Protective Effects of Selected Botanical Agents on Bone
Abstract
1. Introduction
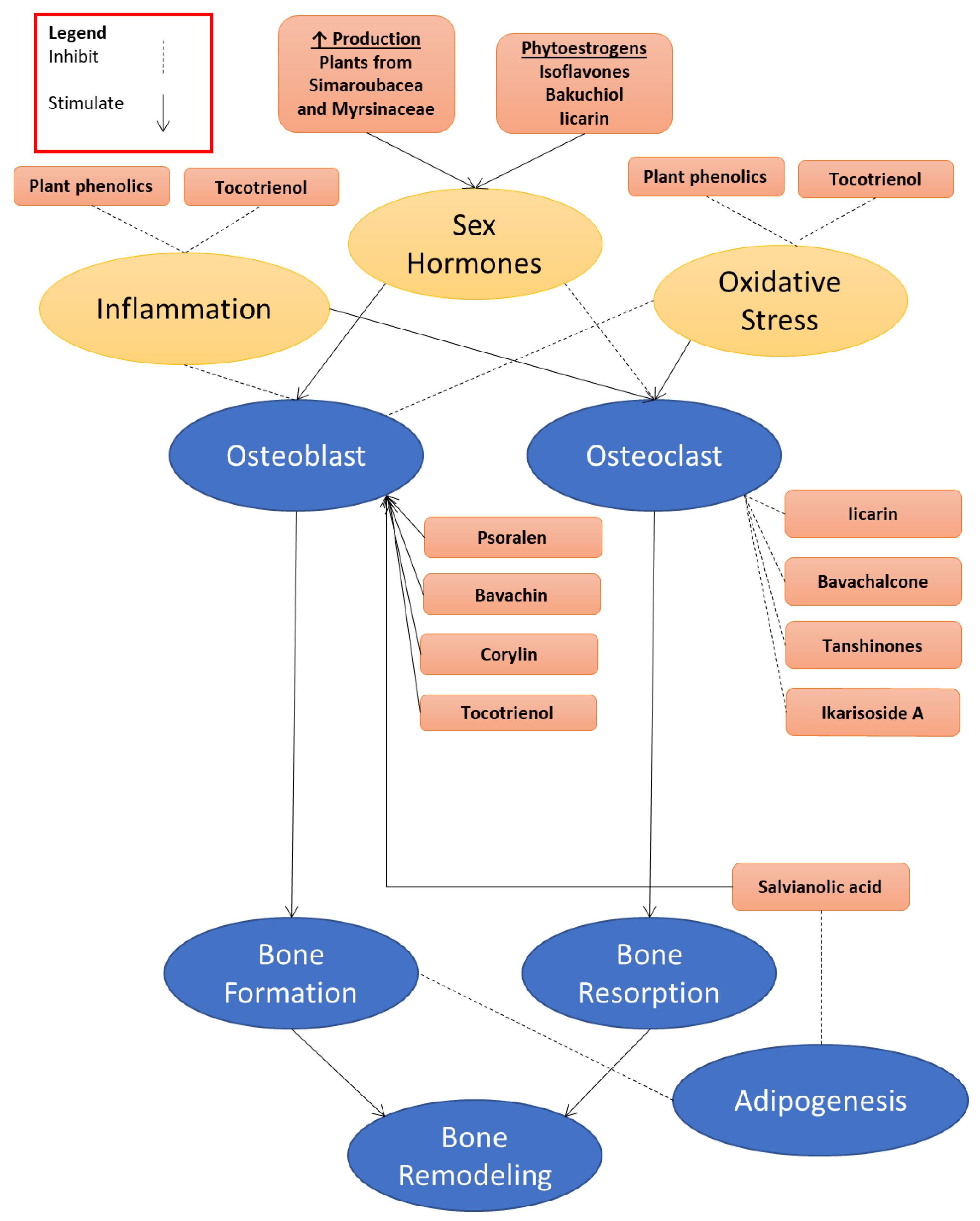
2. Antiosteoporotic Constituents Extracted from Natural Plants
2.1. The Berberidaceae Family
2.2. The Fabaceae Family
2.3. The Arecaceae Family
2.4. The Labiatae Family
2.5. The Simaroubaceae Family
2.6. The Myrsinaceae Family
3. Perspectives
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | Alkaline phosphatase |
| BMP-2/4 | Bone morphogenetic protein-2/4 |
| M-CSF | Macrophage colony stimulating factor |
| OPG | Osteoprotegerin |
| RANKL | Receptor activator of nuclear factor-κB ligand |
| Cbfα1 | Core binding factorα1 |
| SMAD4 | Signaling effectors mothers against decapentaplegic protein 4 |
| Wnt-signaling | Wingless-type signaling |
| cyclinD | Cyclin dependent |
| OVX | Ovariectomized |
| ER | Estrogen receptor |
| TRAP | Tartrate-resistant acid phosphatase |
| LPS | Lipopolysaccharides |
| IL-6/1 | Interleukin-6/1 |
| TNF-α | Tumor necrosis factor |
| COX-2 | Cyclooxygenasetype-2 |
| HIF-1α | Hypoxia inducible factor-1α |
| p38 | Protein 38 |
| JNK | Jun N-terminal kinase |
| ERK1/2 | Extracellular regulated-kinases 1/2 |
| Iκ-BαLPS | ikappa-Balpha lipopolysaccharide |
| MMP-9 | Matrix metalloproteinase-9 |
| Akt | Protein Kinase B |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| RANK | receptor activator of NF-κB |
| COL I | collagen type I |
| NFATc1 | nuclear factor of activated T cells c1 |
| c-Fos | Chromosome-Fos |
| B-lymphopoiesis | Bone marrow-lymphopoiesis |
| ERα/ERβ | Estrogen receptor alpha/beta |
| BMD | Bone mineral density |
| Osx | Osteoblast-specific transcription factor osterix |
| MDA | malondialdehyde |
| NO | nitric oxide |
| mRNA | Messenger ribonucleic acid |
| Dickkopf-1 | DKK-1 |
| Runx2 | Runt-related transcription factor 2 |
| PPAR-γ | Peroxisome proliferator-activated receptor gamma |
| β-catenin | Beta-cateni |
| MSC | Mesenchymal stem cell |
| EL | Eurycoma longifolia |
| ERT | estrogen replacement therapy |
References
- Lama, A.; Santoro, A.; Corrado, B.; Pirozzi, C.; Paciello, O.; Pagano, T.B.; Russo, S.; Calignano, A.; Mattace Raso, G.; Meli, R. Extracorporeal shock waves alone or combined with raloxifene promote bone formation and suppress resorption in ovariectomized rats. PLoS ONE 2017, 12, e0171276. [Google Scholar] [CrossRef] [PubMed]
- Sucuoglu, H.; Koyuncu, H. Distribution of male osteoporosis patients according to age, classification, and fracture. Istanb. Med. J. 2017, 18, 13–17. [Google Scholar] [CrossRef]
- Poole, K.E.S.; Skingle, L.; Gee, A.H.; Turmezei, T.D.; Johannesdottir, F.; Blesic, K.; Rose, C.; Vindlacheruvu, M.; Donell, S.; Vaculik, J.; et al. Focal osteoporosis defects play a key role in hip fracture. Bone 2017, 94, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Iseme, R.A.; Mcevoy, M.; Kelly, B.; Agnew, L.; Walker, F.R.; Attia, J. Is osteoporosis an autoimmune mediated disorder? Bone Rep. 2017, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Christiansen, C.; Grauer, A.; Kutilek, S.; Libanati, C.; McClung, M.R.; Reid, I.R.; Resch, H.; Siris, E.; Uebelhart, D. Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. J. Bone Miner. Res. 2011, 26, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P.; Delmas, P. Bone loss in elderly men: Increased endosteal bone loss and stable periosteal apposition. The prospective minos study. Osteoporos. Int. 2007, 18, 495–503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Misiorowski, W. Osteoporosis in men. Prz. Menopauzalny 2017, 16, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Marconi, E.; Dionello, C.F.; Morel, D.S.; Sá-Caputo, D.C.; Souza-Gonçalves, C.R.; Paineiras-Domingos, L.L.; Guedes-Aguiar, E.O.; Marin, P.J.; del Pozo Cruz, B.; Bernardo-Filho, M. Could whole body vibration exercises influence the risk factors for fractures in women with osteoporosis? Osteoporos. Sarcopenia 2016, 2, 214–220. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef]
- Orcel, P.; Funck-Brentano, T. Medical management following an osteoporotic fracture. Orthop. Traumatol. Surg. Res. 2011, 97, 860–869. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khosla, S.; Hofbauer, L.C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017, 5, 898–907. [Google Scholar] [CrossRef]
- Wu, L.; Ling, Z.; Feng, X.; Mao, C.; Xu, Z. Herb medicines against osteoporosis: Active compounds & relevant biological mechanisms. Curr. Top. Med. Chem. 2017, 17, 1670–1691. [Google Scholar] [PubMed]
- Augustine, M.; Horwitz, M.J. Parathyroid hormone and parathyroid hormone-related protein analogs as therapies for osteoporosis. Curr. Osteoporos. Rep. 2013, 11, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.Y.; Pelousse, F.; Bruyere, O. Safety concerns with the long-term management of osteoporosis. Expert Opin. Drug Saf. 2013, 12, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Hough, F.S.; Brown, S.L.; Cassim, B.; Davey, M.R.; de Lange, W.; de Villiers, T.J.; Ellis, G.C.; Lipschitz, S.; Lukhele, M.; Pettifor, J.M. The safety of osteoporosis medication. S. Afr. Med. J. 2014, 104, 279–282. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deal, C. Potential new drug targets for osteoporosis. Nat. Rev. Rheumatol. 2009, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T.; Christopoulos, A. Signalling bias in new drug discovery: Detection, quantification and therapeutic impact. Nat. Rev. Drug Discov. 2013, 12, 205. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.-M.; Youssef, A.R. Antiosteoporotic activity of salvadora persica sticks extract in an estrogen deficient model of osteoporosis. Osteoporos. Sarcopenia 2017, 3, 132–137. [Google Scholar] [CrossRef]
- Zhang, N.-D.; Han, T.; Huang, B.-K.; Rahman, K.; Jiang, Y.-P.; Xu, H.-T.; Qin, L.-P.; Xin, H.-L.; Zhang, Q.-Y.; Li, Y.-M. Traditional chinese medicine formulas for the treatment of osteoporosis: Implication for antiosteoporotic drug discovery. J. Ethnopharmacol. 2016, 189, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Xu, W.; Xu, Y.L.; Chen, X.; Huang, M.; Lu, J.J. Therapeutic potential of rhizoma alismatis: A review on ethnomedicinal application, phytochemistry, pharmacology, and toxicology. Ann. N. Y. Acad. Sci. 2017, 1401, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, Y.J.; Han, T.; Zhao, L.; Lv, L.; He, Y.Q.; Zhang, Q.Y.; Xin, H.L. Metabolites of curculigoside in rats and their antiosteoporotic activities in osteoblastic mc3t3-e1 cells. Fitoterapia 2017, 117, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Babaria, P.; Nakarani, M.; Shah, K.; Paranjape, A. Antiosteoporotic effect of hemidesmus indicus linn. On ovariectomised rats. J. Ethnopharmacol. 2017, 199, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Chillara, R.; Kushwaha, P.; Khedgikar, V.; Karvande, A.; Choudhary, D.; Adhikary, S.; Maurya, R.; Trivedi, R. Evaluation of anti-osteoporotic activity of butanolic fraction from passiflora foetida in ovariectomy-induced bone loss in mice. Biomed. Pharmacother. 2017, 88, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Tasadduq, R.; Gordon, J.; Al-Ghanim, K.A.; Lian, J.B.; Van Wijnen, A.J.; Stein, J.L.; Stein, G.S.; Shakoori, A.R. Ethanol extract of cissus quadrangularis enhances osteoblast differentiation and mineralization of murine pre-osteoblastic mc3t3-e1 cells. J. Cell. Physiol. 2017, 232, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Karvande, A.; Khedgikar, V.; Kushwaha, P.; Ahmad, N.; Kothari, P.; Verma, A.; Kumar, P.; Nagar, G.K.; Mishra, P.R.; Maurya, R. Heartwood extract from dalbergia sissoo promotes fracture healing and its application in ovariectomy-induced osteoporotic rats. J. Pharm. Pharmacol. 2017, 69, 1381–1397. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Nie, Y.; Cao, D.-P.; Xue, Y.-Y.; Wang, J.-S.; Zhao, L.; Rahman, K.; Zhang, Q.-Y.; Qin, L.-P. Potential antiosteoporotic agents from plants: A comprehensive review. Evid.-Based Complement. Altern. Med. 2012, 2012, 364604. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Li, J.H.; Yang, Y.F.; Zhu, J.Y. Floral development of gymnospermium microrrhynchum (berberidaceae) and its systematic significance in the nandinoideae. Flora 2017, 228, 10–16. [Google Scholar] [CrossRef]
- Sheng, M.; Chen, Q.; Wang, L.; Tian, X. Hybridization among epimedium (berberidaceae) species native to china. Sci. Hortic. 2011, 128, 342–351. [Google Scholar] [CrossRef]
- Indran, I.R.; Liang, R.L.Z.; Min, T.E.; Yong, E.-L. Preclinical studies and clinical evaluation of compounds from the genus epimedium for osteoporosis and bone health. Pharmacol. Ther. 2016, 162, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.P.; Sheu, S.Y.; Sun, J.S.; Chen, M.H.; Liu, M.H. Icariin isolated from epimedium pubescens regulates osteoblasts anabolism through bmp-2, smad4, and cbfa1 expression. Phytomedicine 2010, 17, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Tantry, M.A.; Dar, J.A.; Idris, A.; Akbar, S.; Shawl, A.S. Acylated flavonol glycosides from epimedium elatum, a plant endemic to the western himalayas. Fitoterapia 2012, 83, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, S.; Okamoto, Y.; Miyazaki, K.; Uesugi, T. Evaluation of a soybean product fujiflavone p40 as an antiosteoporotic agent in rats. Phytother. Res. 2003, 17, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.F.; Saga, I.; Ichimura, K.; Nagai, T.; Shinoda, M.; Matsuzaki, S. Coumestrol as well as isoflavones in soybean extract prevent bone resorption in ovariectomized rats. Endocr. Regul. 2003, 37, 145–152. [Google Scholar] [PubMed]
- Chin, K.Y.; Ima-Nirwana, S. Can soy prevent male osteoporosis? A review of the current evidence. Curr. Drug Targets 2013, 14, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.N.T.; Thybo, C.B.; Lykkeboe, S.; Rasmussen, L.M.; Frette, X.; Christensen, L.P.; Jeppesen, P.B. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Ricci, E.; Cipriani, S.; Chiaffarino, F.; Malvezzi, M.; Parazzini, F. Soy isoflavones and bone mineral density in perimenopausal and postmenopausal western women: A systematic review and meta-analysis of randomized controlled trials. J. Womens Health (Larchmt) 2010, 19, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Taku, K.; Melby, M.K.; Kurzer, M.S.; Mizuno, S.; Watanabe, S.; Ishimi, Y. Effects of soy isoflavone supplements on bone turnover markers in menopausal women: Systematic review and meta-analysis of randomized controlled trials. Bone 2010, 47, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Arcoraci, V.; Atteritano, M.; Squadrito, F.; D’Anna, R.; Marini, H.; Santoro, D.; Minutoli, L.; Messina, S.; Altavilla, D.; Bitto, A. Antiosteoporotic activity of genistein aglycone in postmenopausal women: Evidence from a post-hoc analysis of a multicenter randomized controlled trial. Nutrients 2017, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Sun, J.; Yu, B.; Wang, Y.; Sun, W.J.; Yang, J.; Huang, S.H.; Xie, W.L. Daidzein stimulates osteogenesis facilitating proliferation, differentiation, and antiapoptosis in human osteoblast-like mg-63 cells via estrogen receptor–dependent mek/erk and pi3k/akt activation. Nutr. Res. 2017, 42, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Li, Y.; Wang, Y.; Cui, J.; Feng, K.; Kong, X.; Chen, L. Psoralidin, a prenylated coumestan, as a novel anti-osteoporosis candidate to enhance bone formation of osteoblasts and decrease bone resorption of osteoclasts. Eur. J. Pharmacol. 2017, 801, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Chopra, B.; Dhingra, A.K.; Dhar, K.L. Psoralea corylifolia l. (buguchi)—Folklore to modern evidence: Review. Fitoterapia 2013, 90, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.-B.; Gao, Q.-Q.; Wang, F.; Zhao, G.-H.; Yin, F.-Z.; Cai, B.-C.; Chen, Z.-P.; Li, W.-D. Positive skeletal effect of two ingredients of Psoralea corylifolia L. On estrogen deficiency-induced osteoporosis and the possible mechanisms of action. Mol. Cell. Endocrinol. 2015, 417, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.-Z.; Yang, F.; Yang, Z.; Huang, J.; Shi, Q.; Chen, D.; Wang, Y.-J. Psoralen stimulates osteoblast differentiation through activation of bmp signaling. Biochem. Biophys. Res. Commun. 2011, 405, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, Q.; Huang, X.; Wang, Y.; Ge, C.; Qi, Y.; Guo, W.; Sun, H. Psoralen stimulates osteoblast proliferation through the activation of nuclear factor-κb-mitogen-activated protein kinase signaling. Exp. Ther. Med. 2017, 14, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Rink, C.; Khanna, S.; Palm o Chen, R.; Zhang, Y.; Dong, L.; Huang, J.; Hua, S.; Fu, X. Current Persperctive in the Discovery of Anti-aging Agents from Natural Products. Int. J. Curr. Adv. Res. 2017, 27, 335–404. [Google Scholar]
- Sen, C.K.; Rink, C.; Khanna, S. Palm oil–derived natural vitamin e α-tocotrienol in brain health and disease. J. Am. Coll. Nutr. 2010, 29, 314S–323S. [Google Scholar] [CrossRef] [PubMed]
- Sundram, K.; Sambanthamurthi, R.; Tan, Y.-A. Palm fruit chemistry and nutrition. Asia Pac. J. Clin. Nutr. 2003, 12, 355–362. [Google Scholar] [PubMed]
- Reeves, J., III; Weihrauch, J.L. Composition of Foods. Fats and Oils, Raw-Processed-Prepared; Chigago, IL, USA. 1979. Available online: https://www.cabdirect.org/cabdirect/abstract/19811420761 (accessed on 10 May 2018).
- Mustapa, A.; Manan, Z.; Azizi, C.M.; Setianto, W.; Omar, A.M. Extraction of β-carotenes from palm oil mesocarp using sub-critical r134a. Food Chem. 2011, 125, 262–267. [Google Scholar] [CrossRef]
- Poku, K. Small-Scale Palm Oil Processing in Africa; Food & Agriculture Org.: Roma, Italy, 2002; Volume 148. [Google Scholar]
- Peh, H.Y.; Tan, W.D.; Liao, W.; Wong, W.F. Vitamin e therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Fang, X.; Marshall, M.R.; Chung, S. Regulation of obesity and metabolic complications by gamma and delta tocotrienols. Molecules 2016, 21, 344. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Klein, A.; Chin, K.Y.; Mo, H.; Tsai, P.; Yang, R.S.; Chyu, M.C.; Ima-Nirwana, S. Tocotrienols for bone health: A translational approach. Ann. N. Y. Acad. Sci. 2017, 1401, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.H.; Choo, Y.M.; Ma, A.N.; Chuah, C.H.; Hashim, M.A. Separation of vitamin e (tocopherol, tocotrienol, and tocomonoenol) in palm oil. Lipids 2004, 39, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Frega, N.; Mozzon, M.; Bocci, F. Identification and estimation of tocotrienols in the annatto lipid fraction by gas chromatography-mass spectrometry. J. Am. Oil Chem. Soc. 1998, 75, 1723–1727. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Ima-Nirwana, S. The biological effects of tocotrienol on bone: A review on evidence from rodent models. Drug Des. Dev. Ther. 2015, 9, 2049. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Ahmad, F.; Ima-Nirwana, S. The effects of palm tocotrienol on metabolic syndrome and bone loss in male rats induced by high-carbohydrate high-fat diet. J. Funct. Foods 2018, 44, 246–254. [Google Scholar] [CrossRef]
- Ginaldi, L.; Di Benedetto, M.C.; De Martinis, M. Osteoporosis, inflammation and ageing. Immun. Ageing 2005, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [PubMed]
- Fatokun, A.A.; Stone, T.W.; Smith, R.A. Responses of differentiated mc3t3-e1 osteoblast-like cells to reactive oxygen species. Eur. J. Pharmacol. 2008, 587, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Kwak, H.B.; Lee, S.W.; Jin, H.M.; Kim, H.-M.; Kim, H.-H.; Lee, Z.H. Reactive oxygen species mediate rank signaling in osteoclasts. Exp. Cell Res. 2004, 301, 119–127. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.R. Proinflammatory cytokines and osteoporosis. Curr. Osteoporos. Rep. 2009, 7, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Hermizi, H.; Faizah, O.; Ima-Nirwana, S.; Nazrun, S.A.; Norazlina, M. Beneficial effects of tocotrienol and tocopherol on bone histomorphometric parameters in sprague–dawley male rats after nicotine cessation. Calcif. Tissue Int. 2009, 84, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Aktifanus, A.T.; Shuid, A.N.; Rashid, N.H.A.; Ling, T.H.; Loong, C.Y.; Saat, N.M.; Muhammad, N.; Mohamed, N.; Soelaiman, I.N. Comparison of the effects of tocotrienol and estrogen on the bone markers and dynamic changes in postmenopausal osteoporosis rat model. Asian J. Anim. Vet. Adv. 2012, 7, 225–234. [Google Scholar]
- Soelaiman, I.N.; Ming, W.; Abu Bakar, R.; Hashnan, N.A.; Mohd Ali, H.; Mohamed, N.; Muhammad, N.; Shuid, A.N. Palm tocotrienol supplementation enhanced bone formation in oestrogen-deficient rats. Int. J. Endocrinol. 2012, 2012, 532862. [Google Scholar] [CrossRef] [PubMed]
- Norazlina, M.; Ima-Nirwana, S.; Gapor, M.; Khalid, B. Palm vitamin e is comparable to α-tocopherol in maintaining bone mineral density in ovariectomised female rats. Exp. Clin. Endocrinol. Diabetes 2000, 108, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Ima-Nirwana, S.; Kiftiah, A.; Zainal, A.; Norazlina, M.; Gapor, M.; Khalid, B. Palm vitamin e prevents osteoporosis in orchidectomized growing male rats. Nat. Prod. Sci. 2000, 6, 155–160. [Google Scholar]
- Ima, S.N.; Fakhrurazi, H. Palm vitamin eprotects bone against dexamethasone-induced osteoporosis in male rats. Med. J. Malaysia 2002, 57, 136–144. [Google Scholar]
- Chin, K.-Y.; Gengatharan, D.; Mohd Nasru, F.S.; Khairussam, R.A.; Ern, S.L.H.; Aminuddin, S.A.W.; Ima-Nirwana, S. The effects of annatto tocotrienol on bone biomechanical strength and bone calcium content in an animal model of osteoporosis due to testosterone deficiency. Nutrients 2016, 8, 808. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.-V.; Ima-Nirwana, S.; Chin, K.-Y. Effect of tocotrienol from bixa orellana (annatto) on bone microstructure, calcium content, and biomechanical strength in a model of male osteoporosis induced by buserelin. Drug Des. Dev. Ther. 2018, 12, 555. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. Effects of tocotrienol from bixa orellana (annatto) on bone histomorphometry in a male osteoporosis model induced by buserelin. Biomed. Pharmacother. 2018, 103, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ima-Nirwana, S. Effects of annatto-derived tocotrienol supplementation on osteoporosis induced by testosterone deficiency in rats. Clin. Interv. Aging 2014, 9, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ima-Nirwana, S. The effects of alpha-tocopherol on bone: A double-edged sword? Nutrients 2014, 6, 1424–1441. [Google Scholar] [CrossRef] [PubMed]
- Guralp, O. Effects of vitamin e on bone remodeling in perimenopausal women: Mini review. Maturitas 2014, 79, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Yang, S.; Tomison, M.D.; Romero, A.W.; Felton, C.K.; Mo, H. Tocotrienol supplementation suppressed bone resorption and oxidative stress in postmenopausal osteopenic women: A 12-week randomized double-blinded placebo-controlled trial. Osteoporos. Int. 2018, 29, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, X.; Wang, J.; Yu, H.; Wang, X.; Yang, H.; Xu, H.; Tang, S.; Li, Y.; Yang, L. A system-level investigation into the mechanisms of chinese traditional medicine: Compound danshen formula for cardiovascular disease treatment. PLoS ONE 2012, 7, e43918. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Y.; Wang, Y.-P. Pharmacological actions and therapeutic applications of salvia miltiorrhiza depside salt and its active components. Acta Pharmacol. Sin. 2012, 33, 1119. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Xue, L.; Severino, R.P.; Gao, S.; Niu, J.; Qin, L.-P.; Zhang, D.; Brömme, D. Salvia miltiorrhiza: An ancient chinese herbal medicine as a source for anti-osteoporotic drugs. J. Ethnopharmacol. 2014, 155, 1401–1416. [Google Scholar] [CrossRef] [PubMed]
- Baricevic, D.; Bartol, T.V. Pharmacology 11. The biological/pharmacological activity of the salvia genus. In The Genus Salvia; Kintzios, S.E., Ed.; Harwood Academic Publishers: Amsterdam, The Netherland, 2000; pp. 143–184. [Google Scholar]
- Kim, H.-K.; Woo, E.-R.; Lee, H.-W.; Park, H.-R.; Kim, H.-N.; Jung, Y.-K.; Choi, J.-Y.; Chae, S.-W.; Kim, H.-R.; Chae, H.-J. The correlation of salvia miltiorrhiza extract–induced regulation of osteoclastogenesis with the amount of components tanshinone i, tanshinone iia, cryptotanshinone, and dihydrotanshinone. Immunopharmacol. Immunotoxicol. 2008, 30, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Choi, D.-Y.; Woo, E.-R. Inhibition of osteoclast differentiation by tanshinones from the root ofsalvia miltiorrhiza bunge. Arch. Pharm. Res. 2005, 28, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.B.; Yang, D.; Ha, H.; Lee, J.-H.; Kim, H.-N.; Woo, E.-R.; Lee, S.; Kim, H.-H.; Lee, Z.H. Tanshinone iia inhibits osteoclast differentiation through down-regulation of c-fos and nfatc1. Exp. Mol. Med. 2006, 38, 256. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Liu, Y.-Y.; Wu, T.; Ai, C.-M.; Chen, H.-Q. Osteogenic effects of d (+) β-3, 4-dihydroxyphenyl lactic acid (salvianic acid a, saa) on osteoblasts and bone marrow stromal cells of intact and prednisone-treated rats. Acta Pharmacol. Sin. 2009, 30, 321. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, T.; Liu, Y.; Zhou, L.; Li, P.; Xu, B.; Huang, L.; Chen, Y.; Liu, Y.; Tian, X. Salvianolic acid b prevents bone loss in prednisone-treated rats through stimulation of osteogenesis and bone marrow angiogenesis. PLoS ONE 2012, 7, e34647. [Google Scholar] [CrossRef] [PubMed]
- Thu, H.E.; Mohamed, I.N.; Hussain, Z.; Jayusman, P.A.; Shuid, A.N. Eurycoma longifolia as a potential adoptogen of male sexual health: A systematic review on clinical studies. Chin. J. Nat. Med. 2017, 15, 71–80. [Google Scholar] [CrossRef]
- Edwards, S.E.; da Costa Rocha, I.; Williamson, E.M.; Heinrich, M. Tongkat ali eurycoma longifolia jack. In Phytopharmacy: An Evidence-Based Guide to Herbal Medicinal Products; John Wiley & Sons: Hoboken, NJ, USA, 2015; p. 375. [Google Scholar]
- Faisal, G.G.; Zakaria, S.M.; Najmuldeen, G.F.; Al-Ani, I.M. Antifungal activity of eurycoma longifolia jack (tongkat ali) root extract. J. Int. Dent. Med. Res. 2016, 9, 70–74. [Google Scholar]
- Thu, H.E.; Mohamed, I.N.; Hussain, Z.; Shuid, A.N. Eurycoma longifolia as a potential alternative to testosterone for the treatment of osteoporosis: Exploring time-mannered proliferative, differentiative and morphogenic modulation in osteoblasts. J. Ethnopharmacol. 2017, 195, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Low, B.-S.; Choi, S.-B.; Abdul Wahab, H.; Kumar Das, P.; Chan, K.-L. Eurycomanone, the major quassinoid in eurycoma longifolia root extract increases spermatogenesis by inhibiting the activity of phosphodiesterase and aromatase in steroidogenesis. J. Ethnopharmacol. 2013, 149, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.-Y.; Ima-Nirwana, S. Sex steroids and bone health status in men. Int. J. Endocrinol. 2012, 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.-V.; Soelaiman, I.-N.; Chin, K.-Y. A concise review of testosterone and bone health. Clin. Interv. Aging 2016, 11, 1317. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.M.; Bendixen, A.C.; Pathrose, P.; Srivastava, S.; Dienger, K.M.; Shevde, N.K.; Pike, J.W. Androgens suppress osteoclast formation induced by rankl and macrophage-colony stimulating factor. Endocrinology 2001, 142, 3800–3808. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Ima-Nirwana, S. The effects of orchidectomy and supraphysiological testosterone administration on trabecular bone structure and gene expression in rats. Aging Male 2015, 18, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Sforza, A.; Maggi, M. Testosterone replacement therapy: Long-term safety and efficacy. World J. Men's Health 2017, 35, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Shuid, A.N.; Ping, L.L.; Muhammad, N.; Mohamed, N.; Soelaiman, I.N. The effects of Labisia pumila var. Alata on bone markers and bone calcium in a rat model of post-menopausal osteoporosis. J. Ethnopharmacol. 2011, 133, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Fathilah, S.N.; Nazrun Shuid, A.; Mohamed, N.; Muhammad, N.; Nirwana Soelaiman, I. Labisia pumila protects the bone of estrogen-deficient rat model: A histomorphometric study. J. Ethnopharmacol. 2012, 142, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Nadia, M.; Nazrun, A.; Norazlina, M.; Isa, N.; Norliza, M.; Ima Nirwana, S. The anti-inflammatory, phytoestrogenic, and antioxidative role of Labisia pumila in prevention of postmenopausal osteoporosis. Adv. Pharmacol. Sci. 2012, 2012, 706905. [Google Scholar] [PubMed]
- Mohd Effendy, N.; Abdullah, S.; Yunoh, M.F.; Shuid, A.N. Time and dose-dependent effects of Labisia pumila on the bone strength of postmenopausal osteoporosis rat model. BMC Complement. Altern. Med. 2015, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Fathilah, S.N.; Mohamed, N.; Muhammad, N.; Mohamed, I.N.; Soelaiman, I.N.; Shuid, A.N. Labisia pumila regulates bone-related genes expressions in postmenopausal osteoporosis model. BMC Complement. Altern. Med. 2013, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- Effendy, N.M.; Shuid, A.N. Time and dose-dependent effects of Labisia pumila on bone oxidative status of postmenopausal osteoporosis rat model. Nutrients 2014, 6, 3288–3302. [Google Scholar] [CrossRef] [PubMed]
| Family | Scientific Name | Compound | Pharmacological study |
|---|---|---|---|
| Berberidaceae | E. brevicornum Maxim E. sagittatum Maxim E. pubescens Maxim E. koreanum Nakai E. koreanum |
| |
| Iicarin |
| ||
| Ikarisoside A |
| ||
| Fabaceae | Glycine max L. Psoralea corylifolia L. |
| |
| Genistein |
| ||
| Bavachalcone |
| ||
| Psoralidin, Isobavachin |
| ||
| Bavachin Corylin |
| ||
| Bakuchiol |
| ||
| Psoralen |
| ||
| Arecaceae | Elaeis guineensis | Tocotrienol |
|
| Labiatae | Salvia miltiorrhiza Bunge | In ovariectomized rats:
| |
| Tanshinones |
| ||
| Tanshinones IIA |
| ||
| Salvianolic acid A |
| ||
| Salvianolic acid B |
| ||
| Simaroubaceaea | Eurycoma longifolia |
| |
| Eurycomalactone Eurycomanol |
| ||
| Eurycomanone |
| ||
| Myrsinaceae | Labisia pumila |
| |
| Ascorbic acid Anthocyanin Beta-carotene, Flavonoids phenolic compounds |
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jolly, J.J.; Chin, K.-Y.; Alias, E.; Chua, K.H.; Soelaiman, I.N. Protective Effects of Selected Botanical Agents on Bone. Int. J. Environ. Res. Public Health 2018, 15, 963. https://doi.org/10.3390/ijerph15050963
Jolly JJ, Chin K-Y, Alias E, Chua KH, Soelaiman IN. Protective Effects of Selected Botanical Agents on Bone. International Journal of Environmental Research and Public Health. 2018; 15(5):963. https://doi.org/10.3390/ijerph15050963
Chicago/Turabian StyleJolly, James Jam, Kok-Yong Chin, Ekram Alias, Kien Hui Chua, and Ima Nirwana Soelaiman. 2018. "Protective Effects of Selected Botanical Agents on Bone" International Journal of Environmental Research and Public Health 15, no. 5: 963. https://doi.org/10.3390/ijerph15050963
APA StyleJolly, J. J., Chin, K.-Y., Alias, E., Chua, K. H., & Soelaiman, I. N. (2018). Protective Effects of Selected Botanical Agents on Bone. International Journal of Environmental Research and Public Health, 15(5), 963. https://doi.org/10.3390/ijerph15050963






