Habitat Associated with Ramps/Wild Leeks (Allium tricoccum Ait.) in Pennsylvania, USA: Guidance for Forest Farming Site Selection
Simple Summary
Abstract
1. Introduction
- (1)
- What abiotic site factors are associated with ramp occurrences throughout PA?
- (2)
- How do factors encountered in the field compare with presence-only modeling results?
- (3)
- What flora are associated with ramp occurrences, and which species might be useful for site selection?
2. Materials and Methods
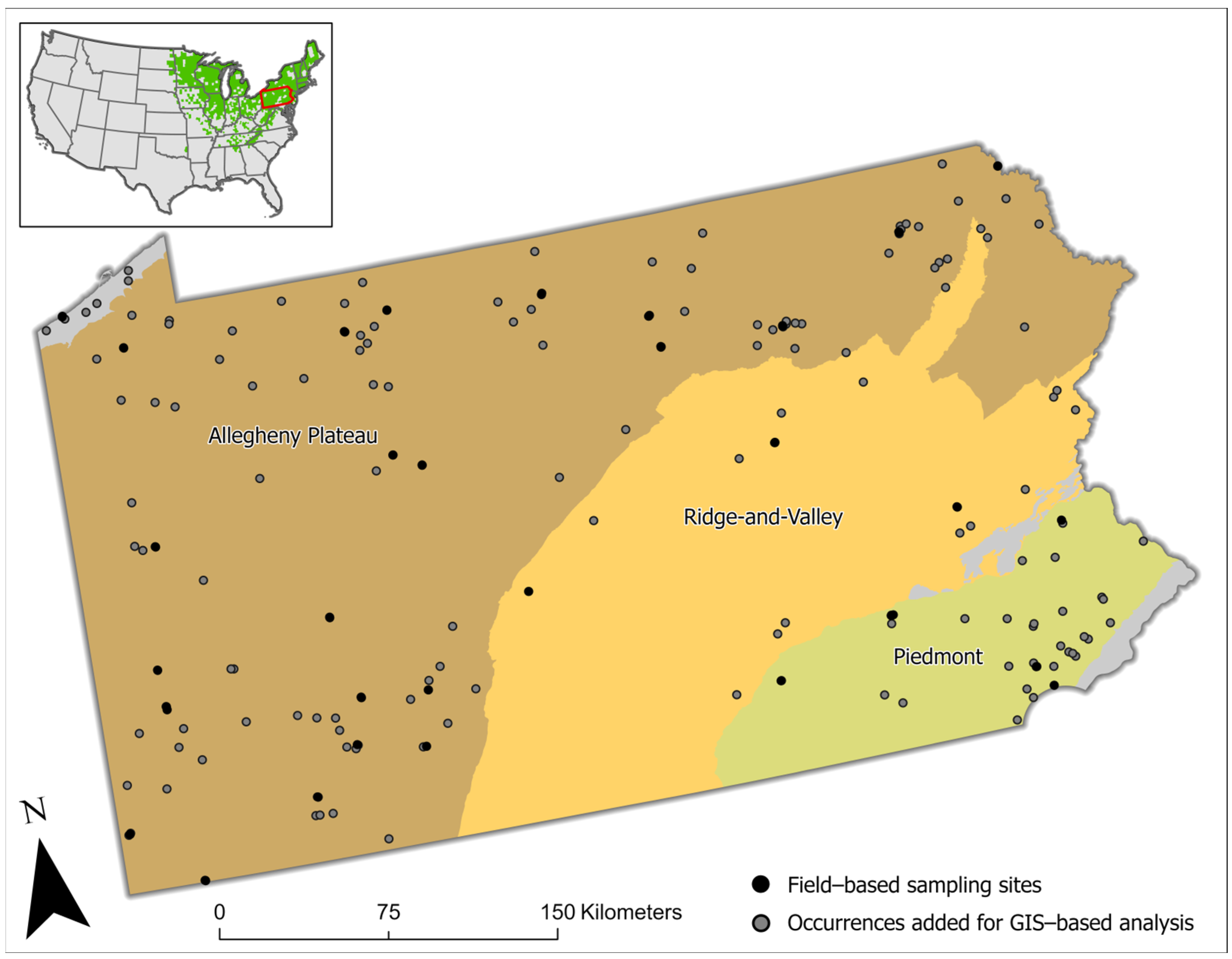
2.1. Study Area
2.2. GIS Methods
2.2.1. Modeling Habitat Suitability
2.2.2. Occurrence Data Inclusion Criteria
2.2.3. Environmental Predictor Variables
2.2.4. Model Evaluation
2.3. Field Methods
2.3.1. Field Sampling Inclusion Criteria
2.3.2. Field Sampling
2.3.3. Statistical Analysis of Field Data
3. Results
3.1. Model Performance
3.2. Topographic Variables
3.3. Edaphic Variables
3.4. Community Analysis
3.4.1. Overall Community Analysis Results
3.4.2. Forest Overstory
3.4.3. Forest Midstory
3.4.4. Forest Understory
4. Discussion
4.1. Topographic Site Factors
4.2. Edaphic Site Factors
4.3. Community Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baumflek, M.; Chamberlain, J.L. Ramps Reporting: What 70 Years of Popular Media Tells Us About A Cultural Keystone Species. Southeast. Geogr. 2019, 59, 77–96. [Google Scholar] [CrossRef]
- Pugh, C. Ramp/Leek “Culture” in Northern Appalachia: A Study of Attitudes, Behaviors, and Knowledge Surrounding a Non-Timber Forest Product. Master’s Thesis, The Pennsylvania State University, University Park, PA, USA, 2022. [Google Scholar]
- Moerman, D.E. Native American Food Plants: An Ethnobotanical Dictionary; Timber Press: Portland, OR, USA, 2010. [Google Scholar]
- Kofiar, M.; Koyuncu, M.; Bafier, K.H.C. Folk Use of Some Wild and Cultivated Allium Species in Turkey. In Proceedings of the Proceedings of the IVth International Congress of Ethnobotany (ICEB 2005), Istanbul, Turkey, 21–26 August 2005; Volume 87, p. 90. [Google Scholar]
- Gębczyński, P.; Bernaś, E.; Słupski, J. Usage of Wild-Growing Plants as Foodstuff. In Cultural Heritage—Possibilities for Land-Centered Societal Development; Hernik, J., Walczycka, M., Sankowski, E., Harris, B.J., Eds.; Environmental History; Springer International Publishing: Cham, Switzerland, 2022; Volume 13, pp. 269–283. ISBN 978-3-030-58091-9. [Google Scholar]
- Yamada, T. The Worldview of the Ainu; Columbia University Press: New York, NY, USA, 2001. [Google Scholar]
- Rivers, B.; Oliver, R.; Resler, L. Pungent Provisions: The Ramp and Appalachian Identity. Mater. Cult. 2014, 46, 1–24. [Google Scholar]
- Bernatchez, A.; Bussières, J.; Lapointe, L. Testing Fertilizer, Gypsum, Planting Season and Varieties of Wild Leek (Allium tricoccum) in Forest Farming System. Agrofor. Syst. 2013, 87, 977–991. [Google Scholar] [CrossRef]
- Rock, J.H.; Beckage, B.; Gross, L.J. Population Recovery Following Differential Harvesting of Allium Tricoccum Ait. in the Southern Appalachians. Biol. Conserv. 2004, 116, 227–234. [Google Scholar] [CrossRef]
- NatureServe. Allium tricoccum | NatureServe Explorer. Available online: https://explorer.natureserve.org/Taxon/ELEMENT_GLOBAL.2.794001/Allium_tricoccum (accessed on 16 October 2024).
- Chamberlain, J.; Beegle, D.; Connette, K. Forest Farming Ramps. Agrofor. Notes 2014, 47, 1–7. [Google Scholar]
- Chamberlain, J.L.; Mitchell, D.; Brigham, T.; Hobby, T.; Zabek, L.; Davis, J. Forest Farming Practices. In ASA, CSSA, and SSSA Books; “Gene” Garrett, H.E., Ed.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 2009; pp. 219–255. ISBN 978-0-89118-190-3. [Google Scholar]
- Strong, N.; Jacobson, M.G. A Case for Consumer-Driven Extension Programming: Agroforestry Adoption Potential in Pennsylvania. Agrofor. Syst. 2006, 68, 43–52. [Google Scholar] [CrossRef]
- McLain, R.; Jones, E. Characteristics of Non-Industrial Private Forest Owners Interested in Managing Their Land for Nontimber Forest Products. J. Ext. 2013, 51, 7. [Google Scholar] [CrossRef]
- Dion, P.-P.; Bussières, J.; Lapointe, L. Sustainable Leaf Harvesting and Effects of Plant Density on Wild Leek Cultivation Plots and Natural Stands in Southern Quebec, Canada. Agrofor. Syst. 2016, 90, 979–995. [Google Scholar] [CrossRef]
- Nilson, S.E.; Burkhart, E.P.; Jordan, R.T.; Lambert, J.D. Ramp (Allium tricoccum Ait.) Weight Differs across the Harvest Season: Implications for Wild Plant Stewardship and Forest Farming. Agrofor. Syst. 2023, 97, 97–107. [Google Scholar] [CrossRef]
- Burkhart, E.P. “Conservation Through Cultivation:” Economic, Socio-Political and Ecological Considerations Regarding the Adoption Of Ginseng Forest Farming In Pennsylvania. Doctoral Dissertation, The Pennsylvania State University, University Park, PA, USA, 2011. [Google Scholar]
- Davis, J.; Persons, W.S. Growing and Marketing Ginseng, Goldenseal and Other Woodland Medicinals; New Society Publishers: Gabriola, BC, Canada, 2014. [Google Scholar]
- Chamberlain, J.L.; Predny, M. Non-Timber Forest Products: Alternative Multiple-Uses for Sustainable Forest Management. Proc. Enhancing South. Appalach. For. Resour. 2003, 1, 1–6. Available online: https://research.fs.usda.gov/treesearch/9179 (accessed on 30 October 2024).
- Weakley, A. Flora of the Southeatern United States: Pennsylvania; University of North Carolina Herbarium, North Carolina Botanical Garden: Chapel Hill, NC, USA, 2023. [Google Scholar]
- Rhoads, A.F.; Block, T.A. The Plants of Pennsylvania: An Illustrated Manual; University of Pennsylvania Press: Philadelphia, PA, USA, 2007. [Google Scholar]
- Vasseur, L.; Gagnon, D. Survival and Growth of Allium tricoccum AIT. Transplants in Different Habitats. Biol. Conserv. 1994, 68, 107–114. [Google Scholar] [CrossRef]
- Geries, L.S.M.; El-Shahawy, T.A.; Moursi, E.A. Cut-off Irrigation as an Effective Tool to Increase Water-Use Efficiency, Enhance Productivity, Quality and Storability of Some Onion Cultivars. Agric. Water Manag. 2021, 244, 106589. [Google Scholar] [CrossRef]
- Davis, J.M.; Greenfield, J. Cultivating Ramps: Wild Leeks of Appalachia. In Trends in New Crops and New Uses; ASHS Press: Alexandria, VA, USA, 2002; Volume 1, pp. 449–452. [Google Scholar]
- Ritchey, K.D.; Schumann, C.M. Response of Woodland-Planted Ramps to Surface-Applied Calcium, Planting Density, and Bulb Preparation. HortScience 2005, 40, 1516–1520. [Google Scholar] [CrossRef]
- Burkhart, E.P. American Ginseng (Panax Quinquefolius L.) Floristic Associations in Pennsylvania: Guidance for Identifying Calcium-Rich Forest Farming Sites. Agrofor. Syst. 2013, 87, 1157–1172. [Google Scholar] [CrossRef]
- Peck, J.E. Multivariate Analysis for Ecologists: Step-by-Step, 2nd ed.; MjM Software Design: Gleneden Beach, OR, USA, 2016. [Google Scholar]
- Gilliam, F. The Herbaceous Layer in Forests of Eastern North America; Oxford University Press: New York, NY, USA, 2014. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species Assemblages and Indicator Species:The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, Q.; Wang, Z.; Guo, Q.; Wang, J.; Liu, N.; Liang, K. Conservation and Possible Reintroduction of an Endangered Plant Based on an Analysis of Community Ecology: A Case Study of Primulina tabacum Hance in China. Plant Species Biol. 2010, 25, 43–50. [Google Scholar] [CrossRef]
- Le Duc, M.G.; Hill, M.O.; Sparks, T.H. A Method for Predicting the Probability of Species Occurrence Using Data from Systematic Surveys. Watsonia 1992, 19, 97–105. [Google Scholar]
- Guisan, A.; Zimmermann, N.E. Predictive Habitat Distribution Models in Ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Hirzel, A.; Guisan, A. Which Is the Optimal Sampling Strategy for Habitat Suitability Modelling. Ecol. Model. 2002, 157, 331–341. [Google Scholar] [CrossRef]
- Bellemare, J.; Motzkin, G.; Foster, D.R. Legacies of the Agricultural Past in the Forested Present: An Assessment of Historical Land-Use Effects on Rich Mesic Forests. J. Biogeogr. 2002, 29, 1401–1420. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of MaxEnt for Ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Yee, T.W.; Mitchell, N.D. Generalized Additive Models in Plant Ecology. J. Veg. Sci. 1991, 2, 587–602. [Google Scholar] [CrossRef]
- Busby, J.R. BIOCLIM-a Bioclimate Analysis and Prediction System. Plant Prot. Q. 1991, 6, 8–9. [Google Scholar]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- The Pennsylvania State Climatologist PA Climate Office. Available online: https://climate.met.psu.edu/ (accessed on 22 October 2024).
- Pennsylvania Bureau of Topographic and Geologic Survey, Department of Conservation and Natural Resources Bedrock Geology of Pennsylvania 2001. Available online: https://www.dcnr.pa.gov/Geology/PublicationsAndData/Pages/default.aspx (accessed on 30 October 2024).
- Pennsylvania Natural Heritage Program Plant Communities of Pennsylvania. Available online: https://www.naturalheritage.state.pa.us/Communities.aspx (accessed on 16 October 2024).
- Albright, T.A.; Butler, B.J.; Caputo, J.; Crocker, S.J.; Goff, T.C.; Kurtz, C.M.; Lehman, S.; Lister, T.W.; Luppold, W.G.; Morin, R.S.; et al. Pennsylvania Forests 2019: Summary Report; Resour. Bull. NRS-131; U.S. Department of Agriculture, Forest Service, Northern Research Station: Madison, WI, USA, 2023; Volume 131, pp. 1–36. [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Blumberg, B.; Blumberg, B.; Cunningham, R.L. An Introduction to Soils of Pennsylvania; Department of Agriculture and Extension Education: University Park, PA, USA, 1982. [Google Scholar]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J.; Hijmans, M.R.J. Package ‘Dismo’. Circles 2017, 9, 1–68. [Google Scholar]
- R Core Team, R. R: A Language and Environment for Statistical Computing, Version 4.2.2; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Hysen, L.; Nayeri, D.; Cushman, S.; Wan, H.Y. Background Sampling for Multi-Scale Ensemble Habitat Selection Modeling: Does the Number of Points Matter? Ecol. Inform. 2022, 72, 101914. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Stark, C. Habitat and Flora Associated with Two Ramp/Leek Species (Allium tricoccum and A. burdickii) in Pennsylvania. Master’s Thesis, The Pennsylvania State University, University Park, PA, USA, 2022. [Google Scholar]
- Brown, J.L. SDMtoolbox: A Python-Based GIS Toolkit for Landscape Genetic, Biogeographic and Species Distribution Model Analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Peters, M.P.; Prasad, A.M.; Matthews, S.N.; Iverson, L.R. Climate Change Tree Atlas; Version 4; U.S. Forest Service, Northern Research Station and Northern Institute of Applied Climate Science: Delaware, OH, USA, 2020. Available online: https://www.nrs.fs.fed.us/atlas (accessed on 30 October 2024).
- Iverson, L.R.; Peters, M.P.; Prasad, A.M.; Matthews, S.N. Analysis of Climate Change Impacts on Tree Species of the Eastern US: Results of DISTRIB-II Modeling. Forests 2019, 10, 302. [Google Scholar] [CrossRef]
- Iverson, L.R.; Prasad, A.M.; Peters, M.P.; Matthews, S.N. Facilitating Adaptive Forest Management under Climate Change: A Spatially Specific Synthesis of 125 Species for Habitat Changes and Assisted Migration over the Eastern United States. Forests 2019, 10, 989. [Google Scholar] [CrossRef]
- Soil Survey Staff. Gridded Soil Survey Geographic (gSSURGO) Database for the United States of America and the Territories, Commonwealths, and Island Nations Served by the USDA-NRCS; Natural Resources Conservation Service: Lincoln, NE, USA, 2014. [Google Scholar]
- Nault, A.; Gagnon, D. Ramet Demography of Allium Tricoccum, A Spring Ephemeral, Perennial Forest Herb. J. Ecol. 1993, 81, 101–119. [Google Scholar] [CrossRef]
- Sitepu, B.S. An Integrative Taxonomic Study of Ramps (Allium tricoccum Aiton) Complex. Master’s Thesis, Ohio University, Athens, OH, USA, 2018. [Google Scholar]
- Iverson, L.R.; Dale, M.E.; Scott, C.T.; Prasad, A. A Gis-Derived Integrated Moisture Index to Predict Forest Composition and Productivity of Ohio Forests (U.S.A.). Landsc. Ecol. 1997, 12, 331–348. [Google Scholar] [CrossRef]
- Beers, T.W.; Dress, P.E.; Wensel, L.C. Notes and Observations: Aspect Transformation in Site Productivity Research. J. For. 1966, 64, 691–692. [Google Scholar]
- Riley, S.J.; DeGloria, S.D.; Elliot, R. Index That Quantifies Topographic Heterogeneity. Intermt. J. Sci. 1999, 5, 23–27. [Google Scholar]
- Weiss, A. Topographic Position and Landforms Analysis. In Proceedings of the Poster Presentation, ESRI User Conference, San Diego, CA, USA, 9-13 July 2001; Volume 200. [Google Scholar]
- Jenness, J.; Majka, D.; Beier, P. Corridor Designer Evaluation Tools; Northern Arizona University: Flagstaff, AZ, USA, 2011; Available online: https://www.jennessent.com/arcgis/corridor.htm (accessed on 30 October 2024).
- PAMAP Program, PA Department of Conservation and Natural Resources, Bureau of Topographic and Geologic Survey. PAMAP Program 3.2 Ft Digital Elevation Model of Pennsylvania. 2008. Available online: https://www.pasda.psu.edu/uci/datasummary.aspx?dataset=1247 (accessed on 30 October 2024).
- Warren, D.L.; Matzke, N.J.; Cardillo, M.; Baumgartner, J.B.; Beaumont, L.J.; Turelli, M.; Glor, R.E.; Huron, N.A.; Simões, M.; Iglesias, T.L.; et al. ENMTools 1.0: An R Package for Comparative Ecological Biogeography. Ecography 2021, 44, 504–511. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silande, J.A., Jr. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Araújo, M.B.; Pearson, R.G.; Thuiller, W.; Erhard, M. Validation of Species–Climate Impact Models under Climate Change. Glob. Chang. Biol. 2005, 11, 1504–1513. [Google Scholar] [CrossRef]
- Phillips, S.J. A Brief Tutorial on Maxent. Att Res. 2005, 190, 231–259. [Google Scholar]
- Eckert, D.; Sims, J.T. Recommended Soil pH and Lime Requirement Tests. Recomm. Soil Test. Proced. Northeast. U.S. Northeast Reg. Bull. 1995, 493, 11–16. [Google Scholar]
- Wolf, A.M.; Beegle, D.B. Recommended Soil Tests for Macronutrients: Phosphorus, Potassium, Calcium, and Magnesium. Recomm. Soil Test. Proced. Northeast. U.S. Northeast Reg. Bull 1995, 493, 25–34. [Google Scholar]
- Causton, D.R. An Introduction to Vegetation Analysis; Springer: Dordrecht, The Netherlands, 1988; ISBN 978-0-04-581025-3. [Google Scholar]
- Kent, M. Vegetation Description and Data Analysis: A Practical Approach; John Wiley & Sons: New York, NY, USA, 2011. [Google Scholar]
- Curtis, J.T.; McIntosh, R.P. An Upland Forest Continuum in the Prairie-Forest Border Region of Wisconsin. Ecology 1951, 32, 476–496. [Google Scholar] [CrossRef]
- Anderson, M.J. Analysis of Ecological Communities: Bruce McCune and James B. Grace, MjM Software Design, Gleneden Beach, USA, 2002, ISBN 0 9721290 0 6, US$ 35 (Pbk). J. Exp. Mar. Biol. Ecol. 2003, 289, 303–305. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. PC-ORD Multivariate Analysis of Ecological Data, Version 6; MjM Software Design: Gleneden Beach, OR, USA, 2011. [Google Scholar]
- Fike, J. Terrestrial & Palustrine Plant Communities of Pennsylvania; Bureau of Forestry, PA, Department of Conservation and Natural Resources: Harrisburg, PA, USA, 1999. [Google Scholar]
- NatureServe. Central Appalachian Rich Cove Forest | NatureServe Explorer. Available online: https://explorer.natureserve.org/Taxon/ELEMENT_GLOBAL.2.687966/Acer_saccharum_-_Fraxinus_americana_-_Tilia_americana_-_Liriodendron_tulipifera_-_Actaea_racemosa_Forest (accessed on 16 October 2024).
- Zimmerman, E. Sugar Maple—Mixed Hardwood Floodplain Forest Factsheet. Pennsylvania Natural Heritage Program. 2022. Available online: https://www.naturalheritage.state.pa.us/Community.aspx?=30017 (accessed on 30 October 2024).
- Zimmerman, E.; Fike, J. Tuliptree—Beech—Maple Forest Factsheet. Pennsylvania Natural Heritage Program. 2022. Available online: https://www.naturalheritage.state.pa.us/Community.aspx?=16065 (accessed on 30 October 2024).
- Perles, S. Bitternut Hickory Floodplain Forest Factsheet. Pennsylvania Natural Heritage Program. 2022. Available online: https://www.naturalheritage.state.pa.us/Community.aspx?=30003 (accessed on 30 October 2024).
- Osman, N.; Barakbah, S.S. The Effect of Plant Succession on Slope Stability. Ecol. Eng. 2011, 37, 139–147. [Google Scholar] [CrossRef]
- Nevo, E.; Fragman, O.; Dafni, A.; Beiles, A. Biodiversity and interslope divergence of vascular plants caused by microclimatic differences at “evolution canyon”, lower nahal oren, mount carmel, Israel. Isr. J. Plant Sci. 1999, 47, 49–59. [Google Scholar] [CrossRef]
- Randall, J.; Anderson, S. Soils Genesis and Geomorphology; Cambridge University Press: Cambridge, UK, 2005; p. 832. ISBN 521812011. [Google Scholar]
- Fletcher, S.W. The Expansion of the Agricultural Frontier. Pa. Hist. J.-Atl. Stud. 1951, 18, 119–129. [Google Scholar]
- Burns, R.M.; Honkala, B.H. Silvics of North America: Volume 2. Hardwoods; U.S. Department of Agriculture, Farm Service Agency: Washington, DC, USA, 1990.
- Caspersen, J.P.; Kobe, R.K. Interspecific Variation in Sapling Mortality in Relation to Growth and Soil Moisture. Oikos 2001, 92, 160–168. [Google Scholar] [CrossRef]
- Ott, N.F.J.; Watmough, S.A. Contrasting Litter Nutrient and Metal Inputs and Soil Chemistry among Five Common Eastern North American Tree Species. Forests 2021, 12, 613. [Google Scholar] [CrossRef]
- Dion, P.-P.; Bussières, J.; Lapointe, L. Late Canopy Closure Delays Senescence and Promotes Growth of the Spring Ephemeral Wild Leek (Allium tricoccum). Botany 2017, 95, 457–467. [Google Scholar] [CrossRef]
- Houston, E. Habitat Modeling of Goldenseal (Hydrastis canadensis L.) and Ramps (Allium tricoccum ait.): Implications for In Situ Conservation and Guidance for Forest Cultivation. Master’s Thesis, The Pennsylvania State University, University Park, PA, USA, 2024. [Google Scholar]
- Davis, J.; McCoy, J.-A. Commercial Goldenseal Cultivation | NC State Extension Publications. Available online: https://content.ces.ncsu.edu/commercial-goldenseal-cultivation (accessed on 16 October 2024).
- Carroll, C.; Apsley, D. Growing American Ginseng in Ohio: An Introduction. Ohio State University Extension. 2004. Available online: https://ohioline.osu.edu/factsheet/F-56 (accessed on 30 October 2024).



| Variable | Description | Percent Contribution | Source |
|---|---|---|---|
| Topographic predictors | |||
| TPI2000 | Topographic position index (2000 m neighborhood) | 57.8 | PA Department of Conservation and Natural Resources |
| ROUGH27 | Variance of elevation (27-pixel neighborhood) | 11.2 | PA Department of Conservation and Natural Resources |
| IMI | Integrated moisture index | 15.7 | Iverson et al., 1997 [58] |
| Edaphic predictors | |||
| DEPTH | Soil depth to underlying bedrock | 8.3 | USDA Natural Resource Conservation Service |
| FORMATIONS | Fertility of bedrock formations | 6.6 | PA Department of Conservation and Natural Resources |
| SILT | % Silt | 0.4 | USDA Natural Resource Conservation Service |
| Nutrients | Mean (SD) | Range |
|---|---|---|
| pH | 5.7 (0.6) | 4.8–7.0 |
| P (ppm) | 17 (13) | 5–76 |
| K (ppm) | 119 (42) | 44–269 |
| Mg (ppm) | 170 (124) | 60–721 |
| Ca (ppm) | 1462 (752) | 356–3399 |
| Scientific Name + | Common Name | Relative Abundance | IV % | ISA | |||||
|---|---|---|---|---|---|---|---|---|---|
| Freq. | Den. | Dom. | Lat | Prov | Aspect | Topo | |||
| Acer saccharum Marshall | Sugar maple | 28.4 | 39 | 29.7 | 32.4% | N ** | AP *** | N ** | |
| Liriodendron tulipifera L. | Tulip poplar | 8.3 | 10.3 | 19.8 | 12.8% | S *** | P *** | ||
| Tilia americana L. | American basswood | 12.3 | 10.8 | 8.6 | 10.6% | ||||
| Prunus serotina Ehrh. | Black cherry | 9.6 | 8.5 | 7.7 | 8.6% | N ** | |||
| Carya cordiformis (Wang) K. Koch | Bitternut hickory | 7.6 | 7 | 6.1 | 6.9% | Flat ** | F *** | ||
| Quercus rubra L. | Northern red oak | 6.6 | 4.7 | 8.5 | 6.6% | ||||
| Fagus grandifolia Ehrh. | American beech | 4.9 | 3.7 | 3.8 | 4.1% | S *** | P *** | ||
| Fraxinus americana L. | White ash | 3.9 | 2.7 | 3.6 | 3.4% | ||||
| Ulmus rubra Muhl. | Slippery elm | 2.9 | 2 | 2.2 | 2.4% | ||||
| Carya ovata (P. Miller) K. Koch | Shagbark hickory | 2.9 | 2 | 1.2 | 2.1% | ||||
| Quercus alba L. | White oak | 1.7 | 2 | 1.2 | 1.6% | ||||
| Platanus occidentalis L. | American sycamore | 0.8 | 1 | 2.1 | 1.3% | ||||
| Juglans nigra L. | Black walnut | 1.2 | 1.7 | 1.1 | 1.3% | ||||
| Acer rubrum L. | Red maple | 1.2 | 1.5 | 0.9 | 1.2% | ||||
| Betula alleghaniensis Britt. | Yellow birch | 1 | 1.5 | 0.6 | 1.0% | ||||
| Betula lenta L. | Black birch | 0.8 | 1.2 | 0.5 | 0.9% | ||||
| Carya tomentosa Sarg. | Mockernut hickory | 0.5 | 0.7 | 1.2 | 0.8% | ||||
| Robinia pseudoacacia L. | Black locust | 0.5 | 0.5 | 0.4 | 0.5% | ||||
| Tsuga canadensis (L.) Carrière | Eastern hemlock | 0.3 | 0.5 | 0.2 | 0.4% | ||||
| Carya glabra Miller | Pignut hickory | 0.3 | 0.5 | 0.2 | 0.3% | ||||
| Quercus montana Willd. | Chestnut oak | 0.2 | 0.2 | 0.2 | 0.2% | ||||
| Magnolia acuminata L. | Cucumber magnolia | 0.2 | 0.2 | 0.2 | 0.2% | ||||
| Sassafras albidum (Nutt.) Nees | Sassafras | 0.2 | 0.2 | 0.2 | 0.2% | ||||
| Ulmus americana L. | American elm | 0.2 | 0.2 | 0.1 | 0.2% | ||||
| Carya sp. Nutt. | Hickory species | 0.2 | 0.2 | 0.1 | 0.2% | ||||
| Scientific Name + | Common Name | % of Sites and (n) |
|---|---|---|
| Rosa multiflora Thunb. Ex. Murr. * | Multiflora rose | 80 (24) |
| Berberis thunbergii A.P. de Candolle * | Japanese barberry | 63 (19) |
| Lindera benzoin (L.) Blume | Spicebush | 50 (15) |
| Ribes sp. L. | Gooseberry | 47 (14) |
| Rubus sp. L. | Blackberry | 47 (14) |
| Sambucus racemosa L. var. pubens (Michx.) Trautv. & C.A. Mey | Red elderberry | 43 (13) |
| Hamamelis virginiana L. | Witch-hazel | 37 (11) |
| Prunus virginiana L. | Choke cherry | 33 (10) |
| Vitis sp. L. | Grape vine | 33 (10) |
| Toxicodendron radicans (L.) Kuntze | Poison ivy | 27 (8) |
| Carpinus caroliniana Walter | Musclewood | 27 (8) |
| Parthenocissus quinquefolia (L.) Planch. | Virginia creeper | 23 (7) |
| Ostrya virginiana (P. Miller) K. Koch | American hophornbeam | 23 (7) |
| Rubus phoenicolasius Maxim. * | Wineberry | 20 (6) |
| Viburnum prunifolium L. | Blackhaw | 20 (6) |
| Celastrus orbiculatus Thunb. * | Oriental bittersweet | 17 (5) |
| Crataegus sp. L. | Hawthorn | 17 (5) |
| Lonicera morrowii A. Gray or bella Zabel * | Morrow’s or Bell’s honeysuckle | 17 (5) |
| Lonicera maackii (Rupr.) Herder * | Amur honeysuckle | 13 (4) |
| Elaeagnus umbellata Thunb. * | Autumn olive | 13 (4) |
| Scientific Name + | Common Name | % of Sites and (n) | ISA | ||||
|---|---|---|---|---|---|---|---|
| Lat | Long | Prov | Aspect | Topo | |||
| Caulophyllum thalictroides. (L.) Michx. | Blue cohosh | 83 (25) | S ** | W *** | N ** | ||
| Erythronium americanum Ker-Gawl. | Yellow trout lily | 83 (25) | N *** | ||||
| Podophyllum peltatum L. | Mayapple | 80 (24) | |||||
| Polystichum acrostichoides (Michx.) Schott | Christmas fern | 77 (23) | AP ** | ||||
| Eurybia divaricata (L.) G.L. Nesom | White wood aster | 73 (22) | N ** | E *** | |||
| Dryopteris intermedia (Muhl. Ex Willd.) A. Gray | Intermediate woodfern | 70 (21) | W *** | ||||
| Osmorhiza claytonii (Michx.) C. B. Clarke | Hairy sweet cicely | 70 (21) | |||||
| Circaea canadensis (L.) Hill | Enchanter’s nightshade | 70 (21) | E *** | Flat ** | F ** | ||
| Polygonatum pubescens (Willd.) Pursh. | Hairy Solomon’s seal | 70 (21) | S ** | ||||
| Alliaria petiolata (Bieb.) Cavara & Grande * | Garlic mustard | 67 (20) | |||||
| Arisaema triphyllum (L.) Schott | Jack-in-the-pulpit | 67 (20) | N ** | E ** | F ** | ||
| Cardamine diphylla (Michx.) Alph. Wood | Broadleaf toothwort | 67 (20) | N *** | W ** | AP ** | ||
| Persicaria virginiana (L.) Gaert. | Jumpseed | 67 (20) | |||||
| Geum canadense Jacquin | White avens | 67 (20) | |||||
| Maianthemum racemosum (L.) Link | False Solomon’s seal | 67 (20) | Flat ** | ||||
| Impatiens spp. | Jewelweed (any species) | 66 (20) | |||||
| Cardamine concatenata (Michx.) O. Schwarz | Cut-leaf toothwort | 63 (19) | W ** | RV ** | |||
| Viola sororia Willde. | Common blue violet | 63 (19) | F ** | ||||
| Trillium erectum L. | Purple trillium | 60 (18) | N ** | W *** | AP ** | ||
| Laportea canadensis (L.) Wedd. | Wood nettle | 56 (17) | RV ** | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houston, E.; Burkhart, E.P.; Stark, C.; Chen, X.; Nilson, S.E. Habitat Associated with Ramps/Wild Leeks (Allium tricoccum Ait.) in Pennsylvania, USA: Guidance for Forest Farming Site Selection. Wild 2024, 1, 63-81. https://doi.org/10.3390/wild1010006
Houston E, Burkhart EP, Stark C, Chen X, Nilson SE. Habitat Associated with Ramps/Wild Leeks (Allium tricoccum Ait.) in Pennsylvania, USA: Guidance for Forest Farming Site Selection. Wild. 2024; 1(1):63-81. https://doi.org/10.3390/wild1010006
Chicago/Turabian StyleHouston, Ezra, Eric P. Burkhart, Cassie Stark, Xin Chen, and Sarah E. Nilson. 2024. "Habitat Associated with Ramps/Wild Leeks (Allium tricoccum Ait.) in Pennsylvania, USA: Guidance for Forest Farming Site Selection" Wild 1, no. 1: 63-81. https://doi.org/10.3390/wild1010006
APA StyleHouston, E., Burkhart, E. P., Stark, C., Chen, X., & Nilson, S. E. (2024). Habitat Associated with Ramps/Wild Leeks (Allium tricoccum Ait.) in Pennsylvania, USA: Guidance for Forest Farming Site Selection. Wild, 1(1), 63-81. https://doi.org/10.3390/wild1010006






