Reducing Neuroinflammation and Risk of Mild Cognitive Impairment and Alzheimer’s Disease by Reducing Dietary Lipopolysaccharides, Arachidonic Acid, and Advanced Glycation End Products
Abstract
1. Introduction
2. LPS, Neuroinflammation, and Cognition
2.1. How LPS Increases Neuroinflammation
2.2. LPS Is Increased in the AD Brain
2.3. LPS Can Increase the Risk of AD and Cognitive Impairment
2.4. LPS Can Work with Arachidonic Acid to Degrade Memory
2.5. How LPS from Food Can Enter the Bloodstream
3. AGEs, Neuroinflammation, and Impaired Cognition
3.1. How AGEs Can Increase Neuroinflammation and Impair Cognition
3.2. AGEs in Food and Neuroinflammation
3.3. AGEs Are Increased in the AD Brain
3.4. How AGEs from Food Can Enter the Bloodstream
3.5. Dietary AGEs Increase Risk of AD and Cognitive Impairment
4. Arachidonic Acid, Neuroinflammation, and Cognitive Impairment
4.1. How Excess Dietary AA Creates Neuroinflammation in AD
4.2. Excess Arachidonic Acid Is Found in AD Brains
4.3. Excess Dietary Arachidonic Acid Can Impair Cognition
4.4. Sources of Arachidonic Acid in Food
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Glossary
References
- Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 1 May 2025).
- Saitgareeva, A.R.; Bulygin, K.V.; Gareev, I.F.; Beylerli, O.A.; Akhmadeeva, L.R. The role of microglia in the development of neurodegeneration. Neurol. Sci. 2020, 41, 3609–3615. [Google Scholar] [CrossRef]
- Gorica, E.; Calderone, V. Arachidonic acid derivatives and neuroinflammation. CNS Neurol. Disord.-Drug Targets Former. Curr. Drug Targets-CNS Neurol. Disord. 2022, 21, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Arnesen, P.M.; Wettergreen, M.; You, P.; Fladby, T. Alzheimer’s disease risk genes are differentially expressed upon Lipopolysaccharide stimulation in a myelogenic cell model. Alzheimer’s Dement. 2023, 19, e080112. [Google Scholar] [CrossRef]
- Blake, S.; Baroni, L.; Sherzai, D.; Blake, C.P.; Harding, T.; Piboolnurak, P.; Borman, P.; Harding, M. Reducing dietary advanced glycation end products to slow progression of cognitive decline and Alzheimer’s disease. Artic. Int. J. Transl. Sci. 2023, 3, 107. [Google Scholar]
- Szczechowiak, K.; Diniz, B.S.; Leszek, J. Diet and Alzheimer’s dementia–Nutritional approach to modulate inflammation. Pharmacol. Biochem. Behav. 2019, 184, 172743. [Google Scholar] [CrossRef]
- Henning, A.L.; Venable, A.S.; Vingren, J.L.; Hill, D.W.; McFarlin, B.K. Consumption of a high-fat meal was associated with an increase in monocyte adhesion molecules, scavenger receptors, and Propensity to Form Foam Cells. Cytom. Part B Clin. Cytom. 2018, 94, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Gayle, D.A.; Ling, Z.; Tong, C.; Landers, T.; Lipton, J.W.; Carvey, P.M. Lipopolysaccharide (LPS)-induced dopamine cell loss in culture: Roles of tumor necrosis factor-alpha, interleukin-1beta, and nitric oxide. Dev. Brain Res. 2002, 133, 27–35. [Google Scholar] [CrossRef]
- Grant, W.B.; Blake, S.M. Diet’s role in modifying risk of Alzheimer’s disease: History and present understanding. J. Alzheimer’s Dis. 2023, 96, 1353–1382. [Google Scholar] [CrossRef]
- Wilson, C.J.; Finch, C.E.; Cohen, H.J. Cytokines and cognition—The case for a head-to-toe inflammatory paradigm. J. Am. Geriatr. Soc. 2002, 50, 2041–2056. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, I.; Lange, J.H. Endotoxin: Is it an environmental factor in the cause of Parkinson’s disease? Occup. Environ. Med. 2003, 60, 378. [Google Scholar] [CrossRef]
- Cunningham, C.; Wilcockson, D.C.; Campion, S.; Lunnon, K.; Perry, V.H. Central and systemic LPS challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J. Neurosci. 2005, 25, 9275–9284. [Google Scholar] [CrossRef]
- Brown, G.C. The endotoxin hypothesis of neurodegeneration. J. Neuroinflamm. 2019, 16, 180. [Google Scholar] [CrossRef]
- Zhao, Y.; Sharfman, N.M.; Jaber, V.R.; Lukiw, W.J. Down-regulation of essential synaptic components by GI-tract microbiome-derived lipopolysaccharide (LPS) in LPS-treated human neuronal-glial (HNG) cells in primary culture: Relevance to Alzheimer’s disease (AD). Front. Cell. Neurosci. 2019, 13, 314. [Google Scholar] [CrossRef]
- Creely, S.J.; McTernan, P.G.; Kusminski, C.M.; Fisher, F.M.; Da Silva, N.F.; Khanolkar, M.; Evans, M.; Harte, A.L.; Kumar, S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol.-Endocrinol. Metab. 2007, 292, E740–E747. [Google Scholar] [CrossRef]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and inflammation in cognitive ageing and Alzheimer’s disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef]
- André, P.; Samieri, C.; Buisson, C.; Dartigues, J.F.; Helmer, C.; Laugerette, F.; Féart, C. Lipopolysaccharide-binding protein, soluble CD14, and the long-term risk of Alzheimer’s disease: A nested case-control pilot study of older community dwellers from the three-city cohort. J. Alzheimer’s Dis. 2019, 71, 751–761. [Google Scholar] [CrossRef]
- André, P.; Laugerette, F.; Féart, C. Metabolic endotoxemia: A potential underlying mechanism of the relationship between dietary fat intake and risk for cognitive impairments in humans? Nutrients 2019, 11, 1887. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lukiw, W.J. Gastrointestinal-tract, microbiome-derived lipopolysaccharide and other pro-inflammatory neurotoxins in sporadic Alzheimer’s disease. Alzheimer’s Dement. 2021, 17, e055635. [Google Scholar]
- Zhang, R.; Miller, R.G.; Gascon, R.; Champion, S.; Katz, J.; Lancero, M.; Narvaez, A.; Honrada, R.; Ruvalcaba, D.; McGrath, M.S. Circulating LPS and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J. Neuroimmunol. 2009, 206, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tapia, M.; Mimenza-Alvarado, A.; Granados-Domínguez, L.; Flores-López, A.; López-Barradas, A.; Ortiz, V.; Pérez-Cruz, C.; Sánchez-Vidal, H.; Hernández-Acosta, J.; Ávila-Funes, J.A.; et al. The gut microbiota–brain axis during aging, mild cognitive impairment and dementia: Role of tau protein, β-amyloid and lps in serum and curli protein in stool. Nutrients 2023, 15, 932. [Google Scholar] [CrossRef]
- Saji, N.; Saito, Y.; Yamashita, T.; Murotani, K.; Tsuduki, T.; Hisada, T.; Sugimoto, T.; Niida, S.; Toba, K.; Sakurai, T. Relationship between plasma lipopolysaccharides, gut microbiota, and dementia: A cross-sectional study. J. Alzheimer’s Dis. 2022, 86, 1947–1957. [Google Scholar] [CrossRef]
- Eikelenboom, P.; Van Exel, E.; Hoozemans, J.J.; Veerhuis, R.; Rozemuller, A.J.; Van Gool, W.A. Neuroinflammation—An early event in both the history and pathogenesis of Alzheimer’s disease. Neurodegener. Dis. 2010, 7, 38–41. [Google Scholar] [CrossRef]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Blasco, G.; Puig, J.; Biarnés, C.; Rivero, M.; Gich, J.; Fernandez-Aranda, F.; Garre-Olmo, J.; Ramio-Torrenta, L.; Alberich-Bayarri, A.; et al. Neuroinflammation in obesity: Circulating lipopolysaccharide-binding protein associates with brain structure and cognitive performance. Int. J. Obes. 2017, 41, 1627–1635. [Google Scholar] [CrossRef]
- DellaGioia, N.; Hannestad, J. A critical review of human endotoxin administration as an experimental paradigm of depression. Neurosci. Biobehav. Rev. 2010, 34, 130–143. [Google Scholar] [CrossRef]
- Yirmiya, R.; Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav. Immun. 2011, 25, 181–213. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Hüll, M.; Lieb, K.; Schumann, G.; Berger, M.; Bauer, J. Potential link between interleukin-6 and arachidonic acid metabolism in Alzheimer’s disease. In Alzheimer’s Disease—From Basic Research to Clinical Applications; Springer Verlag GmbH: Berlin/Heidelberg, Germany, 1998; pp. 269–278. [Google Scholar]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Byrne, M.L. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Laugerette, F.; Alligier, M.; Bastard, J.P.; Drai, J.; Chanséaume, E.; Lambert-Porcheron, S.; Laville, M.; Morio, B.; Vidal, H.; Michalski, M.C. Overfeeding increases postprandial endotoxemia in men: Inflammatory outcome may depend on LPS transporters LBP and sCD14. Mol. Nutr. Food Res. 2014, 58, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- Laugerette, F.; Vors, C.; Peretti, N.; Michalski, M.C. Complex links between dietary lipids, endogenous LPSs and metabolic inflammation. Biochimie 2011, 93, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Vors, C.; Drai, J.; Pineau, G.; Laville, M.; Vidal, H.; Laugerette, F.; Michalski, M.C. Emulsifying dietary fat modulates postprandial endotoxemia associated with chylomicronemia in obese men: A pilot randomized crossover study. Lipids Health Dis. 2017, 16, 97. [Google Scholar] [CrossRef]
- White, A.J.; Wijeyekoon, R.S.; Scott, K.M.; Gunawardana, N.P.; Hayat, S.; Solim, I.H.; McMahon, H.T.; Barker, R.A.; Williams-Gray, C.H. The peripheral inflammatory response to alpha-synuclein and endotoxin in Parkinson’s disease. Front. Neurol. 2018, 9, 946. [Google Scholar] [CrossRef] [PubMed]
- Erridge, C. Accumulation of stimulants of Toll-like receptor (TLR)-2 and TLR4 in meat products stored at 5 C. J. Food Sci. 2011, 76, H72–H79. [Google Scholar] [CrossRef]
- Brown, B.I. Nutritional Management of Metabolic Endotoxemia: A Clinical Review. Altern. Ther. Health Med. 2017, 23, 42–54. [Google Scholar]
- Faraj, T.A. Regulation of Cardiometabolic Risk Factors by Dietary Toll-Like Receptor Stimulants. Ph.D. Thesis, University of Leicester, Leicester, UK, 2017. [Google Scholar]
- Blake, S.; Harding, T.; Baroni, L.; Harding, M.; Blake, C.; Piboolnurak, P.; Grant, W. Reducing dietary lipopolysaccharides to slow progression of cognitive impairment and Alzheimer’s disease. J. Brain Sci. 2024, 7, 10–18488. [Google Scholar] [CrossRef]
- Kellow, N.J.; Coughlan, M.T.; Reid, C.M. Association between habitual dietary and lifestyle behaviours and skin autofluorescence (SAF), a marker of tissue accumulation of advanced glycation endproducts (AGEs), in healthy adults. Eur. J. Nutr. 2018, 57, 2209–2216. [Google Scholar] [CrossRef]
- Blake, S.; King, G.; Kerr, N.; Blake, C.; Harding, T.; Borman, P.; Moss, K.; Adapon, P.; Liow, K.K. Hawaii Dementia Prevention Trial: A Randomized Trial Evaluating a Multifaceted Nutritional Intervention to Slow Cognitive Decline in Mild Cognitive Impairment Patients. J. Brain Sci. 2018, 2, 1–12. [Google Scholar] [CrossRef]
- Li, X.H.; Du, L.L.; Cheng, X.S.; Jiang, X.; Zhang, Y.; Lv, B.L.; Liu, R.; Wang, J.Z.; Zhou, X.W. Glycation exacerbates the neuronal toxicity of beta-amyloid. Cell Death Dis. 2013, 4, e673. [Google Scholar] [CrossRef]
- Xanthis, A.; Hatzitolios, A.; Koliakos, G.; Tatola, V. Advanced glycosylation end products and nutrition—A possible relation with diabetic atherosclerosis and how to prevent it. J. Food Sci. 2007, 72, R125–R129. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mooldijk, S.S.; Licher, S.; Waqas, K.; Ikram, M.K.; Uitterlinden, A.G.; Zillikens, M.C.; Ikram, M.A. Assessment of advanced glycation end products and receptors and the risk of dementia. JAMA Netw. Open 2021, 4, e2033012. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ansari, V.A.; Mahmood, T.; Ahsan, F.; Wasim, R.; Shariq, M.; Parveen, S.; Maheshwari, S. Receptor for advanced glycation end products: Dementia and cognitive impairment. Drug Res. 2023, 73, 247–250. [Google Scholar] [CrossRef]
- Van Puyvelde, K.; Mets, T.; Njemini, R.; Beyer, I.; Bautmans, I. Effect of advanced glycation end product intake on inflammation and aging: A systematic review. Nutr. Rev. 2014, 72, 638–650. [Google Scholar] [CrossRef]
- Li, J.; Liu, D.; Sun, L.; Lu, Y.; Zhang, Z. Advanced glycation end products and neurodegenerative diseases: Mechanisms and perspective. J. Neurol. Sci. 2012, 317, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nedi, O.; Rattan, S.I.; Grune, T.; Trougakos, I.P. Molecular effects of advanced glycation end products on cell signalling pathways, ageing and pathophysiology. Free. Radic. Res. 2013, 47 (Suppl. 1), 28–38. [Google Scholar] [CrossRef] [PubMed]
- Perrone, L.; Grant, W.B. Observational and ecological studies of dietary advanced glycation end products in national diets and Alzheimer’s disease incidence and prevalence. J. Alzheimer’s Dis. 2015, 45, 965–979. [Google Scholar] [CrossRef]
- Vlassara, H.; Cai, W.; Crandall, J.; Goldberg, T.; Oberstein, R.; Dardaine, V.; Peppa, M.; Rayfield, E.J. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc. Natl. Acad. Sci. USA 2002, 99, 15596–15601. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Uribarri, J.; Zhu, L.; Chen, X.; Swamy, S.; Zhao, Z.; Grosjean, F.; Simonaro, C.; Kuchel, G.A.; Schnaider-Beeri, M.; et al. Oral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humans. Proc. Natl. Acad. Sci. USA 2014, 111, 4940–4945. [Google Scholar] [CrossRef]
- Uribarri, J.; del Castillo, M.D.; de la Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macías-Cervantes, M.H.; Bastos, D.H.; Medrano, A.; Menini, T.; et al. Dietary advanced glycation end products and their role in health and disease. Adv. Nutr. 2015, 6, 461–473. [Google Scholar] [CrossRef]
- Vitek, M.P.; Bhattacharya, K.; Glendening, J.M.; Stopa, E.; Vlassara, H.; Bucala, R.; Manogue, K.; Cerami, A. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1994, 91, 47. [Google Scholar] [CrossRef]
- Angeloni, C.; Zambonin, L.; Hrelia, S. Role of methylglyoxal in Alzheimer’s disease. BioMed Res. Int. 2014, 2014, 238485. [Google Scholar] [CrossRef]
- Sharma, A.; Weber, D.; Raupbach, J.; Dakal, T.C.; Fließbach, K.; Ramirez, A.; Grune, T.; Wüllner, U. Advanced glycation end products and protein carbonyl levels in plasma reveal sex-specific differences in Parkinson’s and Alzheimer’s disease. Redox Biol. 2020, 34, 101546. [Google Scholar] [CrossRef]
- Ahmed, N.; Ahmed, U.; Thornalley, P.J.; Hager, K.; Fleischer, G.; Münch, G. Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer’s disease and link to cognitive impairment. J. Neurochem. 2005, 92, 255–263. [Google Scholar] [CrossRef]
- Prasad, C.; Davis, K.E.; Imrhan, V.; Juma, S.; Vijayagopal, P. Advanced glycation end products and risks for chronic diseases: Intervening through lifestyle modification. Am. J. Lifestyle Med. 2019, 13, 384–404. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.U.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 2010, 110, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Beisswenger, B.G.; Delucia, E.M.; Lapoint, N.; Sanford, R.J.; Beisswenger, P.J. Ketosis leads to increased methylglyoxal production on the Atkins diet. Ann. N. Y. Acad. Sci. 2005, 1043, 201–210. [Google Scholar] [CrossRef]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef]
- Palimeri, S.; Palioura, E.; Diamanti-Kandarakis, E. Current perspectives on the health risks associated with the consumption of advanced glycation end products: Recommendations for dietary management. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 415–426. [Google Scholar]
- Abate, G.; Marziano, M.; Rungratanawanich, W.; Memo, M.; Uberti, D. Nutrition and AGE-ing: Focusing on Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2017, 2017, 7039816. [Google Scholar] [CrossRef] [PubMed]
- Tabara, Y.; Yamanaka, M.; Setoh, K.; Segawa, H.; Kawaguchi, T.; Kosugi, S.; Nakayama, T.; Matsuda, F.; Nagahama Study Group. Advanced glycation end product accumulation is associated with lower cognitive performance in an older general population: The Nagahama study. J. Alzheimer’s Dis. 2020, 74, 741–746. [Google Scholar] [CrossRef]
- Igase, M.; Ohara, M.; Igase, K.; Kato, T.; Okada, Y.; Ochi, M.; Tabara, Y.; Kohara, K.; Ohyagi, Y. Skin autofluorescence examination as a diagnostic tool for mild cognitive impairment in healthy people. J. Alzheimer’s Dis. 2017, 55, 1481–1487. [Google Scholar] [CrossRef]
- Chou, P.S.; Wu, M.N.; Yang, C.C.; Shen, C.T.; Yang, Y.H. Effect of advanced glycation end products on the progression of Alzheimer’s disease. J. Alzheimer’s Dis. 2019, 72, 191–197. [Google Scholar] [CrossRef]
- Mooldijk, S.S.; Lu, T.; Waqas, K.; Chen, J.; Vernooij, M.W.; Ikram, M.K.; Zillikens, M.C.; Ikram, M.A. Skin advanced glycation end products and the risk of dementia. Alzheimer’s Dement. 2022, 18, e061469. [Google Scholar] [CrossRef]
- Thomas, M.H.; Olivier, J.L. Arachidonic acid in Alzheimer’s disease. J. Neurol. Neuromed. 2016, 1, 1–6. [Google Scholar]
- Thomas, M.H.; Pelleieux, S.; Vitale, N.; Olivier, J.L. Dietary arachidonic acid as a risk factor for age-associated neurodegenerative diseases: Potential mechanisms. Biochimie 2016, 130, 168–177. [Google Scholar] [CrossRef]
- Fujimi, K.; Noda, K.; Sasaki, K.; Wakisaka, Y.; Tanizaki, Y.; Iida, M.; Kiyohara, Y.; Kanba, S.; Iwaki, T. Altered expression of COX-2 in subdivisions of the hippocampus during aging and in Alzheimer’s disease the Hisayama Study. Dement. Geriatr. Cogn. Disord. 2007, 23, 423–431. [Google Scholar] [CrossRef]
- Stephenson, D.T.; Lemere, C.A.; Selkoe, D.J.; Clemens, J.A. Cytosolic phospholipase A2 (cPLA2) immunoreactivity is elevated in Alzheimer’s disease brain. Neurobiol. Dis. 1996, 3, 51–63. [Google Scholar] [CrossRef]
- Bao, Y.; Shen, Y.; Wu, Z.; Tao, S.; Yang, B.; Zhu, T.; Zhao, W.; Zhang, Y.; Zhao, X.; Jiao, L.; et al. High dietary arachidonic acid produces excess eicosanoids, and induces hepatic inflammatory responses, oxidative stress and apoptosis in juvenile Acanthopagrus schlegelii. Aquac. Rep. 2023, 29, 101506. [Google Scholar] [CrossRef]
- Michael, J.; Marschallinger, J.; Aigner, L. The leukotriene signaling pathway: A druggable target in Alzheimer’s disease. Drug Discov. Today 2019, 24, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Ikonomovic, M.D.; Abrahamson, E.E.; Uz, T.; Manev, H.; DeKosky, S.T. Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer’s disease. J. Histochem. Cytochem. 2008, 56, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.Y.; Ouk, M.; Wu, C.Y.; Rabin, J.S.; Lanctôt, K.L.; Herrmann, N.; Black, S.E.; Edwards, J.D.; Swardfager, W. Leukotriene receptor antagonist use is associated with slower cognitive decline in Alzheimer’s disease. Alzheimer’s Dement. 2021, 17, e057523. [Google Scholar] [CrossRef]
- Thomas, M.; Pelleïeux, S.; Allouche, A.; Colin, J.; Oster, T.; Malaplate-Armand, C.; Olivier, J.L. Control of Intracellular Levels of Free Arachidonic Acid: A Target in Therapeutic or Preventive Strategies against Alzheimer’s desease? In Arachidonic Acid: Sources, Biosynthesis and Health Effects; Nova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 83–126. [Google Scholar]
- Rapoport, S.I.; Carson, R.E.; Bhattacharjee, A.; Schapiro, M.B.; Herscovitch, P.; Eckelman, W.; Esposito, G. Imaging neuroinflammation in Alzheimer disease with [1–11C] arachidonic acid and positron emission tomography. Alzheimer’s Dement. 2005, 1, S41. [Google Scholar] [CrossRef]
- Hammouda, S.; Ghzaiel, I.; Khamlaoui, W.; Hammami, S.; Mhenni, S.Y.; Samet, S.; Hammami, M.; Zarrouk, A. Genetic variants in FADS1 and ELOVL2 increase level of arachidonic acid and the risk of Alzheimer’s disease in the Tunisian population. Prostaglandins Leukot. Essent. Fat. Acids 2020, 160, 102159. [Google Scholar] [CrossRef]
- Abdullah, L.; Evans, J.E.; Emmerich, T.; Crynen, G.; Shackleton, B.; Keegan, A.P.; Luis, C.; Tai, L.; LaDu, M.J.; Mullan, M.; et al. APOE ε4 specific imbalance of arachidonic acid and docosahexaenoic acid in serum phospholipids identifies individuals with preclinical Mild Cognitive Impairment/Alzheimer’s Disease. Aging 2017, 9, 964. [Google Scholar] [CrossRef]
- Trares, K.; Gào, X.; Perna, L.; Rujescu, D.; Stocker, H.; Möllers, T.; Beyreuther, K.; Brenner, H.; Schöttker, B. Associations of urinary 8-iso-prostaglandin F2α levels with all-cause dementia, Alzheimer’s disease, and vascular dementia incidence: Results from a prospective cohort study. Alzheimer’s Dement. 2020, 16, 804–813. [Google Scholar] [CrossRef]
- Maccarrone, M.; Melino, G.; Finazzi-Agro, A. Lipoxygenases and their involvement in programmed cell death. Cell Death Differ 2001, 8, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, A.; Nelson, G.J.; Schmidt, P.C.; Kelley, D.S.; Bartolini, G.; Flanagan, V.P. Increased dietary arachidonic acid enhances the synthesis of vasoactive eicosanoids in humans. Lipids 1997, 32, 435–439. [Google Scholar] [CrossRef]
- Blake, S.; Piboolnurak, P.; Borman, P.; Harding, T.; Blake, C. Reducing Neuroinflammation in Parkinson’s Disease with Dietary Compounds. GSC Biol. Pharm. Sci. 2022, 18, 026–037. [Google Scholar] [CrossRef]
- Pinchaud, K.; Maguin-Gaté, K.; Olivier, J.L. Dietary arachidonic acid: A Janus face actor in brain and Alzheimer’s disease? OCL 2018, 25, D406. [Google Scholar] [CrossRef]
- Whelan, J.; Fritsche, K. Linoleic acid. Adv. Nutr. 2013, 4, 311–312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rett, B.S.; Whelan, J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: A systematic review. Nutr. Metab. 2011, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Taber, L.; Chiu, C.H.; Whelan, J. Assessment of the arachidonic acid content in foods commonly consumed in the American diet. Lipids 1998, 33, 1151–1157. [Google Scholar] [CrossRef]
- Hyvönen, L.; Lampi, A.M.; Varo, P.; Koivistoinen, P. Fatty acid analysis, TAG equivalents as net fat value, and nutritional attributes of commercial fats and oils. J. Food Compos. Anal. 1993, 6, 24–40. [Google Scholar] [CrossRef]
- Li, D.; Ng, A.; Mann, N.J.; Sinclair, A.J. Contribution of meat fat to dietary arachidonic acid. Lipids 1998, 33, 437–440. [Google Scholar] [CrossRef] [PubMed]




| Food | ng/g of LPS | Serving Size g | LPS per Serving in ng |
|---|---|---|---|
| Macaroni cheese | 6500 ng/g | 340 g | 2,200,000 ng |
| Minced turkey | 7800 ng/g | 230 g | 1,800,000 ng |
| Cheese and onion rolls | 17,000 ng/g | 74 g | 1,300,000 ng |
| Minced pork | 10,000 ng/g | 110 g | 1,100,000 ng |
| Minced beef | 7000 ng/g | 98 g | 690,000 ng |
| Pork sausage rolls | 4200 ng/g | 2 rolls 120 g | 520,000 ng |
| Turkey | 300 ng/g | 230 g | 510,000 ng |
| Hamburger patty | 3090 ng/g | 98 g | 300,000 ng |
| Infant formula milk powder | 2800 ng/g | 100 g | 280,000 ng |
| Lobster | 1200 ng/g | 145 g | 170,000 ng |
| Pork | 1100 ng/g | 110 g | 120,000 ng |
| Spaghetti bolognese | 220 ng/g | 400 g | 90,000 ng |
| Skim milk, one cup | 75 ng/mL | 240 g | 18,000 ng |
| Milk, one cup | 50 ng/g | 240 g | 12,000 ng |
| Effect on Risk of AD and Cognitive Impairment | Ref. |
|---|---|
| LPS | |
| Increased levels of LPS in the hippocampus of patients with AD. | [4] |
| High levels of plasma LPS can worsen inflammatory neurodegeneration in AD. Neuronal cell loss may be induced by LPS via TNFα and IL-1β. | [8] |
| Increased microglial expression of IL-1β and neuronal apoptosis in the brain with higher levels of LPS. | [12] |
| Higher LPS caused a significant doubling of IL-6 secretion and greatly increased TNFα in a human study. | [15] |
| LPS co-localizes with amyloid-β in amyloid plaques in AD brains. In a nested case–control design, LPS was significantly associated with a thirty percent higher risk of developing AD (12-year trial). | [17] |
| AD patients were found to have elevated LPS in their blood and brain. | [19] |
| Some patients with AD were found to have three times higher plasma LPS levels, when compared to controls. | [20] |
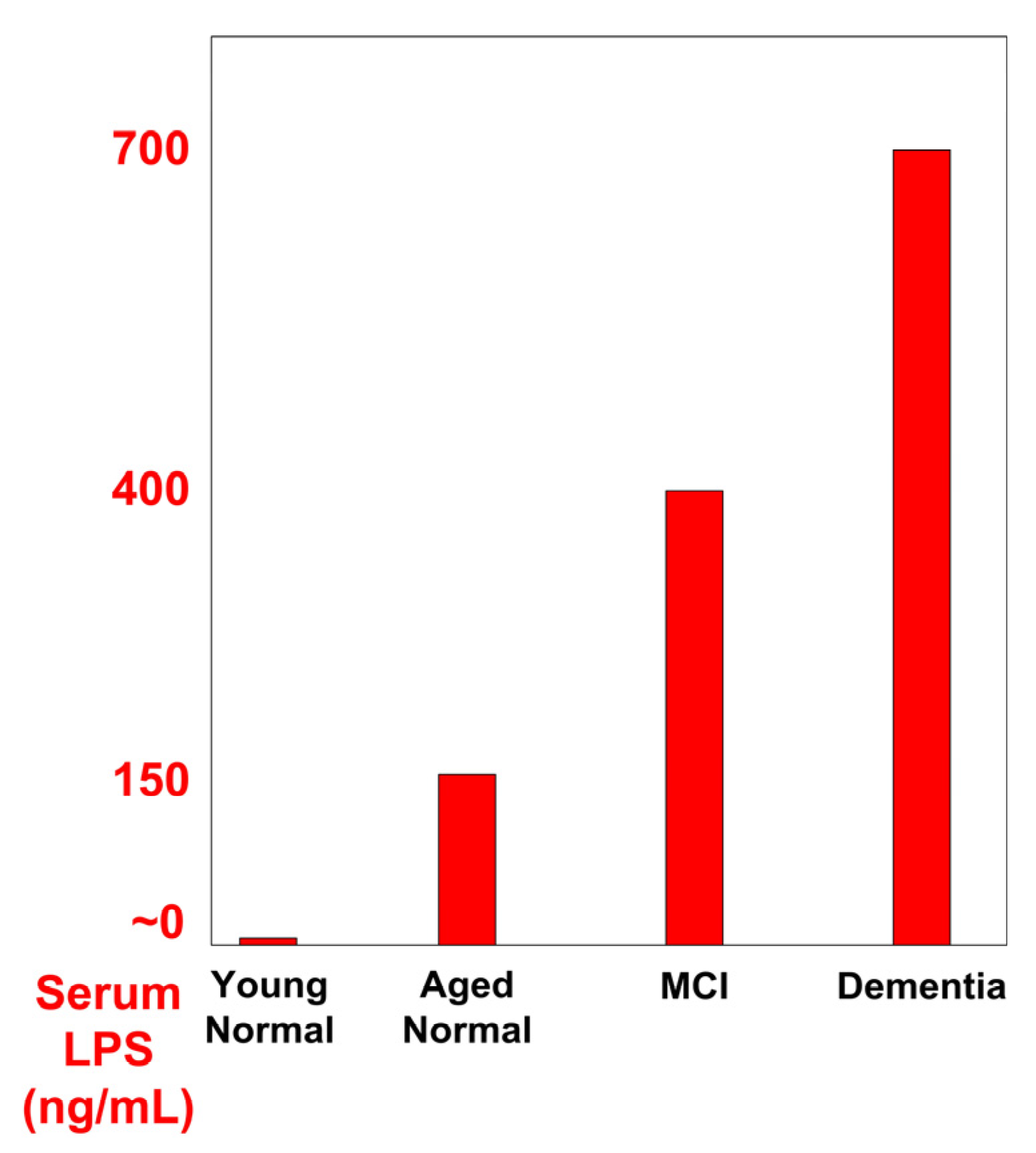
| Those with MCI had almost double the LPS serum levels and those with AD had 4× the LPS serum levels, compared to normal aging controls. | [21] |
| Higher LPS in plasma doubled the incidence of MCI in those without dementia, in a cross-sectional prospective cohort study, | [22] |
| Working memory/short-term verbal memory and the Digit Span Test were negatively impacted by higher levels of lipopolysaccharide binding protein. | [25] |
| LPS treatment of microglia produced 14 times the proinflammatory prostaglandin-E2 (PGE2) and increased IL-6 inflammation, compared with untreated microglia. | [28] |
| Milk fat triggered an early and sharp increase in LPS-laden chylomicrons at 60 min after ingestion in a randomized crossover trial. | [32] |
| High plasma LPS levels resulted in a strong cytokine response. | [33] |
| Higher intakes of fruit and legumes were associated with 34% and 20% less circulating LPS, respectively. | [36] |
| AGEs | |
| It was found that cell death from oxidation of cellular membranes due to AGEs may be one of the neuropathological mechanisms of AD. | [39] |
| When amyloid-β is crosslinked to AGEs, the amyloid-β is more toxic to neurons. | [40] |
| Higher levels of AGEs were associated with lower global cognitive function, while lower levels of AGEs were associated with better cognition. | [42] |
| Dietary AGEs appear to be important risk factors for AD. RAGE and AGE-amyloid-β can lead to reduced cerebral blood flow. | [47] |
| AGE content has been consistently reported higher in amyloid-β plaques in AD brains, compared to normal brains. | [49] |
| AD brains were found to contain about 3-fold more AGE adducts and 5- to 10-fold more amyloid plaques than healthy brains. | [51] |
| In elder subjects, those with less methylglyoxal, an AGE precursor, in serum exhibited a slower rate of cognitive decline. | [52] |
| AGE-modified amyloid-β was found to be more toxic to synaptic proteins than amyloid-β without AGEs. | [53] |
| AGEs were increased in subjects with Alzheimer’s disease. The decline in cognitive function in AD subjects has been correlated to the amount of protein glycation and AGEs. | [54] |
| In a large study of elderly Japanese, those with the lowest cognitive scores had the highest levels of AGE, while those with the highest cognitive scores had the lowest levels of AGEs. | [61] |
| Even half of the maximum levels of AGEs found in normal people resulted in a 640% greater risk of MCI, compared to the lowest AGE levels. | [62] |
| Patients with lower AGEs declined more slowly in regard to cognition. Higher AGE levels were closely associated with a worse score in the clinical dementia rating. | [63] |
| AA | |
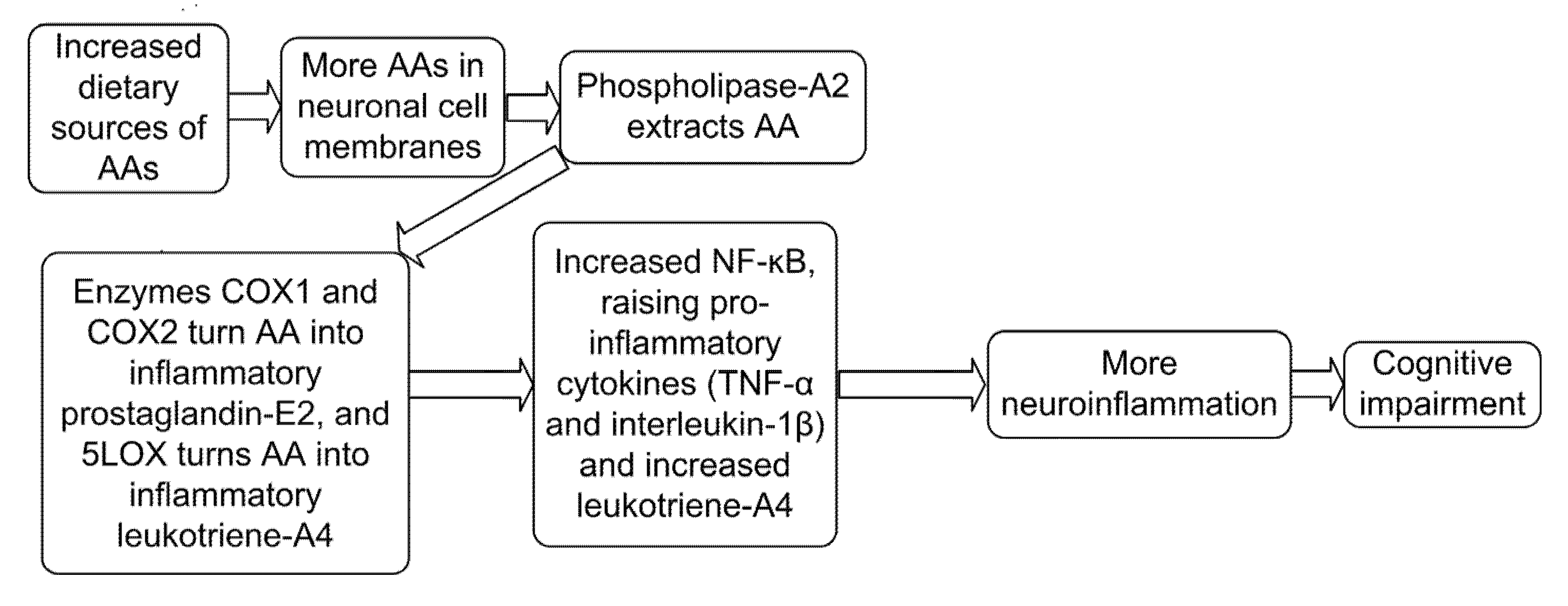
| AA is converted by 5LOX into the highly inflammatory leukotriene-A4, increasing amyloid-β production and tau phosphorylation in neuronal cells. | [65] |
| Over-expression of COX-2 (which makes the inflammatory PGE2) in the brain of AD patients has been correlated with the progression of AD. | [67] |
| Elevated levels of AA and phospholipid-A2 in AD brains increases inflammation of amyloid-β 1–42 peptide in the brain in AD. | [68] |
| 5LOX levels are high in amyloid-beta-containing plaques. AA processed by 5LOX into inflammatory leukotrienes is involved in both pathological inflammatory degeneration and loss of neurons and synapses in AD. | [71] |
| Decreasing dietary AA can 1) reduce neuroinflammation, 2) reduce amyloid-beta oligomer production, 3) reduce the amyloid-beta oligomer-induced apoptotic neuronal death. | [73] |
| AA is elevated in the AD brain, particularly in regions reported to have high densities of senile plaques and activated microglia. | [74] |
| An accumulation of AA (37% higher) was observed in both the periphery and in the brain in patients with AD. | [75] |
| Blood lipid analyses show that higher AA in cell membrane phospholipids is able to identify patients at higher risk of AD. | [76] |
| Those with higher prostaglandin-F2-alpha, an eicosanoid made from AA, had a 45% increased risk of all-cause dementia. | [77] |
| The high AA group had a 41% increase in thromboxane, which, when made from AA, is a powerful inducer of platelet aggregation and vasoconstriction that may contribute to thrombosis, vascular dementia, or stroke risk. | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blake, S.; Baroni, L.; Piboolnurak, P.; Harding, T.; Harding, M.; Blake, C. Reducing Neuroinflammation and Risk of Mild Cognitive Impairment and Alzheimer’s Disease by Reducing Dietary Lipopolysaccharides, Arachidonic Acid, and Advanced Glycation End Products. J. Dement. Alzheimer's Dis. 2025, 2, 27. https://doi.org/10.3390/jdad2030027
Blake S, Baroni L, Piboolnurak P, Harding T, Harding M, Blake C. Reducing Neuroinflammation and Risk of Mild Cognitive Impairment and Alzheimer’s Disease by Reducing Dietary Lipopolysaccharides, Arachidonic Acid, and Advanced Glycation End Products. Journal of Dementia and Alzheimer's Disease. 2025; 2(3):27. https://doi.org/10.3390/jdad2030027
Chicago/Turabian StyleBlake, Steven, Luciana Baroni, Panida Piboolnurak, Thomas Harding, Maile Harding, and Catherine Blake. 2025. "Reducing Neuroinflammation and Risk of Mild Cognitive Impairment and Alzheimer’s Disease by Reducing Dietary Lipopolysaccharides, Arachidonic Acid, and Advanced Glycation End Products" Journal of Dementia and Alzheimer's Disease 2, no. 3: 27. https://doi.org/10.3390/jdad2030027
APA StyleBlake, S., Baroni, L., Piboolnurak, P., Harding, T., Harding, M., & Blake, C. (2025). Reducing Neuroinflammation and Risk of Mild Cognitive Impairment and Alzheimer’s Disease by Reducing Dietary Lipopolysaccharides, Arachidonic Acid, and Advanced Glycation End Products. Journal of Dementia and Alzheimer's Disease, 2(3), 27. https://doi.org/10.3390/jdad2030027









