Heparin-Binding Epidermal-like Growth Factor (HB-EGF) Reduces Cell Death in an Organoid Model of Retinal Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Stem Cell Cultures and Retinal Organoid Differentiation
2.2. 4-OHT and HB-EGF Treatment of Retinal Organoids
2.3. Immunofluorescence and Cell Analysis
2.4. Image Analyses
2.5. Reverse Transcription and Quantitative PCR
3. Results
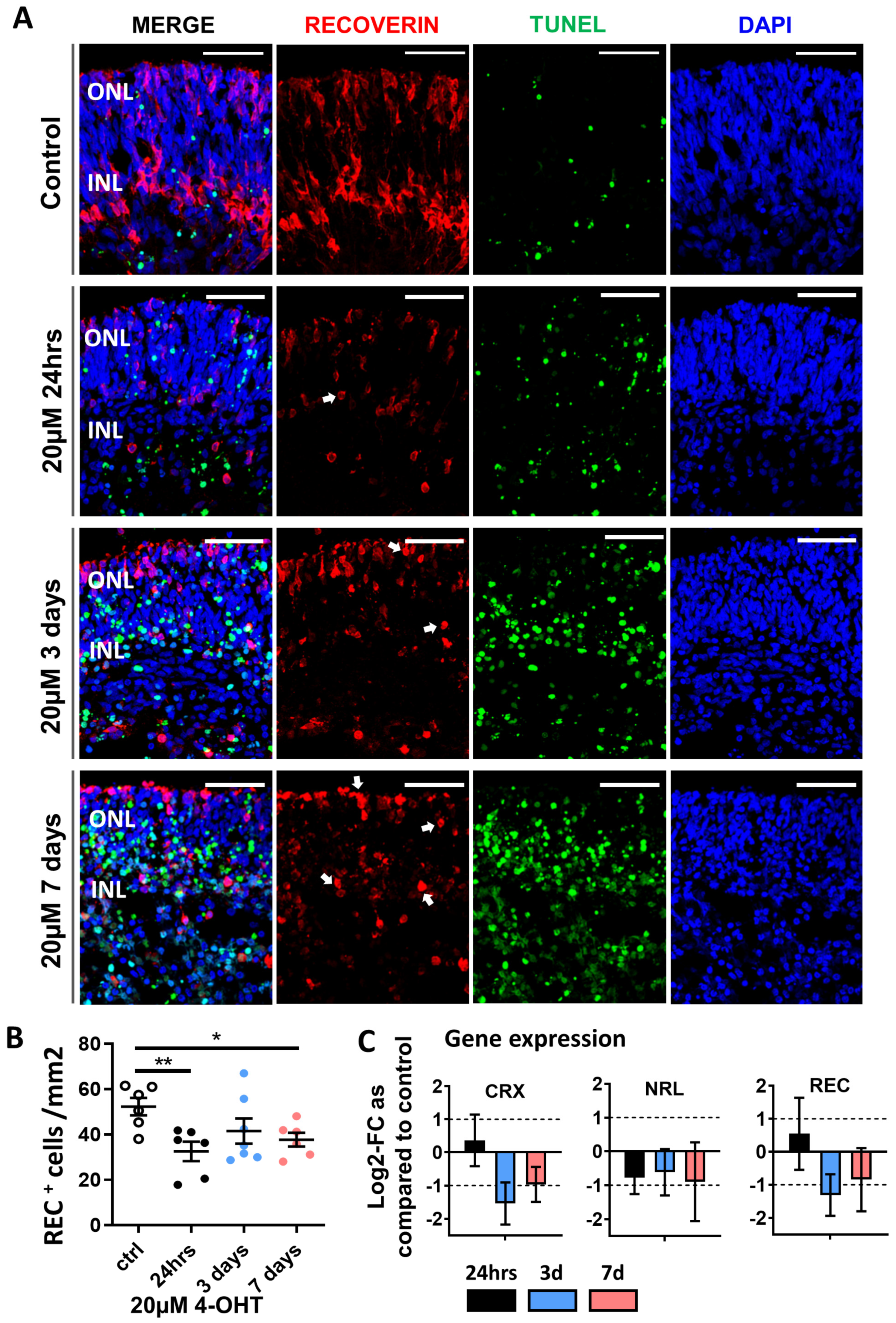
3.1. 4-OHT Dose Response and Changes in Retinal Morphology
3.2. Confirmation of Photoreceptor Degeneration Induced by 20 µM 4-OHT
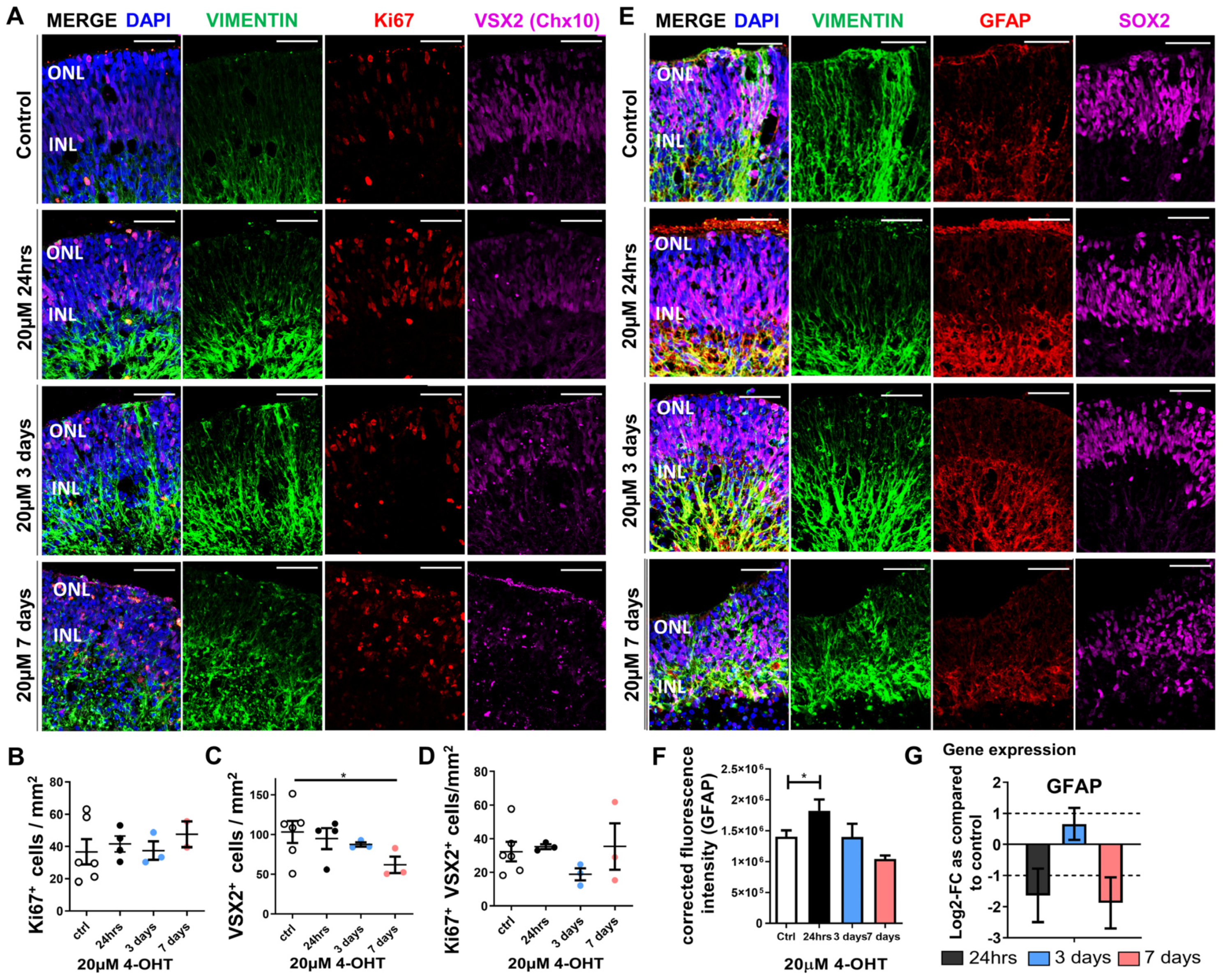
3.3. Müller Glia Responses to 4-OHT Treatment of Retinal Organoids
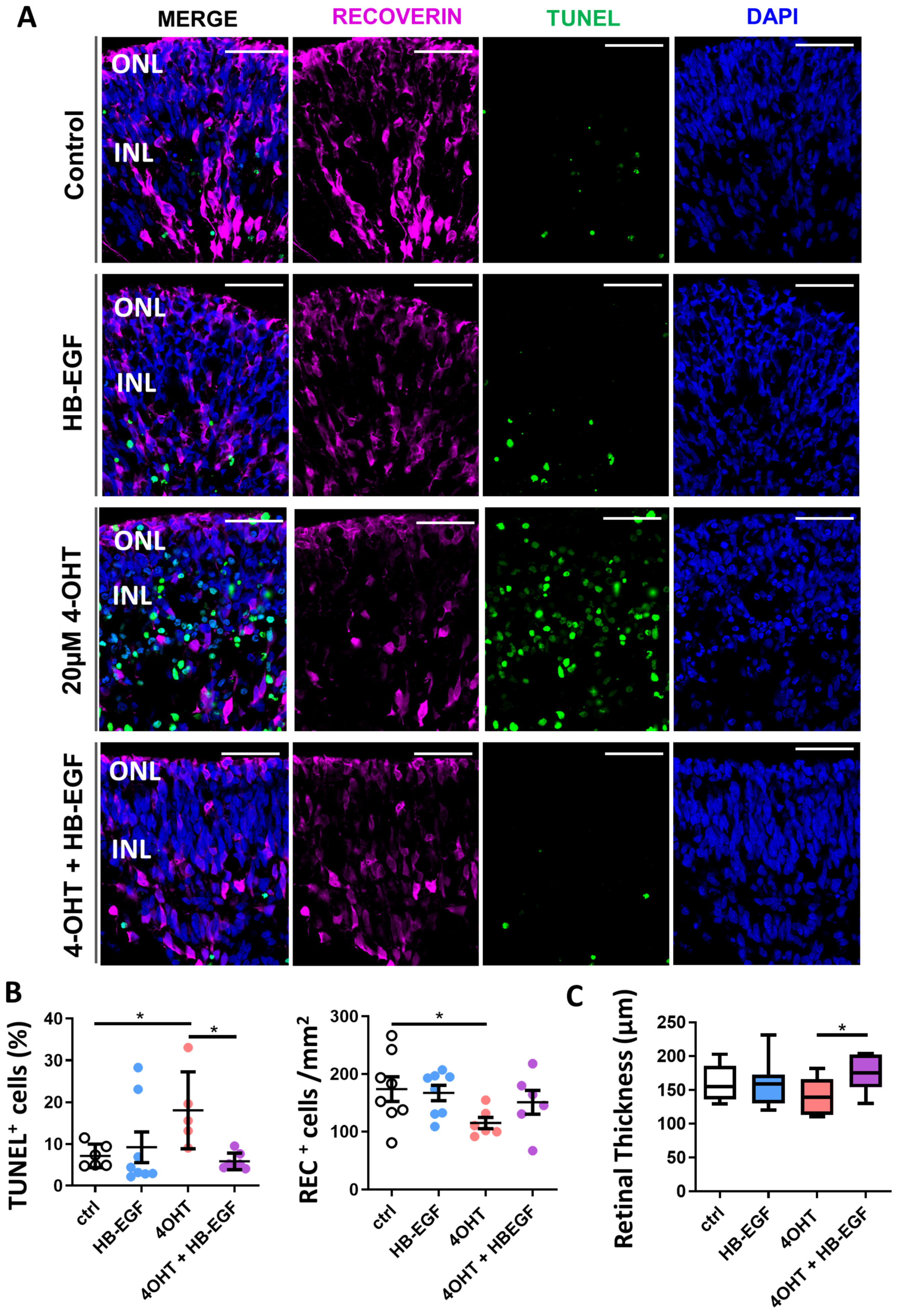
3.4. Partial Restoration of Retinal Organoid Morphology upon Incubation of 4-OHT-Damaged Organoids with HB-EGF
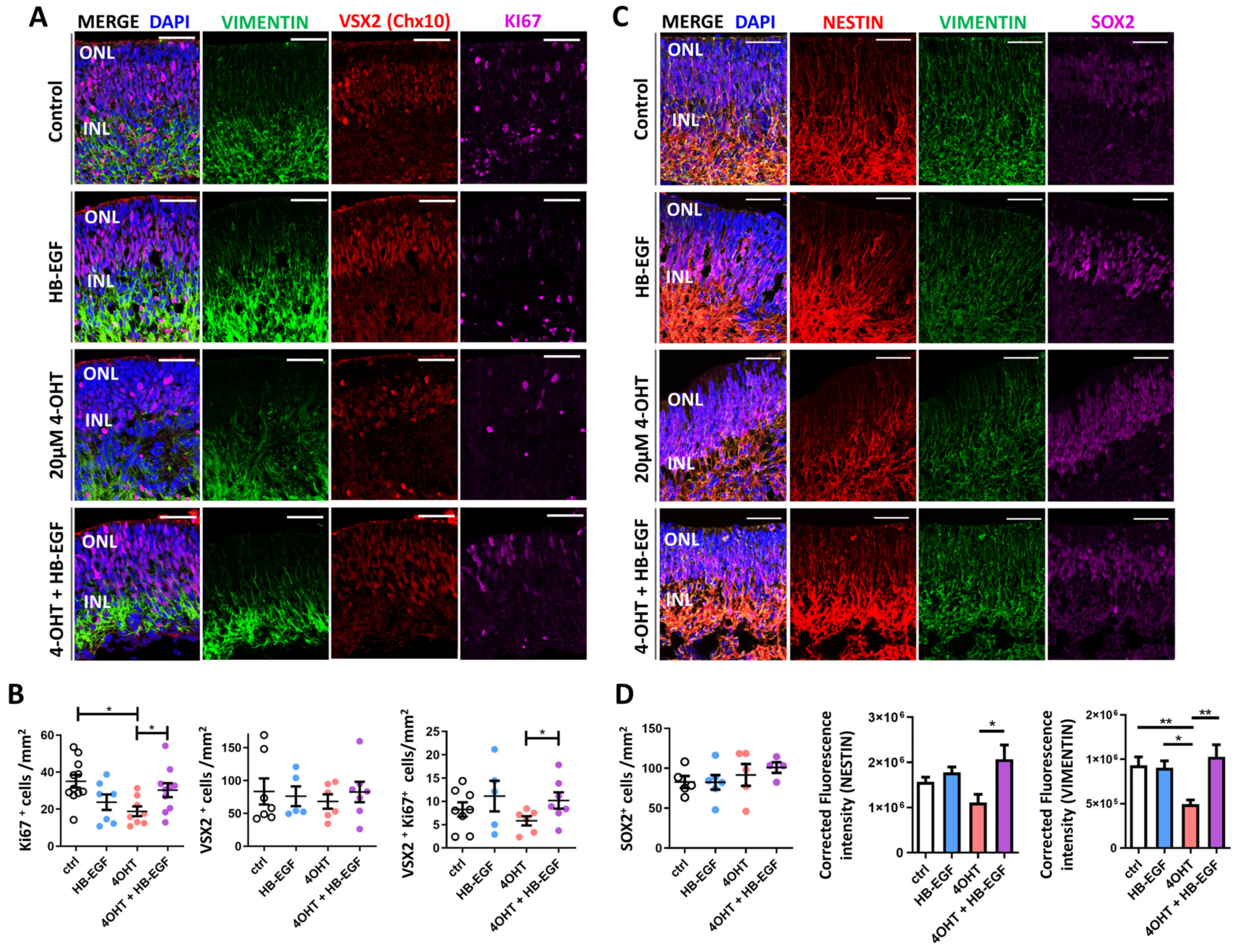
3.5. Müller Glial Cell Responses to 4-OHT and HB-EGF Incubation over 7 Days
3.6. Gene Expression Analysis of Downstream Targets of HB-EGF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, S.; Singhal, S.; Jones, M.F.; Eastlake, K.; Cottrill, P.B.; Jayaram, H.; Limb, G.A. Acquisition of RGC phenotype in human Muller glia with stem cell characteristics is accompanied by upregulation of functional nicotinic acetylcholine receptors. Mol. Vis. 2013, 19, 1925–1936. [Google Scholar] [PubMed]
- Eastlake, K.; Wang, W.; Jayaram, H.; Murray-Dunning, C.; Carr, A.J.F.; Ramsden, C.M.; Vugler, A.; Gore, K.; Clemo, N.; Stewart, M.; et al. Phenotypic and Functional Characterization of Müller Glia Isolated from Induced Pluripotent Stem Cell-Derived Retinal Organoids: Improvement of Retinal Ganglion Cell Function upon Transplantation. Stem Cells Transl. Med. 2019, 8, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Pannicke, T.; Grosche, J.; Francke, M.; Wiedemann, P.; Skatchkov, S.N.; Osborne, N.N.; Reichenbach, A. Müller cells in the healthy and diseased retina. Prog. Retin. Eye Res. 2006, 25, 397–424. [Google Scholar] [CrossRef] [PubMed]
- Yurco, P.; Cameron, D.A. Responses of Müller glia to retinal injury in adult zebrafish. Vision. Res. 2005, 45, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Langhe, R.; Chesneau, A.; Colozza, G.; Hidalgo, M.; Ail, D.; Locker, M.; Perron, M. Müller glial cell reactivation in Xenopus models of retinal degeneration. Glia 2017, 65, 1333–1349. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.J.; Reh, T.A. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat. Neurosci. 2001, 4, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Karl, M.O.; Hayes, S.; Nelson, B.R.; Tan, K.; Buckingham, B.; Reh, T.A. Stimulation of neural regeneration in the mouse retina. Proc. Natl. Acad. Sci. USA 2008, 105, 19508–19513. [Google Scholar] [CrossRef]
- Bhatia, B.; Singhal, S.; Lawrence, J.M.; Khaw, P.T.; Limb, G.A. Distribution of Müller stem cells within the neural retina: Evidence for the existence of a ciliary margin-like zone in the adult human eye. Exp. Eye Res. 2009, 89, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Singhal, S.; Bhatia, B.; Keegan, D.J.; Reh, T.A.; Luthert, P.J.; Khaw, P.T.; Limb, G.A. MIO-M1 cells and similar muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells 2007, 25, 2033–2043. [Google Scholar] [CrossRef]
- Singhal, S.; Bhatia, B.; Jayaram, H.; Becker, S.; Jones, M.F.; Cottrill, P.B.; Khaw, P.T.; Salt, T.E.; Limb, G.A. Human Müller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem Cells Transl. Med. 2012, 1, 188–199. [Google Scholar] [CrossRef]
- Eastlake, K.; Heywood, W.E.; Tracey-White, D.; Aquino, E.; Bliss, E.; Vasta, G.R.; Mills, K.; Khaw, P.T.; Moosajee, M.; Limb, G.A. Comparison of proteomic profiles in the zebrafish retina during experimental degeneration and regeneration. Sci. Rep. 2017, 7, 44601. [Google Scholar] [CrossRef]
- Stanchfield, M.L.; Webster, S.E.; Webster, M.K.; Linn, C.L. Involvement of HB-EGF/Ascl1/Lin28a Genes in Dedifferentiation of Adult Mammalian Müller Glia. Front. Mol. Biosci. 2020, 7, 200. [Google Scholar] [CrossRef] [PubMed]
- Elsaeidi, F.; Macpherson, P.; Mills, E.A.; Jui, J.; Flannery, J.G.; Goldman, D. Notch Suppression Collaborates with Ascl1 and Lin28 to Unleash a Regenerative Response in Fish Retina, But Not in Mice. J. Neurosci. 2018, 38, 2246–2261. [Google Scholar] [CrossRef]
- Gorsuch, R.A.; Lahne, M.; Yarka, C.E.; Petravick, M.E.; Li, J.; Hyde, D.R. Sox2 regulates Muller glia reprogramming and proliferation in the regenerating zebrafish retina via Lin28 and Ascl1a. Exp. Eye Res. 2017, 161, 174–192. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Yokoyama, T.; Takenouchi, T.; Munekata, E. The chemical synthesis and binding affinity to the EGF receptor of the EGF-like domain of heparin-binding EGF-like growth factor (HB-EGF). J. Pept. Sci. 2003, 9, 244–250. [Google Scholar] [CrossRef]
- Wan, J.; Ramachandran, R.; Goldman, D. HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration. Dev. Cell 2012, 22, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Todd, L.; Volkov, L.I.; Zelinka, C.; Squires, N.; Fischer, A.J. Heparin-binding EGF-like growth factor (HB-EGF) stimulates the proliferation of Müller glia-derived progenitor cells in avian and murine retinas. Mol. Cell Neurosci. 2015, 69, 54–64. [Google Scholar] [CrossRef]
- Hollborn, M.; Tenckhoff, S.; Jahn, K.; Iandiev, I.; Biedermann, B.; Schnurrbusch, U.E.; Limb, G.A.; Reichenbach, A.; Wolf, S.; Wiedemann, P.; et al. Changes in retinal gene expression in proliferative vitreoretinopathy: Glial cell expression of HB-EGF. Mol. Vis. 2005, 11, 397–413. [Google Scholar]
- Onishi, A.; Peng, G.-H.; Poth, E.M.; Lee, D.A.; Chen, J.; Alexis, U.; de Melo, J.; Chen, S.; Blackshaw, S. The orphan nuclear hormone receptor ERRbeta controls rod photoreceptor survival. Proc. Natl. Acad. Sci. USA 2010, 107, 11579–11584. [Google Scholar] [CrossRef]
- Ito, S.I.; Onishi, A.; Takahashi, M. Chemically-induced photoreceptor degeneration and protection in mouse iPSC-derived three-dimensional retinal organoids. Stem Cell Res. 2017, 24, 94–101. [Google Scholar] [CrossRef]
- De Sousa, P.; Tye, B.; Bruce, K.; Dand, P.; Russell, G.; Collins, D.; Greenshields, A.; McDonald, K.; Bradburn, H.; Canham, M.; et al. Derivation of the clinical grade human embryonic stem cell line RCe013-A (RC-9). Stem Cell Res. 2016, 17, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Ando, S.; Takata, N.; Kawada, M.; Muguruma, K.; Sekiguchi, K.; Saito, K.; Yonemura, S.; Eiraku, M.; Sasai, Y. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 2012, 10, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.; Jovanovic, K.; Shortall, C.; Ottaviani, D.; Panes, A.B.; Schwarz, N.; Guarascio, R.; Hayes, M.J.; Palfi, A.; Chadderton, N.; et al. Modeling and Rescue of RP2 Retinitis Pigmentosa Using iPSC-Derived Retinal Organoids. Stem Cell Rep. 2020, 15, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D.; Thompson, J.A.; Chen, S.; Huang, Z.; Jennings, L.; McLaren, T.L.; Lamey, T.M.; De Roach, J.N.; Chen, F.K.; et al. Gene correction of the CLN3 c.175G>A variant in patient-derived induced pluripotent stem cells prevents pathological changes in retinal organoids. Mol. Genet. Genom. Med. 2021, 9, e1601. [Google Scholar] [CrossRef] [PubMed]
- Lukovic, D.; Castro, A.A.; Kaya, K.D.; Munezero, D.; Gieser, L.; Davó-Martínez, C.; Corton, M.; Cuenca, N.; Swaroop, A.; Ramamurthy, V.; et al. Retinal Organoids derived from hiPSCs of an AIPL1-LCA Patient Maintain Cytoarchitecture despite Reduced levels of Mutant AIPL1. Sci. Rep. 2020, 10, 5426. [Google Scholar] [CrossRef] [PubMed]
- Capowski, E.E.; Samimi, K.; Mayerl, S.J.; Phillips, M.J.; Pinilla, I.; Howden, S.E.; Saha, J.; Jansen, A.D.; Edwards, K.L.; Jager, L.D.; et al. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development 2019, 146, dev171686. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Nasonkin, I.O. Limitations and Promise of Retinal Tissue from Human Pluripotent Stem Cells for Developing Therapies of Blindness. Front. Cell Neurosci. 2020, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, A.; Bringmann, A. Muller Cells in the Healthy and Diseased Retina, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Bringmann, A.; Wiedemann, P. Müller Glial Cells in Retinal Disease. Ophthalmologica 2012, 227, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Ross, C.T.; Hosseinzadeh, Z. Functional 3-Dimensional Retinal Organoids: Technological Progress and Existing Challenges. Front. Neurosci. 2021, 15, 668857. [Google Scholar] [CrossRef]
- Völkner, M.; Kurth, T.; Schor, J.; Ebner, L.J.A.; Bardtke, L.; Kavak, C.; Hackermüller, J.; Karl, M.O. Mouse Retinal Organoid Growth and Maintenance in Longer-Term Culture. Front. Cell Dev. Biol. 2021, 9, 645704. [Google Scholar] [CrossRef]
- Qiao, H.; Zhao, W.; Guo, M.; Zhu, L.; Chen, T.; Wang, J.; Xu, X.; Zhang, Z.; Wu, Y.; Chen, P. Cerebral Organoids for Modeling of HSV-1-Induced-Amyloid β Associated Neuropathology and Phenotypic Rescue. Int. J. Mol. Sci. 2022, 23, 5981. [Google Scholar] [CrossRef]
- Wahlin, K.J.; Maruotti, J.A.; Sripathi, S.R.; Ball, J.; Angueyra, J.M.; Kim, C.; Grebe, R.; Li, W.; Jones, B.W.; Zack, D.J. Photoreceptor Outer Segment-like Structures in Long Term 3D retinas from human pluripotent stem cells. Sci. Rep. 2017, 7, 766. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.N.H.; Moosajee, M.; Sharif, N.A.; Limb, G.A.; Eastlake, K. Heparin-Binding Epidermal-like Growth Factor (HB-EGF) Reduces Cell Death in an Organoid Model of Retinal Damage. Organoids 2024, 3, 148-164. https://doi.org/10.3390/organoids3030010
Tang MNH, Moosajee M, Sharif NA, Limb GA, Eastlake K. Heparin-Binding Epidermal-like Growth Factor (HB-EGF) Reduces Cell Death in an Organoid Model of Retinal Damage. Organoids. 2024; 3(3):148-164. https://doi.org/10.3390/organoids3030010
Chicago/Turabian StyleTang, Michelle N. H., Mariya Moosajee, Najam A. Sharif, G. Astrid Limb, and Karen Eastlake. 2024. "Heparin-Binding Epidermal-like Growth Factor (HB-EGF) Reduces Cell Death in an Organoid Model of Retinal Damage" Organoids 3, no. 3: 148-164. https://doi.org/10.3390/organoids3030010
APA StyleTang, M. N. H., Moosajee, M., Sharif, N. A., Limb, G. A., & Eastlake, K. (2024). Heparin-Binding Epidermal-like Growth Factor (HB-EGF) Reduces Cell Death in an Organoid Model of Retinal Damage. Organoids, 3(3), 148-164. https://doi.org/10.3390/organoids3030010








