Sludge Biochar Amendment and Alfalfa Revegetation Improve Soil Physicochemical Properties and Increase Diversity of Soil Microbes in Soils from a Rare Earth Element Mining Wasteland
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Soil, Preparation of Sludge Biochar, and Revegetation Plant
2.2. Trial Set-Up and Sampling
2.3. Analysis of Soil Physicochemical Properties
2.4. Analysis of Soil Microbiota
2.4.1. Extraction of Soil DNA
2.4.2. Gene Amplification by Polymerase Chain Reaction (PCR) and Illumina Sequencing
2.4.3. High-Throughput Sequencing Data Processing
2.5. Analysis of Plant Growth and Their Root Morphology
2.6. Statistical Analysis
3. Results and Discussion
3.1. Response of Soil Physicochemical Properties to Alfalfa Revegetation and Sludge Biochar Amendment
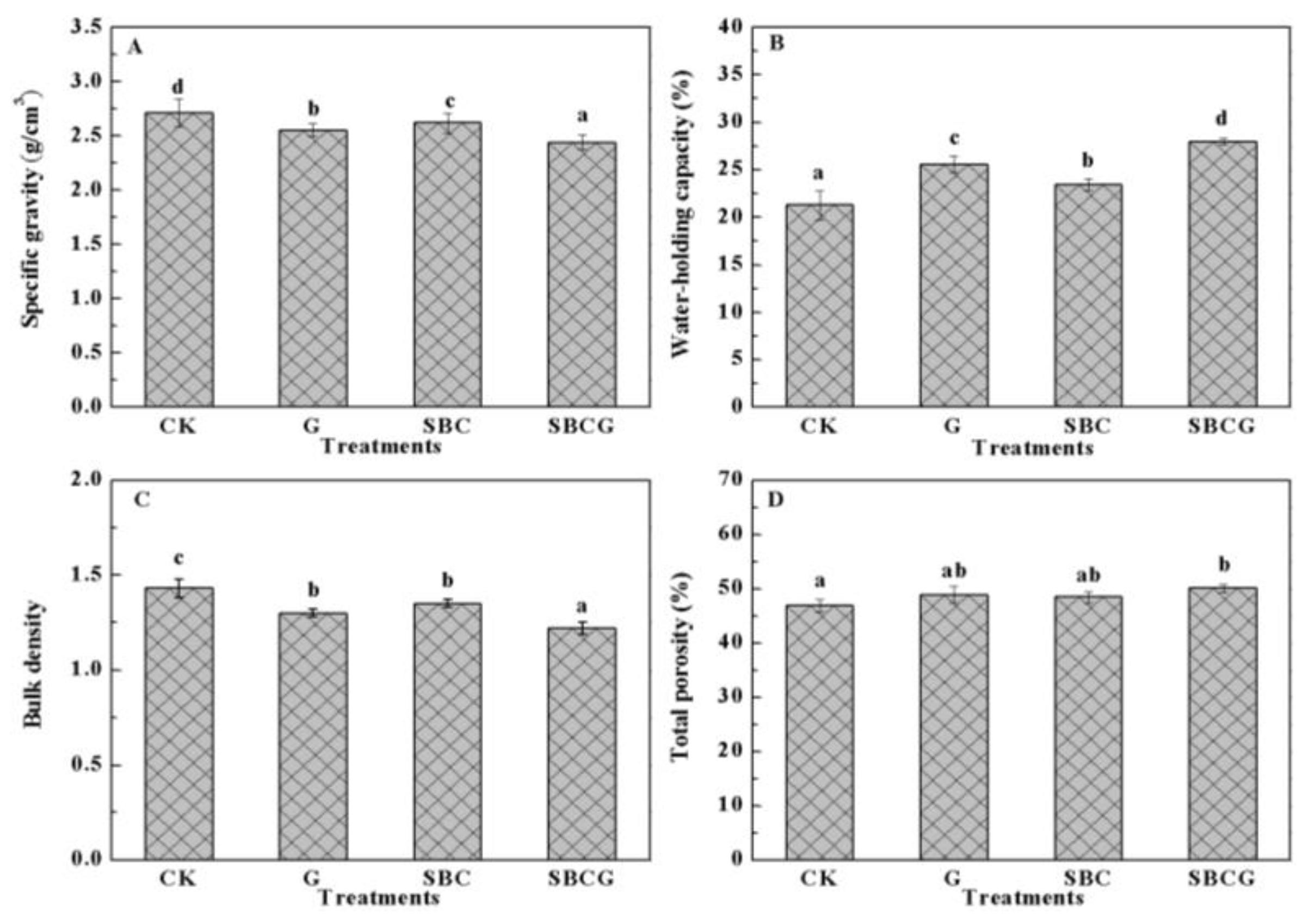
3.1.1. Effects on Soil Physical Properties
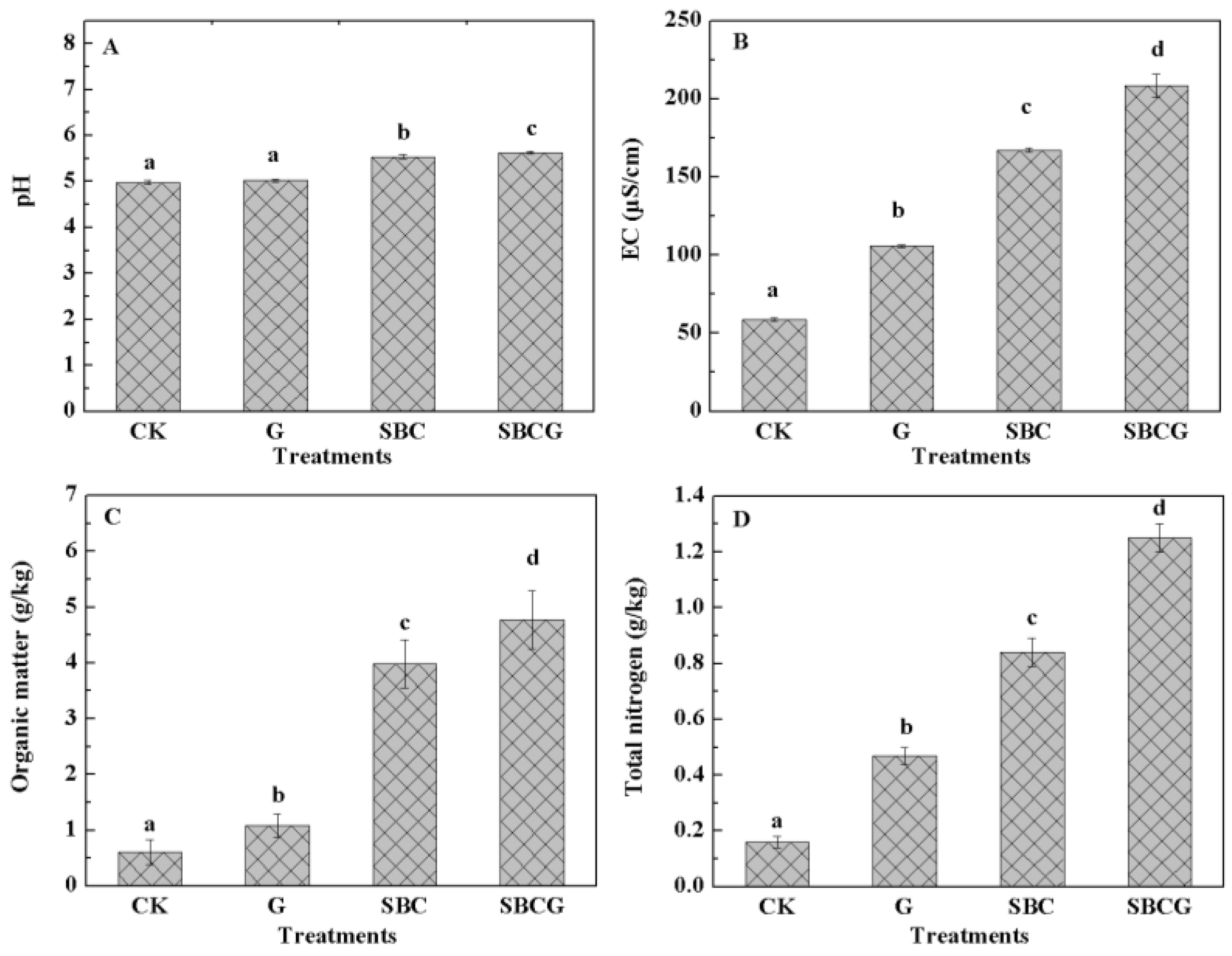
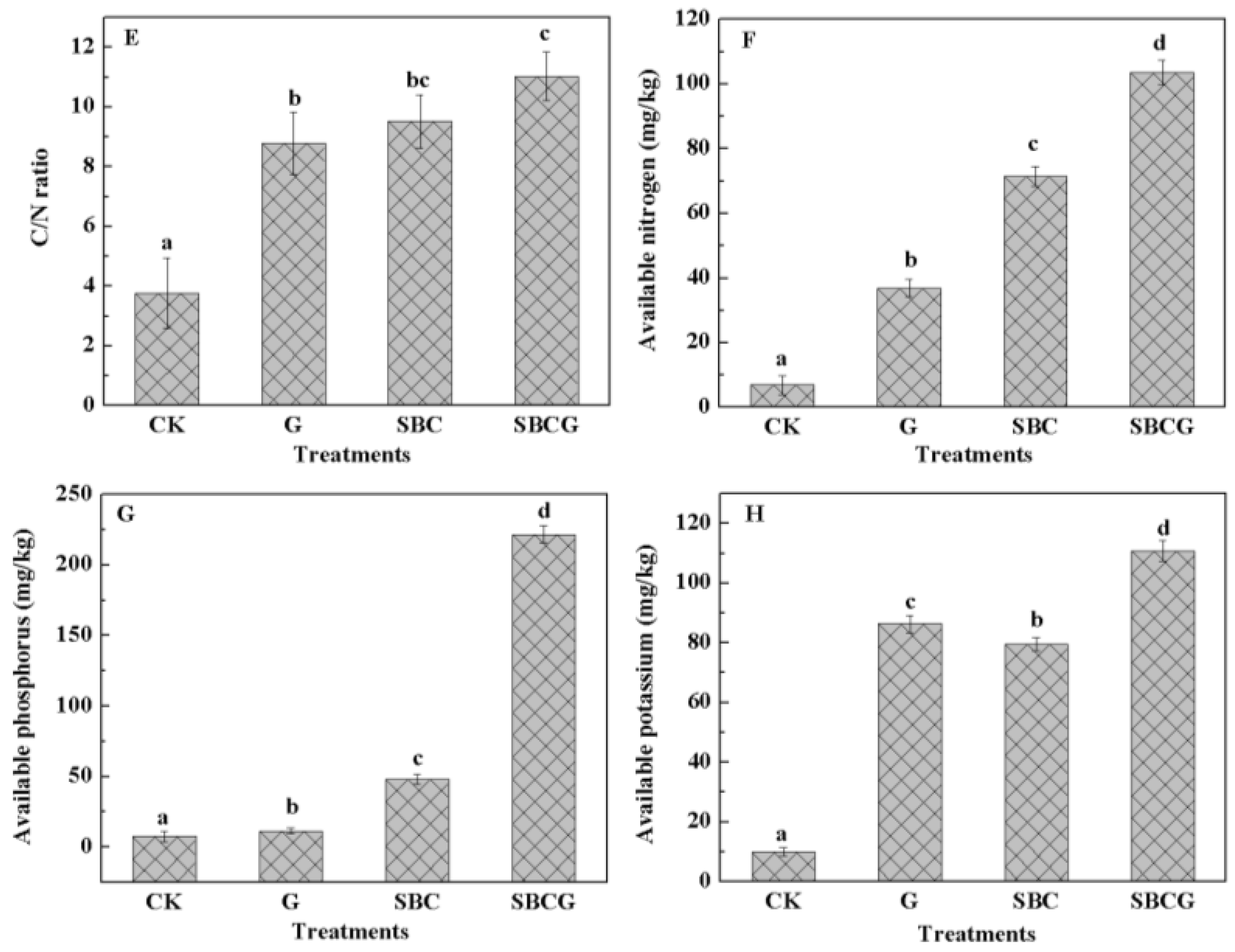
3.1.2. Effect on Soil Chemical Properties
3.2. Response of Soil Microbial Community to Alfalfa Revegetation and Sludge Biochar Amendment
3.2.1. Soil Microbial Alpha Diversity
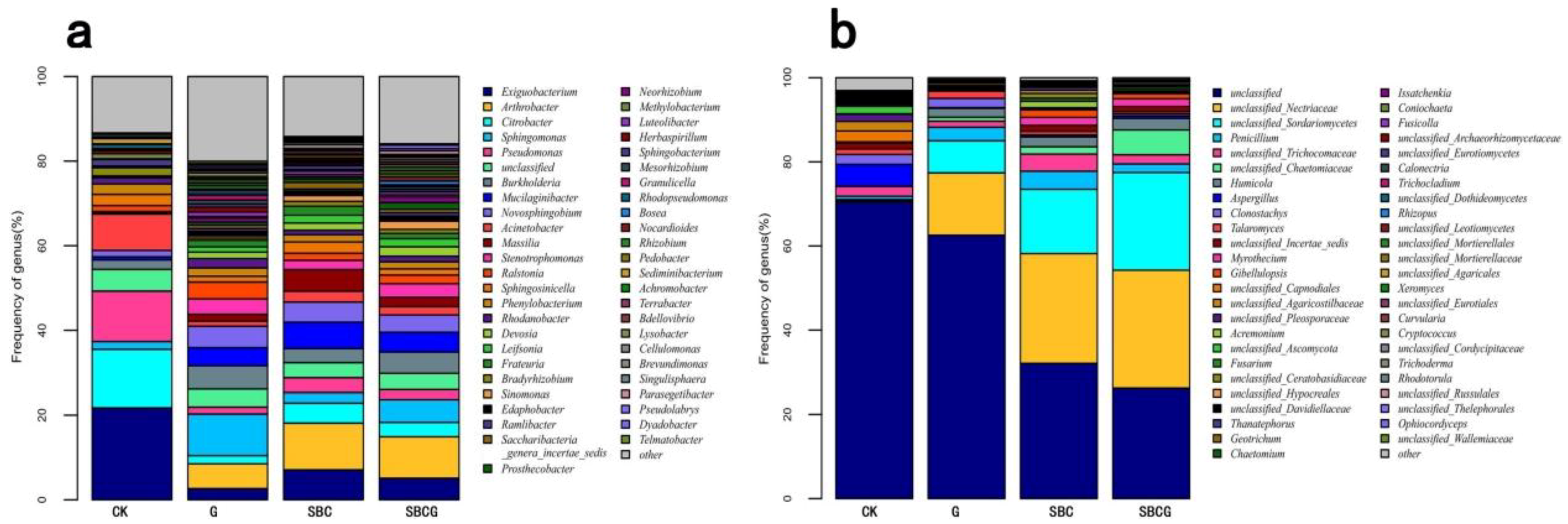
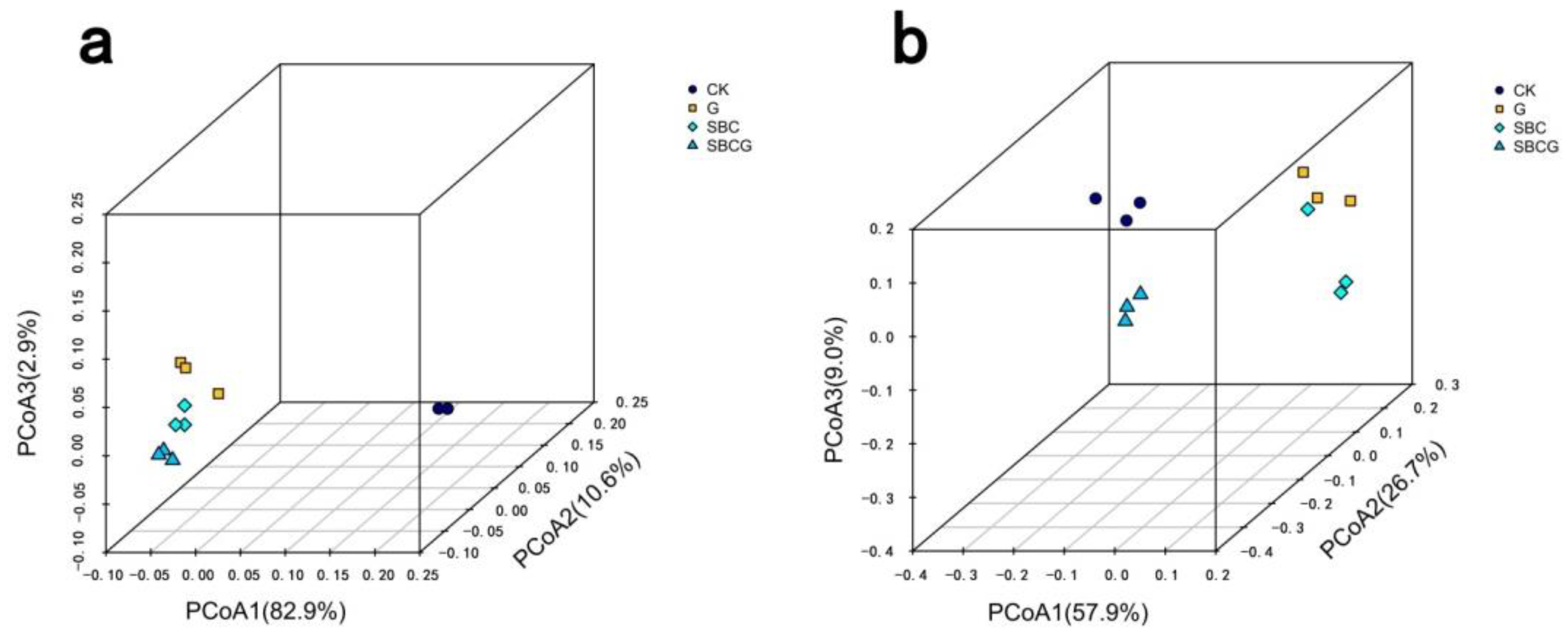
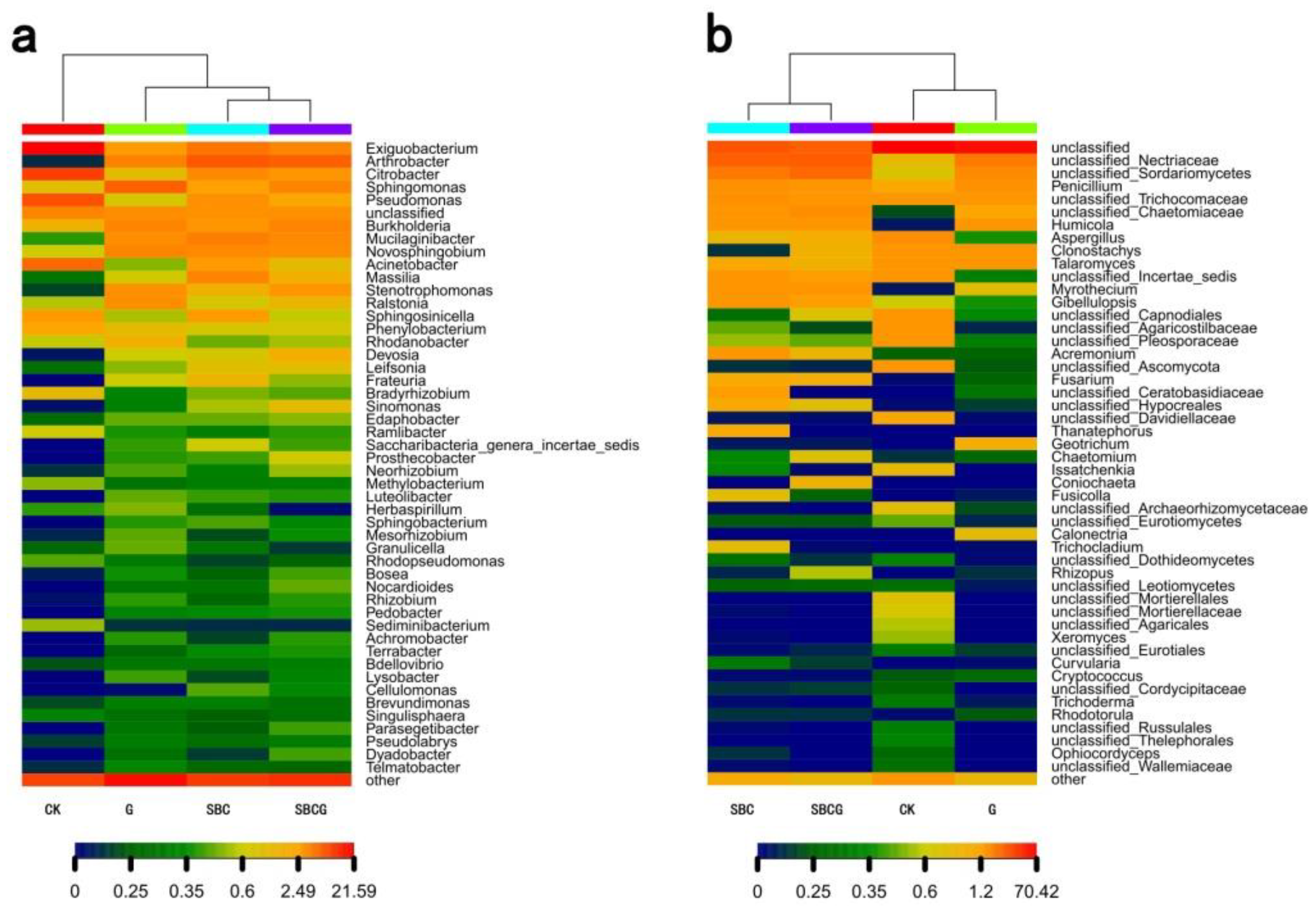
3.2.2. Soil Microbial Community Structure
3.3. The Complex Relationship between Soil Physicochemical Properties and Microbial Communities, and the Response of Plant Growth to Remediation
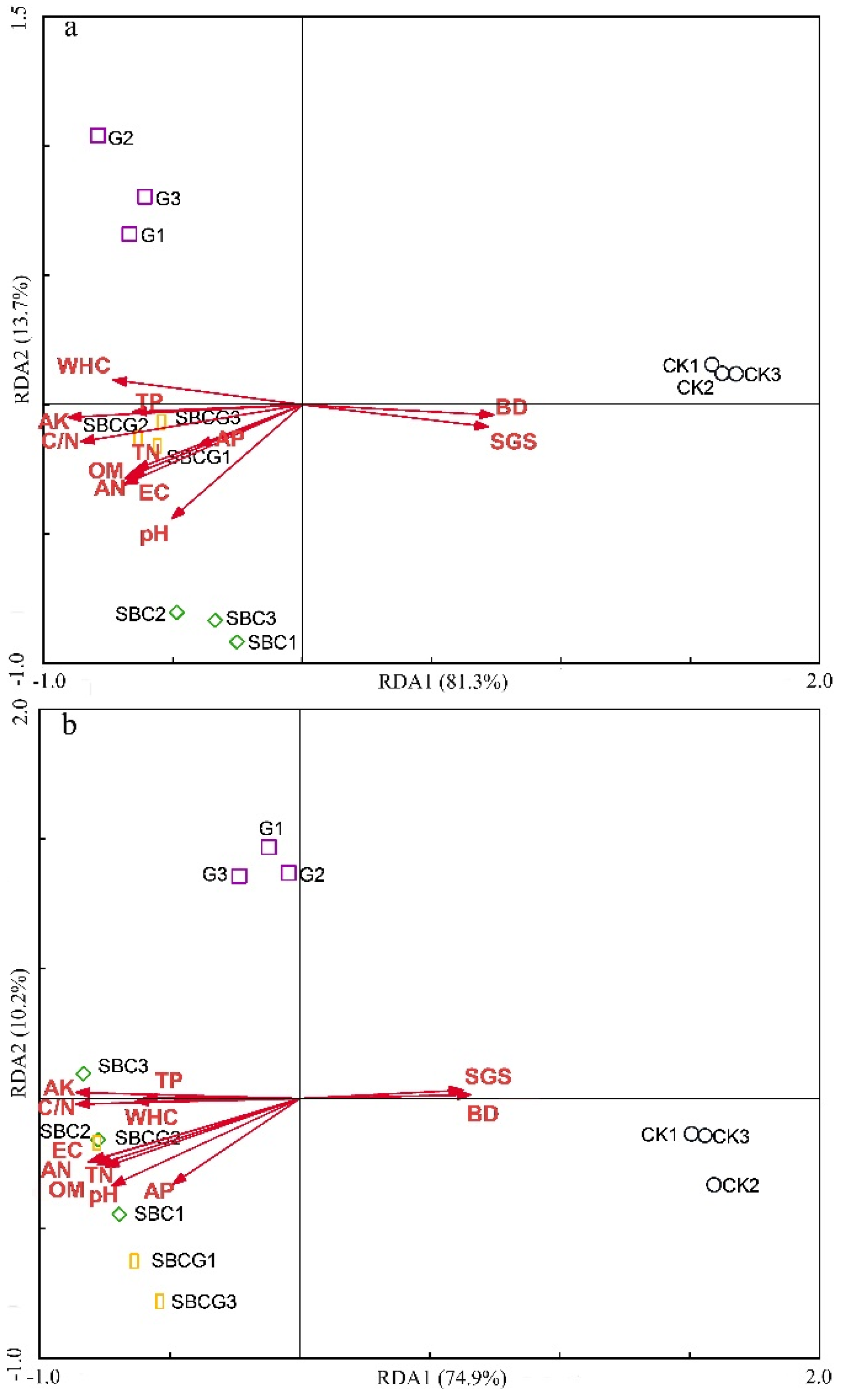
3.3.1. Redundancy Analyses of Soil Physicochemical Properties and Microbial Community
3.3.2. Responses of Plant Growth and Root Morphology
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiao, Y.; Huang, L.; Long, Z.; Feng, Z.; Wang, L. Adsorption ability of rare earth elements on clay minerals and its practical performance. J. Rare Earths 2016, 34, 543–548. [Google Scholar] [CrossRef]
- Yang, X.J.; Lin, A.; Li, X.-L.; Wu, Y.; Zhou, W.; Chen, Z. China’s ion-adsorption rare earth resources, mining consequences and preservation. Environ. Dev. 2013, 8, 131–136. [Google Scholar] [CrossRef]
- Luo, C.; Luo, X.; Su, J.; Chen, C.; Han, D. Environmental Problems and Treatment Measures in Ionic-type rare earth mine. Met. Mine 2014, 6, 91–96. [Google Scholar]
- Initiative, I.B. Standardized Product Definition and Product Testing Guidelines for Biochar That is Used in Soil; International Biochar Initiative: Seattle, WA, USA, 2013. [Google Scholar]
- Wu, H.; Lai, C.; Zeng, G.; Liang, J.; Chen, J.; Xu, J.; Dai, J.; Li, X.; Liu, J.; Chen, M.; et al. The interactions of composting and biochar and their implications for soil amendment and pollution remediation: A review. Crit. Rev. Biotechnol. 2017, 37, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Singla, A.; Iwasa, H.; Inubushi, K. Effect of biogas digested slurry based-biochar and digested liquid on N2O, CO2 flux and crop yield for three continuous cropping cycles of komatsuna (Brassica rapa var. perviridis). Biol Fert Soils 2014, 50, 1201–1209. [Google Scholar] [CrossRef]
- Singla, A.; Dubey, S.K.; Singh, A.; Inubushi, K. Effect of biogas digested slurry-based biochar on methane flux and methanogenic archaeal diversity in paddy soil. Agric. Ecosyst. Environ. 2014, 19 (Suppl. C), 278–287. [Google Scholar] [CrossRef]
- Alpana, S.; Vishwakarma, P.; Adhya, T.K.; Inubushi, K.; Dubey, S.K. Molecular ecological perspective of methanogenic archaeal community in rice agroecosystem. Sci. Total Environ. 2017, 596 (Suppl. C), 136–146. [Google Scholar] [CrossRef] [PubMed]
- Delwiche, K.B.; Lehmann, J.; Walter, M.T. Atrazine leaching from biochar-amended soils. Chemosphere 2014, 95 (Suppl. C), 346–352. [Google Scholar] [CrossRef] [PubMed]
- Fungo, B.; Lehmann, J.; Kalbitz, K.; Tenywa, M.; Thionģo, M.; Neufeldt, H. Emissions intensity and carbon stocks of a tropical Ultisol after amendment with Tithonia green manure, urea and biochar. Field Crops Res. 2017, 209 (Suppl. C), 179–188. [Google Scholar] [CrossRef] [PubMed]
- Lentz, R.D.; Ippolito, J.A.; Spokas, K.A. Biochar and Manure Effects on Net Nitrogen Mineralization and Greenhouse Gas Emissions from Calcareous Soil under Corn. Soil Sci. Soc. Am. J. 2014, 78, 1641–1655. [Google Scholar] [CrossRef]
- Castellini, M.; Giglio, L.; Niedda, M.; Palumbo, A.D.; Ventrella, D. Impact of biochar addition on the physical and hydraulic properties of a clay soil. Soil Tillage Res. 2015, 154, 1–13. [Google Scholar] [CrossRef]
- Chen, B.; Yuan, M.; Qian, L. Enhanced bioremediation of PAH-contaminated soil by immobilized bacteria with plant residue and biochar as carriers. J. Soils Sediment. 2012, 12, 1350–1359. [Google Scholar] [CrossRef]
- Fungo, B.; Guerena, D.; Thiongo, M.; Lehmann, J.; Neufeldt, H.; Kalbitz, K. N2O and CH4 emission from soil amended with steam-activated biochar. J. Plant Nutr. Soil Sci. 2014, 177, 34–38. [Google Scholar] [CrossRef]
- Wang, B.; Lehmann, J.; Hanley, K.; Hestrin, R.; Enders, A. Adsorption and desorption of ammonium by maple wood biochar as a function of oxidation and pH. Chemosphere 2015, 138, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black Carbon Increases Cation Exchange Capacity in Soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Khan, S.; Chao, C.; Waqas, M.; Arp, H.P.H.; Zhu, Y.-G. Sewage Sludge Biochar Influence upon Rice (Oryza sativa L.) Yield, Metal Bioaccumulation and Greenhouse Gas Emissions from Acidic Paddy Soil. Environ. Sci. Technol. 2013, 47, 8624–8632. [Google Scholar] [CrossRef] [PubMed]
- Ro, K.S. Kinetics and Energetics of Producing Animal Manure-Based Biochar. BioEnergy Res. 2016, 9, 447–453. [Google Scholar] [CrossRef]
- Waqas, M.; Li, G.; Khan, S.; Shamshad, I.; Reid, B.; Qamar, Z.; Chao, C. Application of sewage sludge and sewage sludge biochar to reduce polycyclic aromatic hydrocarbons (PAH) and potentially toxic elements (PTE) accumulation in tomato. Environ. Sci. Pollut. Res. 2015, 22, 12114–12123. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Yuan, H.; Wang, Y.; Huang, H.; Chen, Y. Characteristic of heavy metals in biochar derived from sewage sludge. J. Mater. Cycles Waste Manag. 2016, 18, 725–733. [Google Scholar] [CrossRef]
- Feng, L.; Luo, J.; Chen, Y. Dilemma of sewage sludge treatment and disposal in China. Environ. Sci. Technol. 2015, 49, 4781–4782. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Yao, Y.; Lin, Q.; Li, G.; Zhao, X. The change of heavy metals fractions during hydrochar decomposition in soils amended with different municipal sewage sludge hydrochars. J. Soils Sediment. 2017, 17, 763–770. [Google Scholar] [CrossRef]
- Bondarczuk, K.; Markowicz, A.; Piotrowska-Seget, Z. The urgent need for risk assessment on the antibiotic resistance spread via sewage sludge land application. Environ. Int. 2016, 87, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ferreiro, J.; Gascó, G.; Gutiérrez, B.; Méndez, A. Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biol. Fert Soils 2012, 48, 511–517. [Google Scholar] [CrossRef]
- Méndez, A.; Gómez, A.; Paz-Ferreiro, J.; Gascó, G. Effects of sewage sludge biochar on plant metal availability after application to a Mediterranean soil. Chemosphere 2012, 89, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Méndez, A.; Cárdenas-Aguiar, E.; Paz-Ferreiro, J.; Plaza, C.; Gascó, G. The effect of sewage sludge biochar on peat-based growing media. Biol. Agric. Horticult. 2017, 33, 40–51. [Google Scholar] [CrossRef]
- Deng, L.; Wang, K.B.; Li, J.P.; Shangguan, Z.P.; Sweeney, S. Carbon Storage Dynamics in Alfalfa (Medicago sativa) Fields in the Hilly-Gully Region of the Loess Plateau, China. Clean-Soil Air Water 2014, 42, 1253–1262. [Google Scholar] [CrossRef]
- He, H.; Peng, Q.; Wang, X.; Fan, C.; Pang, J.; Lambers, H.; Zhang, X. Growth, morphological and physiological responses of alfalfa (Medicago sativa) to phosphorus supply in two alkaline soils. Plant Soil 2017, 416, 265–584. [Google Scholar] [CrossRef]
- Agnello, A.C.; Huguenot, D.; van Hullebusch, E.D.; Esposito, G. Citric acid- and Tween® 80-assisted phytoremediation of a co-contaminated soil: Alfalfa (Medicago sativa L.) performance and remediation potential. Environ. Sci. Pollut. Res. 2016, 23, 9215–9226. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-W.; Du, Y.-L.; Turner, N.C.; Wang, B.-R.; Fang, Y.; Xi, Y.; Guo, X.-R.; Li, F.-M. Changes in root morphology and physiology to limited phosphorus and moisture in a locally-selected cultivar and an introduced cultivar of Medicago sativa L. growing in alkaline soil. Plant Soil 2015, 392, 215–226. [Google Scholar] [CrossRef]
- Bastida, F.; Torres, I.F.; Andrésabellán, M.; Baldrian, P.; Lópezmondéjar, R.; Větrovský, T.; Richnow, H.H.; Starke, R.; Ondoño, S.; García, C. Differential sensitivity of total and active soil microbial communities to drought and forest management. Glob. Chang. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.-Y.; Wang, M.-W.; Zhu, H.-W.; Guo, Z.-H.; Han, X.-Q.; Zeng, P. Response of soil microbial activities and microbial community structure to vanadium stress. Ecotoxicol. Environ. Saf. 2017, 142, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Deng, Y.; Liang, J.; Zhu, S.; Wei, Z.; Guo, X.; Luo, X. Exogenous rare earth element-yttrium deteriorated soil microbial community structure. J. Rare Earths 2018, 36, 430–439. [Google Scholar] [CrossRef]
- Chao, Y.; Liu, W.; Chen, Y.; Chen, W.; Zhao, L.; Ding, Q.; Wang, S.; Tang, Y.-T.; Zhang, T.; Qiu, R.-L. Structure, Variation, and Co-occurrence of Soil Microbial Communities in Abandoned Sites of a Rare Earth Elements Mine. Environ. Sci. Technol. 2016, 50, 11481–11490. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, Z.; Liu, W.; Liu, S.; Zhang, L.; Zhong, L.; Luo, X.; Liang, H. Restoration of rare earth mine areas: Organic amendments and phytoremediation. Environ. Sci. Pollut. Res. 2015, 22, 17151–17160. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, W.; Yang, M.; Zhou, L.; Liang, H. The genetic diversity of soil bacteria affected by phytoremediation in a typical barren rare earth mined site of South China. SpringerPlus 2016, 5, 1131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, S.; Gao, X. Soil Analysis Technical Specifications; Chinese Industry Publisher: Beijing, China, 2006. [Google Scholar]
- Sinclair, L.; Osman, O.A.; Bertilsson, S.; Eiler, A. Microbial Community Composition and Diversity via 16S rRNA Gene Amplicons: Evaluating the Illumina Platform. PLoS ONE 2015, 10, e0116955. [Google Scholar] [CrossRef] [PubMed]
- Köchling, T.; Sanz, J.L.; Gavazza, S.; Florencio, L. Analysis of microbial community structure and composition in leachates from a young landfill by 454 pyrosequencing. Appl. Microbiol. Biotechnol. 2015, 99, 5657–5668. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, H.; Chen, J.; Shang, S.; Wei, Q.; Yan, J.; Tu, X. Comparison of the fecal microbiota of dholes high-throughput Illumina sequencing of the V3-V4 region of the 16S rRNA gene. Appl. Microbiol. Biotechnol. 2016, 100, 3577–3586. [Google Scholar] [CrossRef] [PubMed]
- Amato, K.R.; Yeoman, C.J.; Kent, A.; Righini, N.; Carbonero, F.; Estrada, A.; Rex Gaskins, H.; Stumpf, R.M.; Yildirim, S.; Torralba, M.; et al. Habitat degradation impacts black howler monkey (Alouatta pigra) gastrointestinal microbiomes. ISME J. 2013, 7, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wang, L.; Zhou, Q. Combined effects of lanthanum(III) and elevated ultraviolet-B radiation on root growth and ion absorption in soybean seedlings. Environ. Sci. Pollut. Res. 2014, 21, 3621–3633. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Patel, A.; Patra, D.D. Biochar ameliorates crop productivity, soil fertility, essential oil yield and aroma profiling in basil (Ocimum basilicum L.). Ecol. Eng. 2016, 90, 361–366. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, G.; Liang, J.; Chen, J.; Xu, J.; Dai, J.; Li, X.; Chen, M.; Xu, P.; Zhou, Y. Responses of bacterial community and functional marker genes of nitrogen cycling to biochar, compost and combined amendments in soil. Appl. Microbiol. Biotechnol. 2016, 100, 8583–8591. [Google Scholar] [CrossRef] [PubMed]
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO. Bull. Ecol. Soc. Am. 2004, 86, 5. [Google Scholar]
- Blackwell, P.S.; Green, T.W.; Mason, W.K. Responses of Biopore Channels from Roots to Compression by Vertical Stresses. Soil Sci. Soc. Am. J. 1990, 54, 1088–1091. [Google Scholar] [CrossRef]
- Malhi, S.S.; Lemke, R.; Schoenau, J.J. Influence of time and method of alfalfa stand termination on yield, seed quality, N uptake, soil properties and greenhouse gas emissions under different N fertility regimes. Nutr. Cycl. Agroecosyst. 2010, 86, 17–38. [Google Scholar] [CrossRef]
- Zong, Y.; Wang, Y.; Sheng, Y.; Wu, C.; Lu, S. Ameliorating soil acidity and physical properties of two contrasting texture Ultisols with wastewater sludge biochar. Environ. Sci. Pollut. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Herath, H.M.S.K.; Camps-Arbestain, M.; Hedley, M. Effect of biochar on soil physical properties in two contrasting soils: An Alfisol and an Andisol. Geoderma 2013, 209, 188–197. [Google Scholar] [CrossRef]
- Busscher, W.J.; Novak, J.M.; Evans, D.E.; Watts, D.W.; Niandou, M.A.S.; Ahmedna, M. Influence of pecan biochar on physical properties of a Norfolk loamy sand. Soil Sci. 2010, 175, 10–14. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X.; Jing, Y.; Li, Q.; Zhang, J.; Huang, Q. Effects of biochar amendment on rapeseed and sweet potato yields and water stable aggregate in upland red soil. CATENA 2014, 123, 45–51. [Google Scholar] [CrossRef]
- Fungo, B.; Lehmann, J.; Kalbitz, K.; Thionģo, M.; Okeyo, I.; Tenywa, M.; Neufeldt, H. Aggregate size distribution in a biochar-amended tropical Ultisol under conventional hand-hoe tillage. Soil Tillage Res. 2017, 165, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.M.; Busscher, W.J.; Watts, D.W.; Amonette, J.E.; Ippolito, J.A.; Lima, I.M.; Gaskin, J.; Das, K.C.; Steiner, C.; Ahmedna, M.; et al. Biochars Impact on Soil-Moisture Storage in an Ultisol and Two Aridisols. Soil Sci. 2012, 177, 310–320. [Google Scholar] [CrossRef]
- Manyà, J.J. Pyrolysis for Biochar Purposes: A Review to Establish Current Knowledge Gaps and Research Needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.Z.; Wang, X.F.; Yang, R.; Lee, J. Effects of sandy desertified land rehabilitation on soil carbon sequestration and aggregation in an arid region in China. J. Environ. Manag. 2010, 91, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, Z.; Fu, S.; Méndez, A.; Gascó, G.; Paz-Ferreiro, J. Combining phytoextraction and biochar addition improves soil biochemical properties in a soil contaminated with Cd. Chemosphere 2015, 119, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.H.; Xu, R.K. The amelioration effects of low temperature biochar generated from nine crop residues on an acidic Ultisol. Soil Use Manag. 2011, 27, 110–115. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential role of biochars in decreasing soil acidification—A critical review. Sci. Total Environ. 2017, 581–582, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Cui, L.; Lin, Q.; Li, G.; Zhao, X. Efficiency of sewage sludge biochar in improving urban soil properties and promoting grass growth. Chemosphere 2017, 173, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Ye, L.L.; Wang, C.H.; Zhou, H.; Sun, B. Temperature- and duration-dependent rice straw-derived biochar: Characteristics and its effects on soil properties of an Ultisol in southern China. Soil Tillage Res. 2011, 112, 159–166. [Google Scholar] [CrossRef]
- Streubel, J.D.; Collins, H.P.; Garcia-Perez, M.; Tarara, J.; Granatstein, D.; Kruger, C.E. Influence of Contrasting Biochar Types on Five Soils at Increasing Rates of Application. Soil Sci. Soc. Am. J. 2011, 75, 1402–1413. [Google Scholar] [CrossRef]
- Laghari, M.; Mirjat, M.S.; Hu, Z.; Fazal, S.; Xiao, B.; Hu, M.; Chen, Z.; Guo, D. Effects of biochar application rate on sandy desert soil properties and sorghum growth. CATENA 2015, 135, 313–320. [Google Scholar] [CrossRef]
- Liu, C.; Wang, H.; Tang, X.; Guan, Z.; Reid, B.J.; Rajapaksha, A.U.; Ok, Y.S.; Sun, H. Biochar increased water holding capacity but accelerated organic carbon leaching from a sloping farmland soil in China. Environ. Sci. Pollut. Res. 2016, 23, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, J.; Wang, S.; Xing, G. Successive straw biochar application as a strategy to sequester carbon and improve fertility: A pot experiment with two rice/wheat rotations in paddy soil. Plant Soil 2014, 378, 279–294. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.; Liang, Y.; Gao, B.; Harris, W. Simultaneous Immobilization of Lead and Atrazine in Contaminated Soils Using Dairy-Manure Biochar. Environ. Sci. Technol. 2011, 45, 4884–4889. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.-H.; Zhang, S.; Rao, X.; Liu, C.-A. Newly-reclaimed alfalfa forage land improved soil properties comparison to farmland in wheat–maize cropping systems at the margins of oases. Ecol. Eng. 2016, 94, 57–64. [Google Scholar] [CrossRef]
- Kim, J.-S.; Sparovek, G.; Longo, R.M.; De Melo, W.J.; Crowley, D. Bacterial diversity of terra preta and pristine forest soil from the Western Amazon. Soil Biol. Biochem. 2007, 39, 684–690. [Google Scholar] [CrossRef]
- O’Neill, B.; Grossman, J.; Tsai, M.T.; Gomes, J.E.; Lehmann, J.; Peterson, J.; Neves, E.; Thies, J.E. Bacterial Community Composition in Brazilian Anthrosols and Adjacent Soils Characterized Using Culturing and Molecular Identification. Microb. Ecol. 2009, 58, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Faragová, N.; Gottwaldová, K.; Faragó, J. Effect of transgenic alfalfa plants with introduced gene for Alfalfa Mosaic Virus coat protein on rhizosphere microbial community composition and physiological profile. Biologia 2011, 66, 768. [Google Scholar] [CrossRef]
- Pascault, N.; Cécillon, L.; Mathieu, O.; Hénault, C.; Sarr, A.; Lévêque, J.; Farcy, P.; Ranjard, L.; Maron, P.-A. In Situ Dynamics of Microbial Communities during Decomposition of Wheat, Rape, and Alfalfa Residues. Microb. Ecol. 2010, 60, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Bruun, E.W.; Arthur, E.; de Jonge, L.W.; Moldrup, P.; Hauggaard-Nielsen, H.; Elsgaard, L. Effect of biochar on aerobic processes, enzyme activity, and crop yields in two sandy loam soils. Biol Fert Soils 2014, 50, 1087–1097. [Google Scholar] [CrossRef]
- Hafner, S.; Wiesenberg, G.L.B.; Stolnikova, E.; Merz, K.; Kuzyakov, Y. Spatial distribution and turnover of root-derived carbon in alfalfa rhizosphere depending on top- and subsoil properties and mycorrhization. Plant Soil 2014, 380, 101–115. [Google Scholar] [CrossRef]
- Song, Y.; Bian, Y.; Wang, F.; Herzberger, A.; Yang, X.; Gu, C.; Jiang, X. Effects of biochar on dechlorination of hexachlorobenzene and the bacterial community in paddy soil. Chemosphere 2017, 186 (Suppl. C), 116–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Meng, J.; Jiang, L.; Yang, X.; Lan, Y.; Cheng, X.; Chen, W. Rice husk biochar impacts soil phosphorous availability, phosphatase activities and bacterial community characteristics in three different soil types. Appl. Soil Ecol. 2017, 116, 12–22. [Google Scholar] [CrossRef]
- Hu, L.; Cao, L.; Zhang, R. Bacterial and fungal taxon changes in soil microbial community composition induced by short-term biochar amendment in red oxidized loam soil. World J. Microbiol. Biotechnol. 2014, 30, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Tu, C.; Ma, L.; Guo, P.; Song, F.; Teng, Y.; Zhang, H.; Luo, Y. Rhizoremediation of a dioxin-like PCB polluted soil by alfalfa: Dynamic characterization at temporal and spatial scale. Chemosphere 2017, 189 (Suppl. C), 517–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Chen, Y.T.; Chen, S.H.; Chang Chien, S.W.; Sunkara, S.V. Phytoremediation of pyrene contaminated soils amended with compost and planted with ryegrass and alfalfa. Chemosphere 2012, 87, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Khodadad, C.L.M.; Zimmerman, A.R.; Green, S.J.; Uthandi, S.; Foster, J.S. Taxa-specific changes in soil microbial community composition induced by pyrogenic carbon amendments. Soil Biol. Biochem. 2011, 43, 385–392. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.; Yu, Z.; Li, Y.; Jin, J.; Liu, X.; Wang, G. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol. Biochem. 2017, 110 (Suppl. C), 56–67. [Google Scholar] [CrossRef]
- Liu, L.; Chen, P.; Sun, M.; Shen, G.; Shang, G. Effect of biochar amendment on PAH dissipation and indigenous degradation bacteria in contaminated soil. J Soils Sediment. 2015, 15, 313–322. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Zhang, W.; Wang, F.; Bian, Y.; Boughner, L.A.; Jiang, X. Novel Biochar-Plant Tandem Approach for Remediating Hexachlorobenzene Contaminated Soils: Proof-of-Concept and New Insight into the Rhizosphere. J. Agric. Food Chem. 2016, 64, 5464–5471. [Google Scholar] [CrossRef] [PubMed]
- Haichar, F.E.Z.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008, 2, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Liu, W.; Huo, R.; Xu, J.; Liang, S.; Li, J.; Zhao, T.; Wang, S. Effects of biochar on nitrogen transformation and heavy metals in sludge composting. Bioresour. Technol. 2017, 235 (Suppl. C), 43–49. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Meng, J.; Xu, E.G.; Chen, W. Microbial community structure and predicted bacterial metabolic functions in biochar pellets aged in soil after 34 months. Appl. Soil Ecol. 2016, 100 (Suppl. C), 135–143. [Google Scholar] [CrossRef]
- Shrestha, B.; Anderson, T.A.; Acosta-Martinez, V.; Payton, P.; Cañas-Carrell, J.E. The influence of multiwalled carbon nanotubes on polycyclic aromatic hydrocarbon (PAH) bioavailability and toxicity to soil microbial communities in alfalfa rhizosphere. Ecotoxicol. Environ. Saf. 2015, 116 (Suppl. C), 143–149. [Google Scholar] [CrossRef] [PubMed]
- Brussaard, L.; de Ruiter, P.C.; Brown, G.G. Soil biodiversity for agricultural sustainability. Agric. Ecosyst. Environ. 2007, 121, 233–244. [Google Scholar] [CrossRef]
- Moore, J.; Berlow, E.; Coleman, D.; Ruiter, P.; Dong, Q.; Hastings, A.; Johnson, N.; McCann, K.; Melville, K.; Morin, P.; et al. Detritus, trophic dynamics and biodiversity. Ecol. Lett. 2004, 7, 584–600. [Google Scholar] [CrossRef]
- Hršelová, H.; Chvátalová, I.; Vosátka, M.; Klír, J.; Gryndler, M. Correlation of abundance of arbuscular mycorrhizal fungi, bacteria and saprophytic microfungi with soil carbon, nitrogen and phsophorus. Folia Microbiol. 1999, 44, 683–687. [Google Scholar] [CrossRef]
- Tu, C.; Teng, Y.; Luo, Y.; Sun, X.; Deng, S.; Li, Z.; Liu, W.; Xu, Z. PCB removal, soil enzyme activities, and microbial community structures during the phytoremediation by alfalfa in field soils. J. Soils Sediment. 2011, 11, 649–656. [Google Scholar] [CrossRef]
- Zeng, G.; Wu, H.; Liang, J.; Guo, S.; Huang, L.; Xu, P.; Liu, Y.; Yuan, Y.; He, X.; He, Y. Efficiency of biochar and compost (or composting) combined amendments for reducing Cd, Cu, Zn and Pb bioavailability, mobility and ecological risk in wetland soil. RSC Adv. 2015, 5, 34541–34548. [Google Scholar] [CrossRef]
- Xu, H.-J.; Wang, X.-H.; Li, H.; Yao, H.-Y.; Su, J.-Q.; Zhu, Y.-G. Biochar Impacts Soil Microbial Community Composition and Nitrogen Cycling in an Acidic Soil Planted with Rape. Environ. Sci. Technol. 2014, 48, 9391–9399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, Q.; Hu, C.; Zheng, C.; Gui, H.; Zeng, W.; Sun, X.; Zhao, X. Differences in responses of soil microbial properties and trifoliate orange seedling to biochar derived from three feedstocks. J. Soils Sediment. 2015, 15, 541–551. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, L.M.; Farrell, M.; Zwieten, L.V.; Krull, E.S. Plant growth responses to biochar addition: An Australian soils perspective. Biol. Fert Soils 2014, 50, 1035–1045. [Google Scholar] [CrossRef]
- Ye, J.; Yin, H.; Peng, H.; Bai, J.; Li, Y. Pyrene removal and transformation by joint application of alfalfa and exogenous microorganisms and their influence on soil microbial community. Ecotoxicol. Environ. Saf. 2014, 110, 129–135. [Google Scholar] [CrossRef] [PubMed]


| Samples | Soil | Sludge Biochar |
|---|---|---|
| Clay (%) | 7.64 ± 0.27 | - |
| Silt (%) | 15.48 ± 0.32 | - |
| Sand (%) | 76.88 ± 0.38 | - |
| pH | 5.44 ± 0.02 | 6.17 ± 0.03 |
| Electrical conductivity (μS/cm) | 45.16 ± 1.03 | 272.67 ± 6.06 |
| Organic carbon (g/kg) | 0.73 ± 0.18 | 97.32 ± 2.78 |
| Total nitrogen (g/kg) | 0.21 ± 0.03 | 2.48 ± 0.02 |
| C/N ratio | 3.44 ± 0.37 | 39.31 ± 0.72 |
| Available nitrogen (mg/kg) | 10.35 ± 2.58 | 378.93 ± 10.19 |
| Available phosphorus (mg/kg) | 9.43 ± 2.31 | 712.61 ± 12.24 |
| Available potassium (mg/kg) | 10.62 ± 1.51 | 172.97 ± 5.92 |
| BET surface area (m2/g) | - | 47.03 ± 0.02 |
| Pore volume (cm3/g) | - | 0.06 ± 0.001 |
| Pore size (nm) | - | 5.16 ± 0.01 |
| Treatments | Mine Soil (kg) | Alfalfa Seed (g) | Sludge Biochar (kg) |
|---|---|---|---|
| CK | 5 | - | - |
| G | 5 | 5 | - |
| SBC | 4.75 | - | 0.25 |
| SBCG | 4.75 | 5 | 0.25 |
| Microbial Group | Indices | CK | G | SBC | SBCG |
|---|---|---|---|---|---|
| Bacteria | Number of OTUs | 927 ± 36 a | 1332 ± 15 b | 1278 ± 62 b | 1341 ± 8 b |
| ACE | 3220 ± 395 a | 3366 ± 192 a | 3270 ± 221 a | 3430 ± 157 a | |
| Chao 1 | 2309 ± 240 a | 2603 ± 166 a | 2565 ± 142 a | 2630 ± 84 a | |
| Shannon | 3.55 ± 0.04 a | 4.89 ± 0.02 c | 4.62 ± 0.02 b | 4.98 ± 0.07 c | |
| Simpson | 0.092 ± 0.00 d | 0.020 ± 0.00 c | 0.028 ± 0.00 c | 0.017 ± 0.00 b | |
| Coverage | 0.99 ± 0.00 a | 0.98 ± 0.00 b | 0.98 ± 0.00 a | 0.98 ± 0.00 a | |
| Fungi | Number of OTUs | 361 ± 17 a | 496 ± 7 a | 427 ± 18 a | 514 ± 91 a |
| ACE | 1074 ± 107 a | 1558 ± 27 b | 1085 ± 89 a | 1798 ± 69 b | |
| Chao 1 | 752 ± 42 a | 1093 ± 111 b | 1079 ± 131 b | 1157 ± 52 b | |
| Shannon | 1.96 ± 0.07 a | 3.07 ± 0.02 b,c | 2.72 ± 0.07 b | 3.32 ± 0.02 c | |
| Simpson | 0.340 ± 0.02 b | 0.107 ± 0.00 a | 0.140 ± 0.00 a | 0.011 ± 0.00 a | |
| Coverage | 0.99 ± 0.00 a | 0.99 ± 0.00 a | 0.99 ± 0.00 a | 0.98 ± 0.00 a |
| Soil Parameters | Bacterial Variation Explains (%) | F-Value | p-Value | Soil Parameters | Fungal Variation Explains (%) | F-Value | p-Value |
|---|---|---|---|---|---|---|---|
| Available potassium | 74.3 | 28.955 | 0.0020 | pH | 72.5 | 26.356 | 0.0020 |
| pH | 67.1 | 20.375 | 0.0080 | Available potassium | 64.9 | 18.496 | 0.0020 |
| C/N | 67.0 | 20.316 | 0.0040 | C/N | 64.6 | 18.283 | 0.0020 |
| Bulk density | 49.9 | 9.950 | 0.0040 | EC (Electrical conductivity) | 59.5 | 14.677 | 0.0020 |
| Water-holding capacity | 49.4 | 9.754 | 0.0040 | Available nitrogen | 57.9 | 13.765 | 0.0020 |
| Specific gravity | 47.5 | 9.061 | 0.0060 | Total nitrogen | 54.8 | 12.128 | 0.0020 |
| EC (Electrical conductivity) | 46.7 | 8.769 | 0.0040 | Organic matter | 51.2 | 10.491 | 0.0020 |
| Available nitrogen | 46.1 | 8.555 | 0.0120 | Bulk density | 39.3 | 6.488 | 0.0240 |
| Total nitrogen | 43.1 | 7.575 | 0.0100 | Water-holding capacity | 37.2 | 5.930 | 0.0180 |
| Organic matter | 40.8 | 6.879 | 0.0120 | Specific gravity | 36.7 | 5.790 | 0.0220 |
| Total porosity | 39.2 | 6.456 | 0.0040 | Total porosity | 32.3 | 4.761 | 0.0440 |
| Available phosphorus | 17.2 | 2.074 | 0.1420 | Available phosphorus | 26.0 | 3.522 | 0.0420 |
| Total | 98.9 | Total | 99.0 |
| Treatments | Plant Height (cm) | Shoot Biomass (g) | Root Biomass (g) | Total Biomass (g) |
|---|---|---|---|---|
| G | 8.98 ± 0.24 a | 5.36 ± 0.35 a | 8.53 ± 0.03 a | 13.89 ± 0.34 a |
| SBCG | 11.09 ± 0.15 b | 7.64 ± 0.26 b | 11.55 ± 0.04 b | 19.20 ± 0.22 b |
| Treatments | TRL (cm) | RSA (cm2) | RV (cm3) | RAD (mm) | RTN | RFN |
|---|---|---|---|---|---|---|
| G | 96.70 ± 1.79 a | 12.05 ± 1.17 a | 0.19 ± 0.04 a | 0.46 ± 0.02 a | 161.67 ± 5.36 a | 293.00 ± 6.56 a |
| SBCG | 120.67 ± 4.35 b | 18.38 ± 0.96 b | 0.53 ± 0.04 b | 0.58 ± 0.01 b | 249.00 ± 5.69 b | 365.00 ± 18.90 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, C.; Deng, Y.; Inubushi, K.; Liang, J.; Zhu, S.; Wei, Z.; Guo, X.; Luo, X. Sludge Biochar Amendment and Alfalfa Revegetation Improve Soil Physicochemical Properties and Increase Diversity of Soil Microbes in Soils from a Rare Earth Element Mining Wasteland. Int. J. Environ. Res. Public Health 2018, 15, 965. https://doi.org/10.3390/ijerph15050965
Luo C, Deng Y, Inubushi K, Liang J, Zhu S, Wei Z, Guo X, Luo X. Sludge Biochar Amendment and Alfalfa Revegetation Improve Soil Physicochemical Properties and Increase Diversity of Soil Microbes in Soils from a Rare Earth Element Mining Wasteland. International Journal of Environmental Research and Public Health. 2018; 15(5):965. https://doi.org/10.3390/ijerph15050965
Chicago/Turabian StyleLuo, Caigui, Yangwu Deng, Kazuyuki Inubushi, Jian Liang, Sipin Zhu, Zhenya Wei, Xiaobin Guo, and Xianping Luo. 2018. "Sludge Biochar Amendment and Alfalfa Revegetation Improve Soil Physicochemical Properties and Increase Diversity of Soil Microbes in Soils from a Rare Earth Element Mining Wasteland" International Journal of Environmental Research and Public Health 15, no. 5: 965. https://doi.org/10.3390/ijerph15050965
APA StyleLuo, C., Deng, Y., Inubushi, K., Liang, J., Zhu, S., Wei, Z., Guo, X., & Luo, X. (2018). Sludge Biochar Amendment and Alfalfa Revegetation Improve Soil Physicochemical Properties and Increase Diversity of Soil Microbes in Soils from a Rare Earth Element Mining Wasteland. International Journal of Environmental Research and Public Health, 15(5), 965. https://doi.org/10.3390/ijerph15050965






