek, R.P.; Converse, S.J. The dilemma of pest suppression in the conservation of endangered species. Conserv. Biol. 2019, 33, 788–796. [Google Scholar] [CrossRef]
- Jaworski, J.A. Factors Influencing Nest Success of Reintroduced Whooping Cranes (Grus americana) in Wisconsin. Master’s Thesis, University of Wisconsin, Stevens Point, WI, USA, 2016. [Google Scholar]
- Amat, J.A.; Fraga, R.M.; Arroyo, G.M. Replacement clutches by Kentish Plovers. Condor Ornithol. Appl. 1999, 101, 746–751. [Google Scholar] [CrossRef]
- Arnold, T.W.; Devries, J.H.; Howerter, D.W. Factors that affect renesting in Mallards (Anas platyrhynchos). Auk 2010, 127, 212–221. [Google Scholar] [CrossRef]
- McNew, L.B.; Gregory, A.J.; Wisely, S.M.; Sandercock, B.K. Reproductive biology of a southern population of Greater Prairie-Chickens. In Ecology, Conservation, and Management of Grouse; Studies in Avian Biology; Sandercock, B.K., Martin, L., Segelbacher, G., Eds.; University of California Press: Berkeley, CA, USA, 2011; Volume 39, pp. 209–221. [Google Scholar]
- Gates, H.R.; Lanctot, R.B.; Powell, A.N. High renesting rates in arctic-breeding Dunlin (Calidris alpina): A clutch-removal experiment. Auk 2013, 130, 372–380. [Google Scholar] [CrossRef]
- Claassen, A.H.; Arnold, T.W.; Roche, E.A.; Saunders, S.P.; Cuthbert, F.J. Factors influencing nest survival and renesting by Piping Plovers in the Great Lakes region. Condor Ornithol. Appl. 2014, 116, 394–407. [Google Scholar] [CrossRef]
- Swift, R.J.; Anteau, M.J.; Ring, M.M.; Toy, D.L.; Sherfy, M.H. Low renesting propensity and reproductive success make renesting unproductive for threatened Piping Plover (Charadrius melodus). Condor Ornithol. Appl. 2020, 122, duz066. [Google Scholar] [CrossRef]
- Martin, K.; Wilson, S.; Hannon, S.J. Mechanisms underlying variation in renesting ability of Willow Ptarmigan. In Ecology, Conservation, and Management of Grouse; Studies in Avian Biology; Sandercock, B.K., Martin, K., Segelbacher, G., Eds.; University of California Press: Berkeley, CA, USA, 2011; Volume 39, pp. 233–246. [Google Scholar]
- U.S. Fish and Wildlife Service [USFWS]. Necedah National Wildlife Refuges Comprehensive Conservation Plan and Environmental Assessment; U.S. Fish and Wildlife Service: Necedah, WI, USA, 2004. [Google Scholar]
- Wisoconsin Department of Natural Resources [WDNR]. White River Marsh Wildlife Area. 2023. Available online: https://dnr.wisconsin.gov/topic/Lands/WildlifeAreas/whiteriver.html (accessed on 17 April 2024).
- U.S. Fish and Wildlife Service [USFWS]. Horicon and Fox River National Wildlife Refuges Comprehensive Conservation Plan; U.S. Fish and Wildlife Service: Mayville, WI, USA, 2007. [Google Scholar]
- Wellington, M.; Burke, A.; Nicolich, J.M.; O’Malley, K. Chick rearing. In Cranes: Their Biology, Husbandry, and Conservation; Ellis, D.H., Gee, G.F., Mirande, C.M., Eds.; Department of the Interior National Biological Service: Washington, DC, USA; International Crane Foundation: Baraboo, WI, USA, 1996; pp. 77–104. [Google Scholar]
- Hartup, B.K. Rearing and release methods for reintroduction of captive-reared whooping cranes. In Whooping Cranes: Biology and Conservation; French, J.B., Converse, S.J., Austin, J.E., Eds.; Academic Press: San Diego, CA, USA, 2019; pp. 433–447. [Google Scholar]
- Griffiths, R.; Double, M.C.; Orr, K.; Dawson, R.J.G. A DNA test to sex most birds. Mol. Ecol. 1998, 7, 1071–1075. [Google Scholar] [CrossRef]
- Duan, W.; Fuerst, P.A. Isolation of a sex-linked DNA sequence in cranes. J. Hered. 2001, 92, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Urbanek, R.P. Color-band identification system of the reintroduced Eastern Migratory Whooping Crane Population. Proc. N. Am. Crane Workshop 2018, 14, 101–109. [Google Scholar]
- McKinney, L.F. Conservation Challenges for Whooping Cranes (Grus americana) and Greater Sandhill Cranes (Grus canadensis) in Wisconsin. Master’s Thesis, University of Wisconsin, Stevens Point, WI, USA, 2014. [Google Scholar]
- Thompson, H.L.; Gordon, N.M. First description of nesting behavior of a same-sex pair of whooping cranes (Grus americana) in the reintroduced Eastern Migratory Population. Waterbirds 2020, 3, 326–332. [Google Scholar] [CrossRef]
- Gordon, N.M.; Bolt, D.P.; Thompson, H.L. Vigilance of nesting whooping cranes in Juneau County, Wisconsin. Proc. N. Am. Crane Workshop 2022, 15, 81–89. [Google Scholar]
- Fair, J.; Paul, E.; Jones, J.; Bies, L. (Eds.) Guidelines to the Use of Wild Birds in Research; Ornithological Council: Washington, DC, USA, 2023. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 4 November 2024).
- Nelder, J.A.; Wedderburn, R.W.M. Generalized linear models. J. R. Stat. Soc. Ser. A (Gen.) 1972, 135, 370–384. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002. [Google Scholar]
- Dobson, A.J.; Barnett, A.G. An Introduction to Generalized Linear Models; Chapman and Hall/CRC: Boca Raton, FL, USA, 2018. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Bartoń, K.; MuMIn: Multi-Model Inference. R Package Version 1.47.5. 2023. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 4 November 2024).
- Dean, C.B.; Nielsen, J.D. Generalized linear mixed models: A review and some extensions. Lifetime Data Anal. 2007, 13, 497–512. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Scheipl, F.; Grothendieck, G.; Green, P.; et al. Package ‘lme4’: Linear Mixed-Effects Models Using ‘Eigen’ and S4. CRAN, R Package Version 1.1-27.1. 2021. Available online: https://cran.r-project.org/web/packages/lme4/index.html (accessed on 4 November 2024).
- Harrell, F.E., Jr. Hmisc: Harrell Miscellaneous. R Package Version 4.5-0. 2021. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 4 November 2024).
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Cramer, H. Mathematical Methods of Statistics, 19th ed.; Princeton University Press: Princeton, NJ, USA, 1999. [Google Scholar]
- Ben-Shachar, M.; Lüdecke, D.; Makowski, D. effectsize: Estimation of Effect Size Indices and Standardized Parameters. J. Open Source Softw. 2020, 5, 2815. [Google Scholar] [CrossRef]
- Rizopoulos, D. ltm: An R package for Latent Variable Modelling and Item Response Theory Analyses. J. Stat. Softw. 2006, 17, 1–25. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Powell, M.J.D. The BOBYQA Algorithm for Bound Constrained Optimization Without Derivatives; Report No. DAMTP 2009/NA06; Centre for Mathematical Sciences, University of Cambridge: Cambridge, UK, 2009. [Google Scholar]
- Afifi, A.; May, S.; Donatello, R.A.; Clark, V.A. Practical Multivariate Analysis, 6th ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2020. [Google Scholar]
- Nakagawa, S.; Johnson, P.C.; Schielzeth, H. The coefficient of determination R2 and intra-class correlation coefficient from generalized linear mixed-effects models revisited and expanded. J. R. Soc. Interface 2017, 14, 20170213. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Weisberg, S. Visualizing fit and lack of fit in complex regression models with Predictor Effect Plots and Partial Residuals. J. Stat. Softw. 2018, 87, 1–27. [Google Scholar] [CrossRef]
- Becker, P.H.; Zhang, H. Renesting of Common Terns in the life history perspective. J. Ornithol. 2010, 152, 213–225. [Google Scholar] [CrossRef]
- Forslund, P.; Pärt, T. Age and reproduction in birds: Hypotheses and tests. Trends Ecol. Evol. 1995, 10, 374–378. [Google Scholar] [CrossRef]
- Devries, J.H.; Brook, R.W.; Howerter, D.W.; Anderson, M.G. Effects of spring body condition and age on reproduction in mallards (Anas platyrhynchos). Auk 2008, 125, 618–628. [Google Scholar] [CrossRef]
- Nagy, L.R.; Holmes, R.T. Factors influencing fecundity in migratory songbirds: Is nest predation the most important? J. Avian Biol. 2004, 35, 487–491. [Google Scholar] [CrossRef]
- Lacy, A.; McElwee, D. Observations of molt in reintroduced whooping cranes. Proc. N. Am. Crane Workshop 2014, 12, 75. [Google Scholar]
- Edwards, H.A.; Bidwell, M.T.; Moehrenschlager, A. A call for structured decision making in conservation programs considering wild egg collection. Biol. Conserv. 2019, 238, 108226. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).