Gray Matter Volume Associations with Montreal Cognitive Assessment Domains in an ADNI Cohort of Early-Onset Mild Cognitive Impairment and Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Data
2.2. T1w-MPRAGE Pre-Processing
2.3. Voxel-Based Morphometry
2.4. Region of Interest (ROI)-Based Morphometry
2.5. Statistical Analyses
3. Results
3.1. Subject Characteristics
3.2. MoCA Total and Domain-Specific Scores
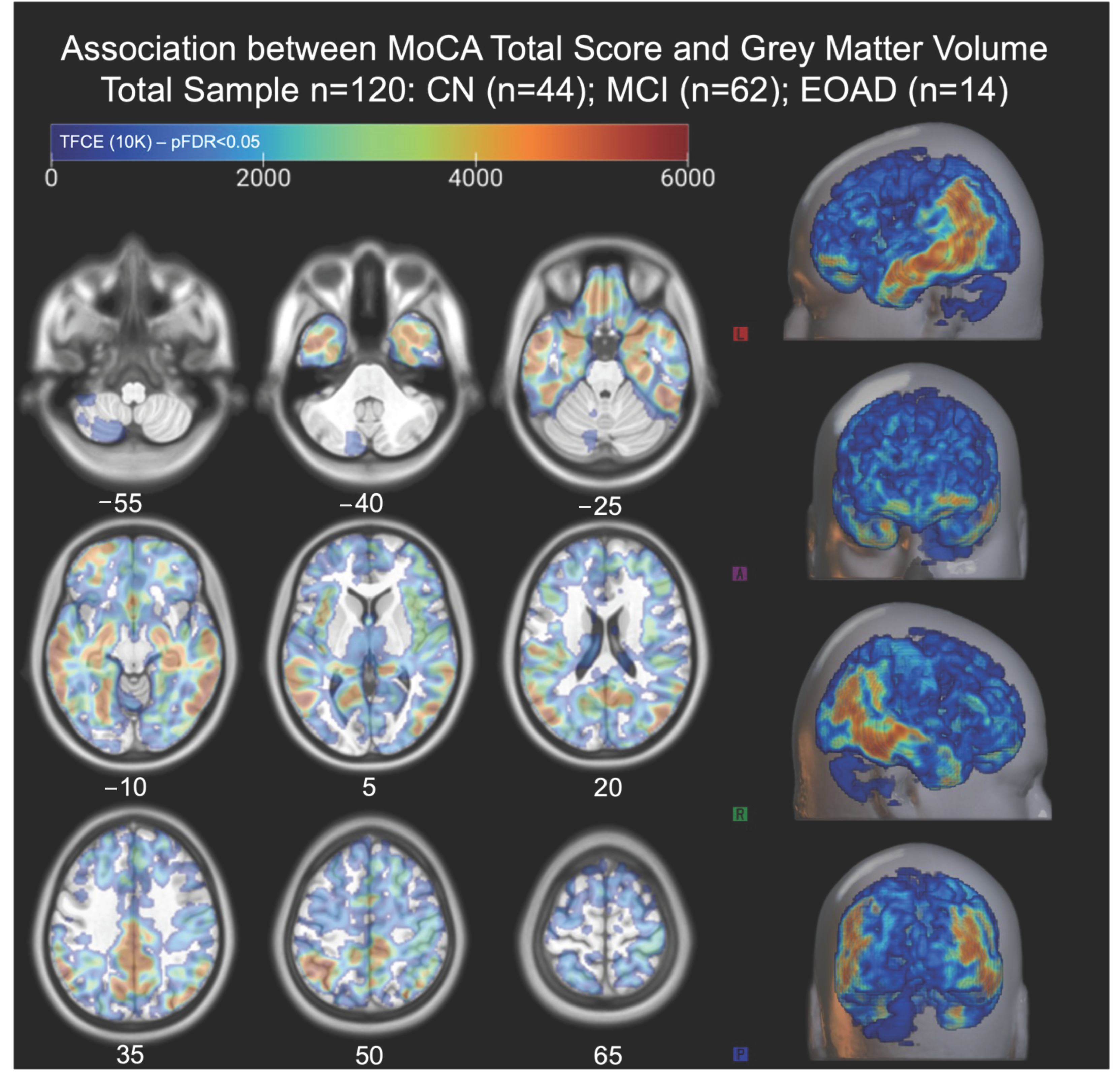
3.3. Global Associations Between MoCA Total Score and Gray Matter Volume
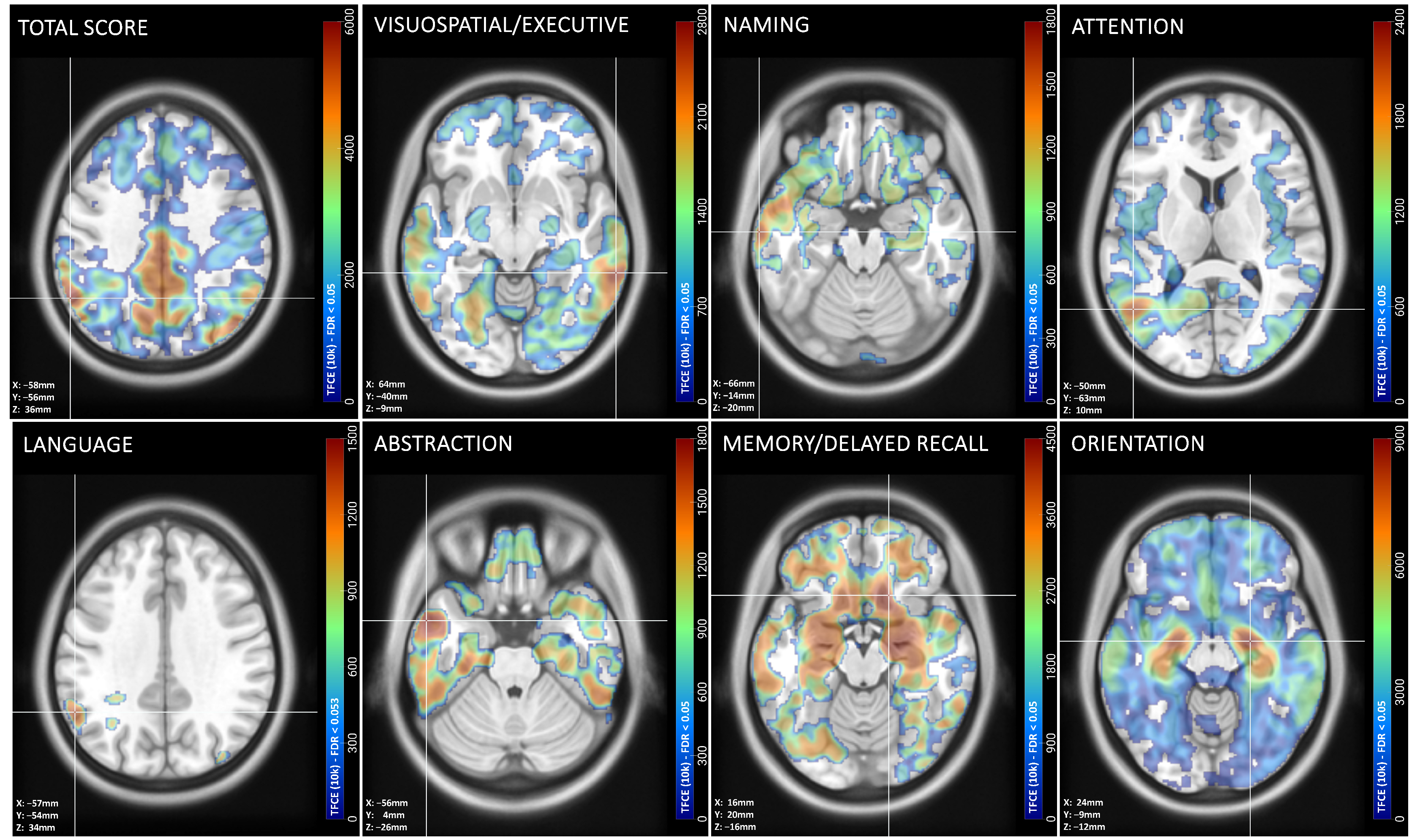
3.4. Peak Associations Between MoCA Domain Scores and Gray Matter Volume
3.5. Significant Spearman’s Correlations Between MoCA Cognitive Domain Scores and Regional Gray Matter Volume
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar]
- Wilson, R.S.; Leurgans, S.E.; Boyle, P.A.; Bennett, D.A. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch. Neurol. 2011, 68, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Gholami, A. Alzheimer’s disease: The role of proteins in formation, mechanisms, and new therapeutic approaches. Neurosci. Lett. 2023, 817, 137532. [Google Scholar]
- Bekris, L.M.; Yu, C.E.; Bird, T.D.; Tsuang, D.W. Genetics of Alzheimer disease. J. Geriatr. Psychiatry Neurol. 2010, 23, 213–227. [Google Scholar] [CrossRef]
- Tanzi, R.E. The genetics of Alzheimer disease. Cold Spring Harb. Perspect Med. 2012, 2, a006296. [Google Scholar] [CrossRef]
- Andrade-Guerrero, J.; Santiago-Balmaseda, A.; Jeronimo-Aguilar, P.; Vargas-Rodríguez, I.; Cadena-Suárez, A.R.; Sánchez-Garibay, C.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Cardenas-Aguayo, M.-D.; Diaz-Cintra, S.; et al. Alzheimer’s Disease: An Updated Overview of Its Genetics. Int. J. Mol. Sci. 2023, 24, 3754. [Google Scholar] [CrossRef] [PubMed]
- Morishima-Kawashima, M.; Ihara, Y. Alzheimer’s disease: β-Amyloid protein and tau. J. Neurosci. Res. 2002, 70, 392–401. [Google Scholar] [CrossRef]
- Ahmad, F.; Javed, M.; Athar, M.; Shahzadi, S. Determination of affected brain regions at various stages of Alzheimer’s disease. Neurosci. Res. 2023, 192, 77–82. [Google Scholar] [CrossRef]
- Mendez, M.F. Early-Onset Alzheimer Disease and Its Variants. Contin. Lifelong Learn. Neurol. 2019, 25, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Chishiki, Y.; Hirano, S.; Li, H.; Kojima, K.; Nakano, Y.; Sakurai, T.; Mukai, H.; Sugiyama, A.; Kuwabara, S. Different Patterns of Gray Matter Volume Reduction in Early-onset and Late-onset Alzheimer Disease. Cogn. Behav. Neurol. 2020, 33, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, G.B.; Pievani, M.; Testa, C.; Sabattoli, F.; Bresciani, L.; Bonetti, M.; Beltramello, A.; Hayashi, K.M.; Toga, A.W.; Thompson, P.M. The topography of grey matter involvement in early and late onset Alzheimer’s disease. Brain 2007, 130, 720–730. [Google Scholar] [CrossRef]
- Möller, C.; Vrenken, H.; Jiskoot, L.; Versteeg, A.; Barkhof, F.; Scheltens, P.; van der Flier, W.M. Different patterns of gray matter atrophy in early- and late-onset Alzheimer’s disease. Neurobiol. Aging 2013, 34, 2014–2022. [Google Scholar] [CrossRef] [PubMed]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and Future Treatments in Alzheimer Disease: An Update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef] [PubMed]
- Tombaugh, T.N.; McIntyre, N.J. The mini-mental state examination: A comprehensive review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Hammers, D.; Spurgeon, E.; Ryan, K.; Persad, C.; Barbas, N.; Heidebrink, J.; Darby, D.; Giordani, B. Validity of a Brief Computerized Cognitive Screening Test in Dementia. J. Geriatr. Psychiatry Neurol. 2012, 25, 89–99. [Google Scholar] [CrossRef]
- Maruff, P.; Lim, Y.Y.; Darby, D.; A Ellis, K.; Pietrzak, R.H.; Snyder, P.J.; I Bush, A.; Szoeke, C.; Schembri, A.; Ames, D.; et al. Clinical utility of the cogstate brief battery in identifying cognitive impairment in mild cognitive impairment and Alzheimer’s disease. BMC Psychol. 2013, 1, 30. [Google Scholar] [CrossRef]
- Sandberg, M.A. Cambridge Neuropsychological Testing Automated Battery. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011; pp. 480–482. [Google Scholar]
- Hoops, S.; Nazem, S.; Siderowf, A.D.; Duda, J.E.; Xie, S.X.; Stern, M.B.; Weintraub, D. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 2009, 73, 1738–1745. [Google Scholar] [CrossRef]
- Smith, T.; Gildeh, N.; Holmes, C. The Montreal Cognitive Assessment: Validity and Utility in a Memory Clinic Setting. Can. J. Psychiatry 2007, 52, 329–332. [Google Scholar] [CrossRef]
- Julayanont, P.; Phillips, N.A.; Chertkow, H.; Nasreddine, Z.S. Montreal Cognitive Assessment (MoCA): Concept and Clinical Review; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Islam, N.; Hashem, R.; Gad, M.; Brown, A.; Levis, B.; Renoux, C.; Thombs, B.D.; McInnes, M.D. Accuracy of the Montreal Cognitive Assessment tool for detecting mild cognitive impairment: A systematic review and meta-analysis. Alzheimer’s Dement. 2023, 19, 3235–3243. [Google Scholar] [CrossRef] [PubMed]
- Bobinski, M.; de Leon, M.; Wegiel, J.; DeSanti, S.; Convit, A.; Louis, L.S.; Rusinek, H.; Wisniewski, H. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer’s disease. Neuroscience 2000, 95, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Fox, N.C.; Sperling, R.A.; Klunk, W.E. Brain imaging in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006213. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Dickson, D.W.; Parisi, J.E.; Xu, Y.C.; Cha, R.H.; O’Brien, P.C.; Edland, S.D.; Smith, G.E.; Boeve, B.F.; Tangalos, E.G.; et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology 2002, 58, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—The methods. Neuroimage 2000, 11, 805–821. [Google Scholar] [CrossRef]
- Ramanoël, S.; Hoyau, E.; Kauffmann, L.; Renard, F.; Pichat, C.; Boudiaf, N.; Krainik, A.; Jaillard, A.; Baciu, M. Gray Matter Volume and Cognitive Performance During Normal Aging. A Voxel-Based Morphometry Study. Front. Aging Neurosci. 2018, 10, 235. [Google Scholar] [CrossRef]
- van de Mortel, L.A.; Thomas, R.M.; van Wingen, G.A. Grey Matter Loss at Different Stages of Cognitive Decline: A Role for the Thalamus in Developing Alzheimer’s Disease. J. Alzheimers Dis. 2021, 83, 705–720. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Yu, J.-T.; Liu, Y.; Yin, R.-H.; Wang, H.-F.; Wang, J.; Tan, L.; Radua, J.; Tan, L. Voxel-based meta-analysis of grey matter changes in Alzheimer’s disease. Transl. Neurodegener. 2015, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Khagi, B.; Lee, K.H.; Choi, K.Y.; Lee, J.J.; Kwon, G.-R.; Yang, H.-D. VBM-Based Alzheimer’s Disease Detection from the Region of Interest of T1 MRI with Supportive Gaussian Smoothing and a Bayesian Regularized Neural Network. Appl. Sci. 2021, 11, 6175. [Google Scholar] [CrossRef]
- Petersen, R.C.; Aisen, P.S.; Beckett, L.A.; Donohue, M.C.; Gamst, A.C.; Harvey, D.J.; Jack, C.R., Jr.; Jagust, W.J.; Shaw, L.M.; Toga, A.W.; et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology 2010, 74, 201–209. [Google Scholar] [CrossRef]
- Pearce, N.; Vandenbroucke, J.P.; Lawlor, D.A. Causal Inference in Environmental Epidemiology: Old and New Approaches. Epidemiology 2019, 30, 311–316. [Google Scholar] [CrossRef]
- Xiao, Y.; Gao, L.; Hu, Y.; Initiative, T.A.D.N. Disrupted single-subject gray matter networks are associated with cognitive decline and cortical atrophy in Alzheimer’s disease. Front. Neurosci. 2024, 18, 1366761. [Google Scholar]
- Lee, P.; Kim, H.-R.; Jeong, Y.; Initiative, F.T.A.D.N. Detection of gray matter microstructural changes in Alzheimer’s disease continuum using fiber orientation. BMC Neurol. 2020, 20, 362. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Abe, O.; Aoki, S.; Hayashi, N.; Ohtsu, H.; Takao, H.; Miyati, T.; Matsuda, H.; Yamashita, F.; Iwatsubo, T.; et al. Longitudinal gray-matter volume change in the default-mode network: Utility of volume standardized with global gray-matter volume for Alzheimer’s disease: A preliminary study. Radiol. Phys. Technol. 2015, 8, 64–72. [Google Scholar] [CrossRef]
- MATLAB, Version: (2023a); The Mathworks, Inc.: Natick, MA, USA, 1993.
- Gaser, C.; Dahnke, R.; Thompson, P.M.; Kurth, F.; Luders, E.; The Alzheimer’s Disease Neuroimaging Initiative. CAT—A Computational Anatomy Toolbox for the Analysis of Structural MRI Data. bioRxiv 2023. bioRxiv:2022.06.11.495736. [Google Scholar]
- Ashburner, J.; Friston, K.J. Unified segmentation. NeuroImage 2005, 26, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Huang, C.-C.; Lin, C.-P.; Feng, J.; Joliot, M. Automated anatomical labelling atlas 3. NeuroImage 2020, 206, 116189. [Google Scholar] [CrossRef]
- Han, H.; Dawson, K.J. JASP, Version 18.3. [Computer Software]. 2020.
- Cajanus, A.; Solje, E.; Koikkalainen, J.; Lötjönen, J.; Suhonen, N.-M.; Hallikainen, I.; Vanninen, R.; Hartikainen, P.; de Marco, M.; Venneri, A.; et al. The Association Between Distinct Frontal Brain Volumes and Behavioral Symptoms in Mild Cognitive Impairment, Alzheimer’s Disease, and Frontotemporal Dementia. Front. Neurol. 2019, 10, 1059. [Google Scholar] [CrossRef]
- Schroeter, M.L.; Stein, T.; Maslowski, N.; Neumann, J. Neural correlates of Alzheimer’s disease and mild cognitive impairment: A systematic and quantitative meta-analysis involving 1351 patients. Neuroimage 2009, 47, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.H.; Creavin, S.T.; Yip, J.L.; Noel-Storr, A.H.; Brayne, C.; Cullum, S. Montreal Cognitive Assessment for the diagnosis of Alzheimer’s disease and other dementias. Cochrane Database Syst Rev. 2015, 2015, Cd010775. [Google Scholar] [CrossRef]
- Razani, J.; Wong, J.T.; Dafaeeboini, N.; Edwards-Lee, T.; Lu, P.; Alessi, C.; Josephson, K. Predicting everyday functional abilities of dementia patients with the Mini-Mental State Examination. J. Geriatr. Psychiatry Neurol. 2009, 22, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Aminoff, E.M.; Kveraga, K.; Bar, M. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 2013, 17, 379–390. [Google Scholar]
- Hölscher, C. Time, space and hippocampal functions. Rev. Neurosci. 2003, 14, 253–284. [Google Scholar]
- Baier, B.; Cuvenhaus, H.S.; Müller, N.; Birklein, F.; Dieterich, M. The importance of the insular cortex for vestibular and spatial syndromes. Eur. J. Neurol. 2021, 28, 1774–1778. [Google Scholar] [CrossRef] [PubMed]
- Seydell-Greenwald, A.; Ferrara, K.; Chambers, C.E.; Newport, E.L.; Landau, B. Bilateral parietal activations for complex visual-spatial functions: Evidence from a visual-spatial construction task. Neuropsychologia 2017, 106, 194–206. [Google Scholar] [CrossRef]
- Possin, K.L. Visual spatial cognition in neurodegenerative disease. Neurocase 2010, 16, 466–487. [Google Scholar] [CrossRef]
- Takeuchi, H.; Taki, Y.; Sassa, Y.; Hashizume, H.; Sekiguchi, A.; Fukushima, A.; Kawashima, R. Brain structures associated with executive functions during everyday events in a non-clinical sample. Brain Struct. Funct. 2013, 218, 1017–1032. [Google Scholar] [PubMed]
- Son, G.; Jahanshahi, A.; Yoo, S.-J.; Boonstra, J.T.; Hopkins, D.A.; Steinbusch, H.W.M.; Moon, C. Olfactory neuropathology in Alzheimer’s disease: A sign of ongoing neurodegeneration. BMB Rep. 2021, 54, 295–304. [Google Scholar] [CrossRef]
- Scheff, S.W.; Price, D.A.; Ansari, M.A.; Roberts, K.N.; Schmitt, F.A.; Ikonomovic, M.D.; Mufson, E.J. Synaptic change in the posterior cingulate gyrus in the progression of Alzheimer’s disease. J. Alzheimers Dis. 2015, 43, 1073–1090. [Google Scholar] [CrossRef]
- Poulin, S.P.; Dautoff, R.; Morris, J.C.; Barrett, L.F.; Dickerson, B.C. Amygdala atrophy is prominent in early Alzheimer’s disease and relates to symptom severity. Psychiatry Res. 2011, 194, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Fouquet, M.; Desgranges, B.; La Joie, R.; Rivière, D.; Mangin, J.-F.; Landeau, B.; Mézenge, F.; Pélerin, A.; de La Sayette, V.; Viader, F.; et al. Role of hippocampal CA1 atrophy in memory encoding deficits in amnestic Mild Cognitive Impairment. Neuroimage 2012, 59, 3309–3315. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, X.; Chen, X.; Zhang, P.; Bao, M.; Zhang, D.; Chen, J.; He, S.; Hu, X. Age-dependent brain activation during forward and backward digit recall revealed by fMRI. Neuroimage 2005, 26, 36–47. [Google Scholar] [CrossRef]
- Hoshi, Y.; Oda, I.; Wada, Y.; Ito, Y.; Yamashita, Y.; Oda, M.; Ohta, K.; Yamada, Y.; Tamura, M. Visuospatial imagery is a fruitful strategy for the digit span backward task: A study with near-infrared optical tomography. Brain Res. Cogn. Brain Res. 2000, 9, 339–342. [Google Scholar] [CrossRef]
- Binder, J.R. The Wernicke area: Modern evidence and a reinterpretation. Neurology 2015, 85, 2170–2175. [Google Scholar] [CrossRef] [PubMed]
- Dell, G.S.; Schwartz, M.F.; Nozari, N.; Faseyitan, O.; Branch Coslett, H. Voxel-based lesion-parameter mapping: Identifying the neural correlates of a computational model of word production. Cognition 2013, 128, 380–396. [Google Scholar] [CrossRef]
- Şahin, M.H.; Akyüz, M.E.; Karadağ, M.K.; Yalçın, A. Supramarginal Gyrus and Angular Gyrus Subcortical Connections: A Microanatomical and Tractographic Study for Neurosurgeons. Brain Sci. 2023, 13, 430. [Google Scholar] [CrossRef]
- McDonough, I.M.; Bischof, G.N.; Kennedy, K.M.; Rodrigue, K.M.; Farrell, M.E.; Park, D.C. Discrepancies between fluid and crystallized ability in healthy adults: A behavioral marker of preclinical Alzheimer’s disease. Neurobiol. Aging 2016, 46, 68–75. [Google Scholar] [CrossRef]
- Binder, J.R.; Westbury, C.F.; McKiernan, K.A.; Possing, E.T.; Medler, D.A. Distinct brain systems for processing concrete and abstract concepts. J. Cogn. Neurosci. 2005, 17, 905–917. [Google Scholar] [CrossRef]
- Gleichgerrcht, E.; Fridriksson, J.; Bonilha, L. Neuroanatomical foundations of naming impairments across different neurologic conditions. Neurology 2015, 85, 284–292. [Google Scholar] [CrossRef]
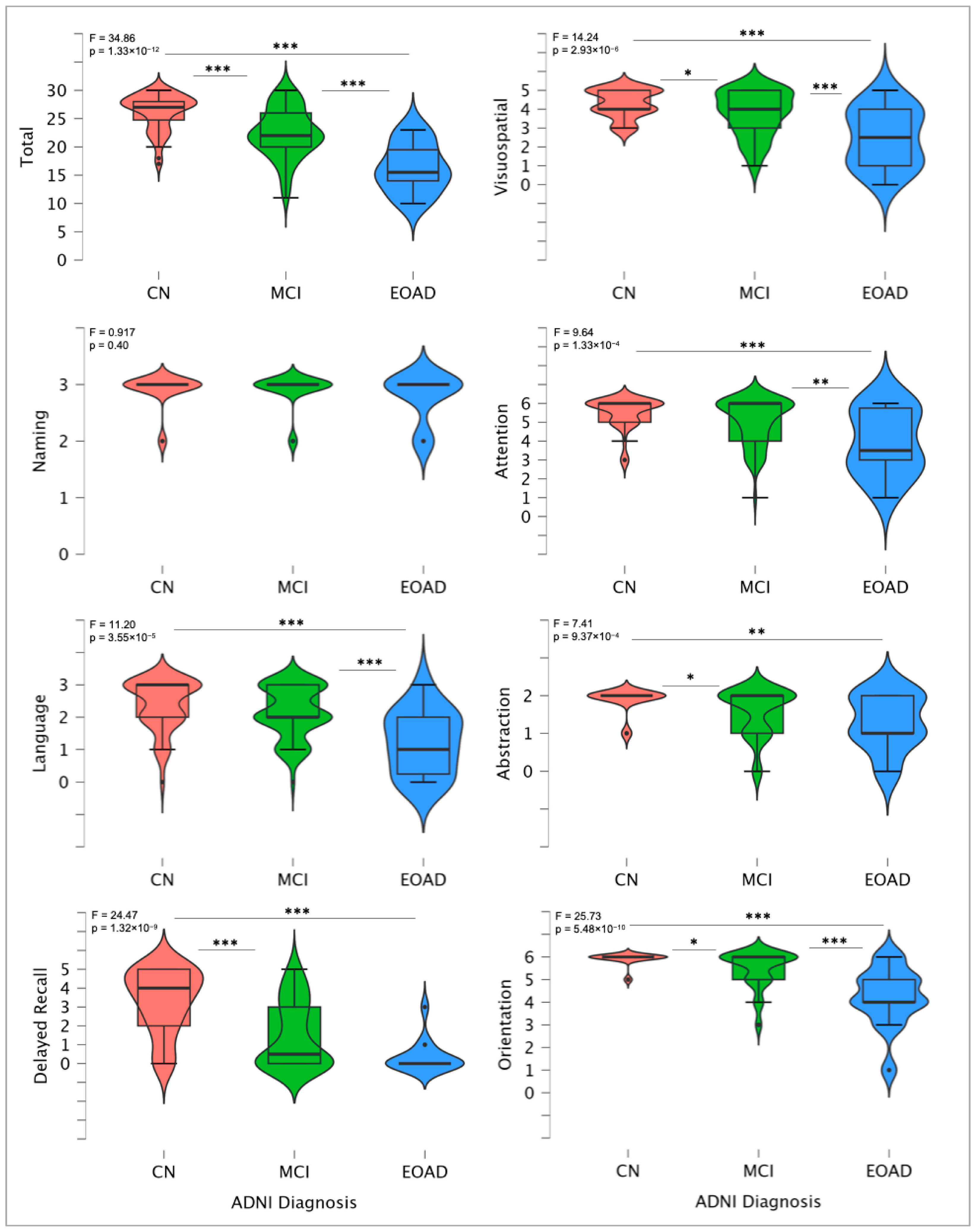
| Characteristics | ADNI Control (CN) [n = 44] | Mildly Cognitively Impaired (MCI) [n = 62] | Early-Onset Alzheimer’s Disease (EOAD) [n = 14] | Whole Sample [n = 120] | F/χ2 | p-Value |
|---|---|---|---|---|---|---|
| Age (Years) | 60.38 [3.66] | 61.45 [2.42] | 59.47 [3.09] | 60.98 [3.07] | 2.937 + | 0.062 |
| Education (School Years) | 15.80 [2.42] | 16.08 [2.34] | 14.86 [2.48] | 15.96 [2.39] | 0.807 | |
| Male | 25% | 31% | 71% | 33% | 10.719 ^ | 0.005 * |
| Race | 32.039 ^ | <0.001 * | ||||
| American Indian/Alaskan Native | 2% | 0% | 0% | 1% | ||
| Asian | 18% | 0% | 0% | 7% | ||
| Native Hawaiian/Pacific Islander | 0% | 0% | 0% | 0% | ||
| Black/African American | 32% | 11% | 7% | 18% | ||
| White | 41% | 84% | 86% | 68% | ||
| More than One Race | 5% | 2% | 7% | 3% | ||
| Unknown | 2% | 3% | 0% | 3% | ||
| Ethnicity | 8.188 ^ | 0.017 * | ||||
| Hispanic or Latino | 25% | 6% | 7% | 13% | ||
| Not Hispanic or Latino | 75% | 94% | 93% | 87% |
| Cognitive Domain | kE (%ICV) | p (FDR) | Cluster aal3-Atlas Associated Regions |
|---|---|---|---|
| Visuospatial/Executive | 193,852 (34.7%) | 0.002 | R middle temporal gyrus; R inferior temporal gyrus; R Crus I of cerebellar hemisphere; L precuneus; L cuneus; L superior parietal gyrus; L superior occipital gyrus |
| Naming | 42,769 (7.6%) | 0.048 | L middle temporal gyrus; L inferior temporal gyrus; L superior temporal gyrus; L insula |
| Attention | 127,919 (22.9%) | 0.016 | L middle temporal gyrus; L middle occipital gyrus; L&R middle cingulate; L&R middle paracingulate; L&R precuneus; L&R paracentral lobule; R supplementary motor cortex |
| Language | 128,743 (23.0%) | 0.053 | L supramarginal gyrus; L angular gyrus; L inferior parietal gyrus; L superior parietal gyrus; L postcentral gyrus |
| Abstraction | 107,176 (19.2%) | 0.026 | L superior temporal gyrus; L middle temporal gyrus; L inferior temporal gyrus |
| Memory/Delayed Recall | 124,686 (22.3%) | 0.007 | R gyrus rectus; R midial orbital gyrus; R posterior orbital gyrus; R putamen; L&R olfactory cortex; R nucleus accumbens; R caudate; nucleus; R insula; R anterior orbital gyrus; L&R superior frontal gyrus-medial orbital; L&R anterior cingulate cortex; R parahippocampal gyrus; R superior temporal gyrus; R amygdala |
| Orientation | 300,363 (53.8%) | 0.000 | L&R hippocampus; R pallidum; L&R putamen; L&R parahippocampal gyrus; L&R amygdala; L fusiform gyrus; L superior temporal gyrus; L olfactory; L insula; L posterior orbital gyrus; L medial orbital gyrus |
| Total Score | 325,370 (58.2%) | 0.000 | L supramarginal gyrus; L angular gyrus; L inferior parietal gyrus; R inferior temporal gyrus; R Crus I of cerebellar hemisphere; R fusiform gyrus; R inferior occipital gyrus |
| Intracranial Volume (ICV): 558,718 voxels | Degrees of freedom = [1.0, 115.0] | ||
| Voxel size: 1.5 1.5 1.5 [mm] | Permutations = 10,000 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kritikos, M.; Rama, T.; Zubair, V.; Huang, C.; Christodoulou, C.; Chen, A.P.F.; Kotov, R.; Mann, F.D.; on behalf of the Alzheimer’s Disease Neuroimaging Initiative. Gray Matter Volume Associations with Montreal Cognitive Assessment Domains in an ADNI Cohort of Early-Onset Mild Cognitive Impairment and Alzheimer’s Disease. J. Dement. Alzheimer's Dis. 2025, 2, 24. https://doi.org/10.3390/jdad2030024
Kritikos M, Rama T, Zubair V, Huang C, Christodoulou C, Chen APF, Kotov R, Mann FD, on behalf of the Alzheimer’s Disease Neuroimaging Initiative. Gray Matter Volume Associations with Montreal Cognitive Assessment Domains in an ADNI Cohort of Early-Onset Mild Cognitive Impairment and Alzheimer’s Disease. Journal of Dementia and Alzheimer's Disease. 2025; 2(3):24. https://doi.org/10.3390/jdad2030024
Chicago/Turabian StyleKritikos, Minos, Taulant Rama, Vania Zubair, Chuan Huang, Christopher Christodoulou, Allen P. F. Chen, Roman Kotov, Frank D. Mann, and on behalf of the Alzheimer’s Disease Neuroimaging Initiative. 2025. "Gray Matter Volume Associations with Montreal Cognitive Assessment Domains in an ADNI Cohort of Early-Onset Mild Cognitive Impairment and Alzheimer’s Disease" Journal of Dementia and Alzheimer's Disease 2, no. 3: 24. https://doi.org/10.3390/jdad2030024
APA StyleKritikos, M., Rama, T., Zubair, V., Huang, C., Christodoulou, C., Chen, A. P. F., Kotov, R., Mann, F. D., & on behalf of the Alzheimer’s Disease Neuroimaging Initiative. (2025). Gray Matter Volume Associations with Montreal Cognitive Assessment Domains in an ADNI Cohort of Early-Onset Mild Cognitive Impairment and Alzheimer’s Disease. Journal of Dementia and Alzheimer's Disease, 2(3), 24. https://doi.org/10.3390/jdad2030024







