Novel Techniques for Mapping DNA Damage and Repair in the Brain
Abstract
1. Introduction
2. DNA Damage Detection Strategies
2.1. Existing Strategies for the Detection of DNA Lesions within the Brain
| Methods | Advantages | Limitations | Refs |
|---|---|---|---|
| INDIRECT | |||
| Immunoblotting | - Multiple DDR and DNA repair proteins - Compatible with surrogate blood markers | - Specificity of antibodies for protein targets | [41,48,49,50,51,52,53] |
| Immunohistochemical | - DDR and DNA repair proteins - Compatible with surrogate tissues or cells | - Specificity of antibodies for targets of interest - Differences in tissue processing methods for detection - Surrogate markers may not reflect 1:1 events in the brain | [1,3,36,37,38,39,40,41,42,43,44,45,46,48,49] |
| ChIP sequencing methods | - Robust capture methods and established techniques - Compatible with DDR proteins or specific DNA repair proteins or pathways | - Position of lesions less precise - Need for high-specificity antibodies - Need for adequate control for evaluating DNA versus DNA damage interactions - Surrogate markers may not reflect 1:1 events in the brain - Significant input of material and limits of detection for low-prevalence events | [1,2] |
| Fluorescent reporters | - Assessment of DNA repair capacity - Exploit specific repair mechanism (PRISM) | - Artificially induces DNA damage (I-SceI) - Post-event monitoring for genotoxic exposures | [54,55,56,57,58] |
| PCR | - Mitochondrial DNA damage assessment - Compatible with surrogate blood markers | - Specific DNA lesions cannot be identified - Surrogate markers may not reflect 1:1 events in the brain | [59,60,61,62,63,64,65,66,67,68,69,70,71] |
| DIRECT | |||
| Immunohistochemical/ Immunofluorescence | - Identify specific lesions | - Specificity of antibodies for DNA lesions - Unwinding of DNA to detect lesions in situ | [61,72,73,74,75,76,77,78,79,80,81,82,83,84] |
| Mass Spectrometry | - Larger number of DNA lesions detected from oxidative to acrolein | - A significant amount of brain tissue for DNA isolation - Need for isotopic standards | [78,85,86,87,88,89,90] |
| Comet Assay | - Strand breaks and abasic sites - Ability for single nucleoid analysis - Specific DNA lesions are detected when combined with DNA repair enzymes or treatment strategies | - Mixture of lesions detected - Crosslinks and DNA protein crosslinks need specific protocols for detection - Not compatible with formalin-fixed samples | [87,88,91,92,93,94,95,96] |
| HPLC | - Lesion detection within genomic DNA - Lesion detection in mitochondrial DNA with specific isolation | - Significant amount of brain tissue genomic or mitochondrial DNA - May require specialty columns or enhanced separation methods - May require standards | [78,97,98,99] |
| Adapter or lesion-specific sequencing techniques | - Robust capture strategies using click or biotin chemistry - Specific labeling of DNA lesion targets through end breaks, lesion sites, or synthesis -Modifiable and adaptable protocols for analysis integration with single cell or other approaches | - Need for high specific antibodies for specific DNA lesions -Enzyme-mediated methods detect lesion classes based on specific enzymes used -Variable amount of DNA may be needed based on the desired analysis method | [100] |
| Enzyme-mediated labeling or sequencing strategies | - DNA repair enzymes allow specific lesion class detection -Modifiable and adaptable protocols compatible with spatial imaging or transcriptomics - Compatible with isolated DNA, cells, or frozen or formalin-fixed tissues - Single nuclei analysis possible | - Lesion classes may be large depending on the enzymes used - Optimization for specific tissue and cell types may be required | [101,102] |
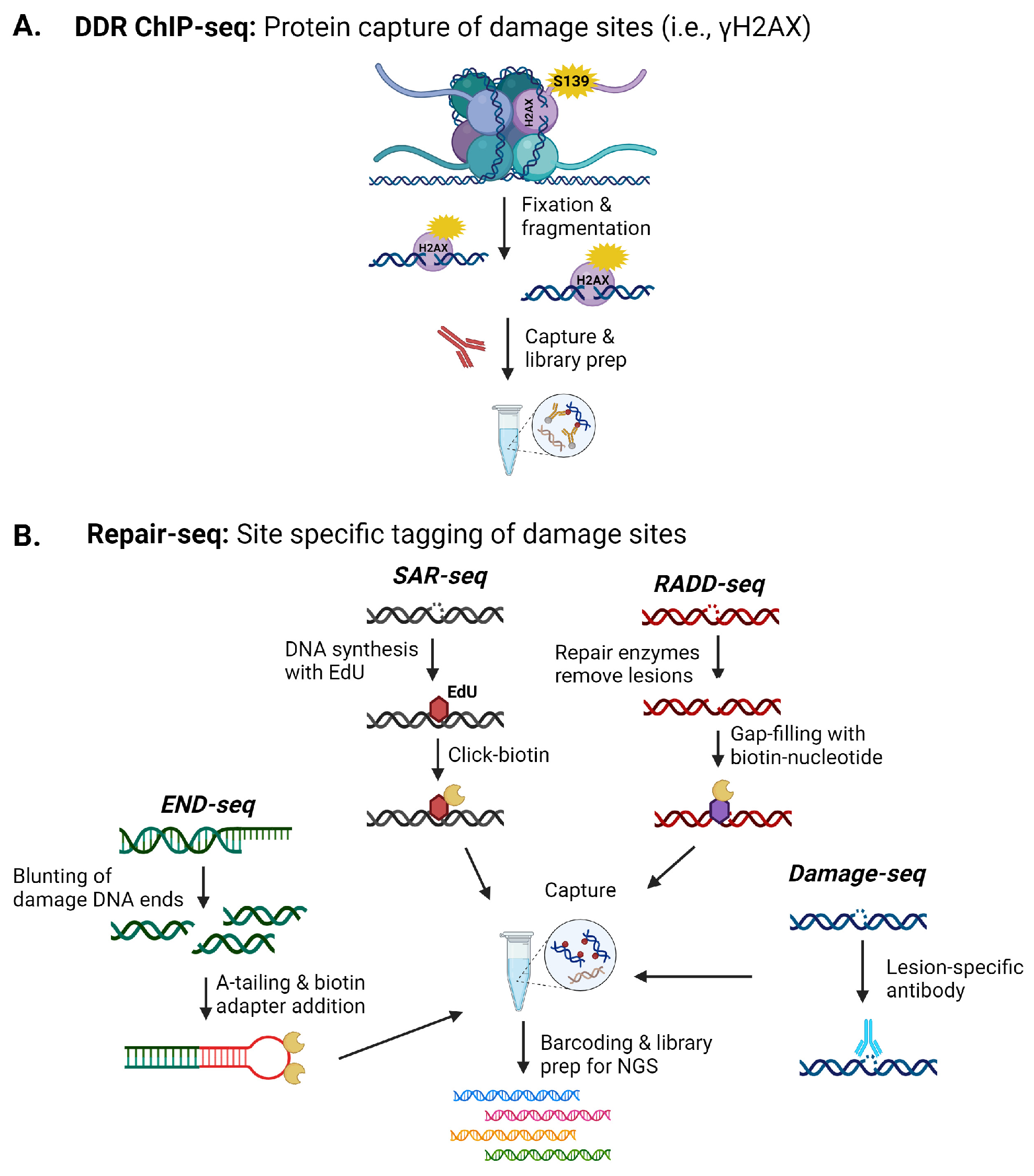
2.2. Sequencing-Based Methods
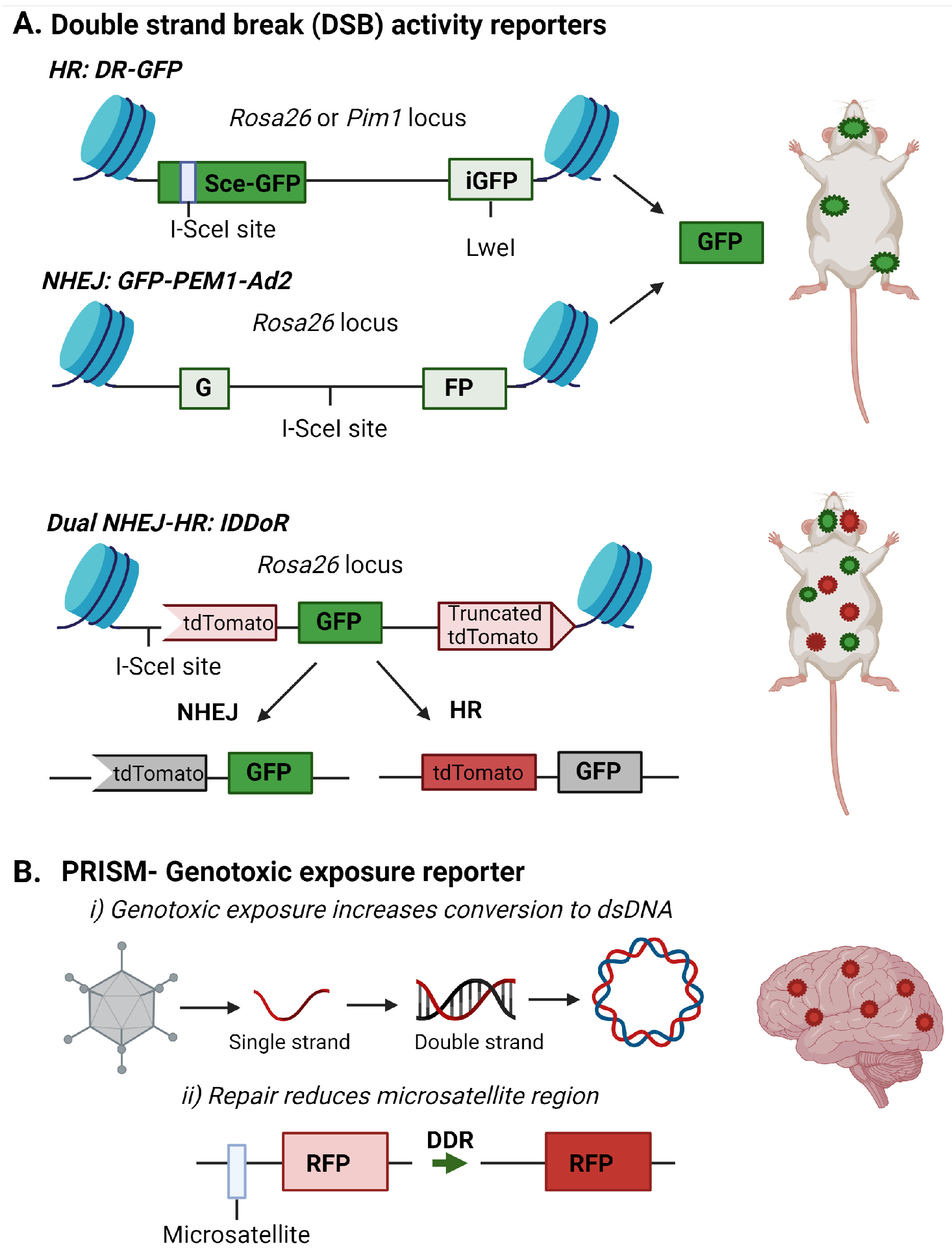
2.3. Fluorescent Reporters for DNA Damage
2.4. Mitochondrial DNA Damage
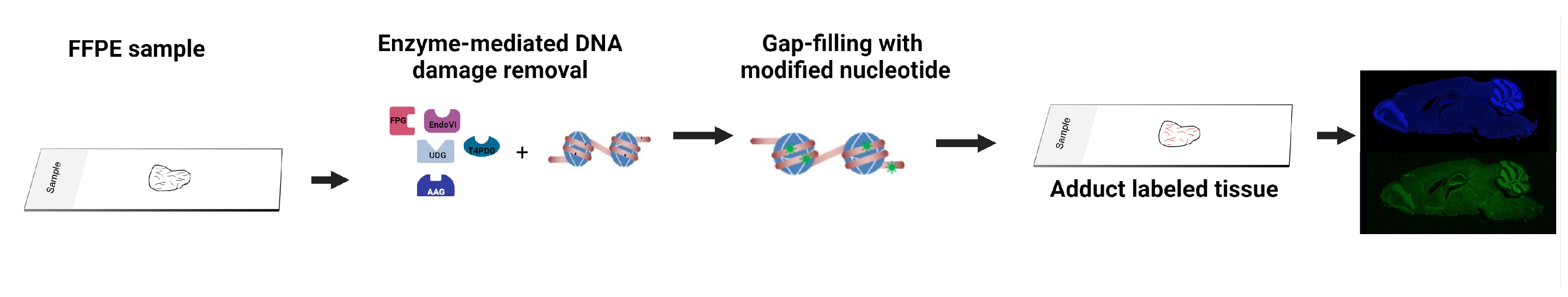
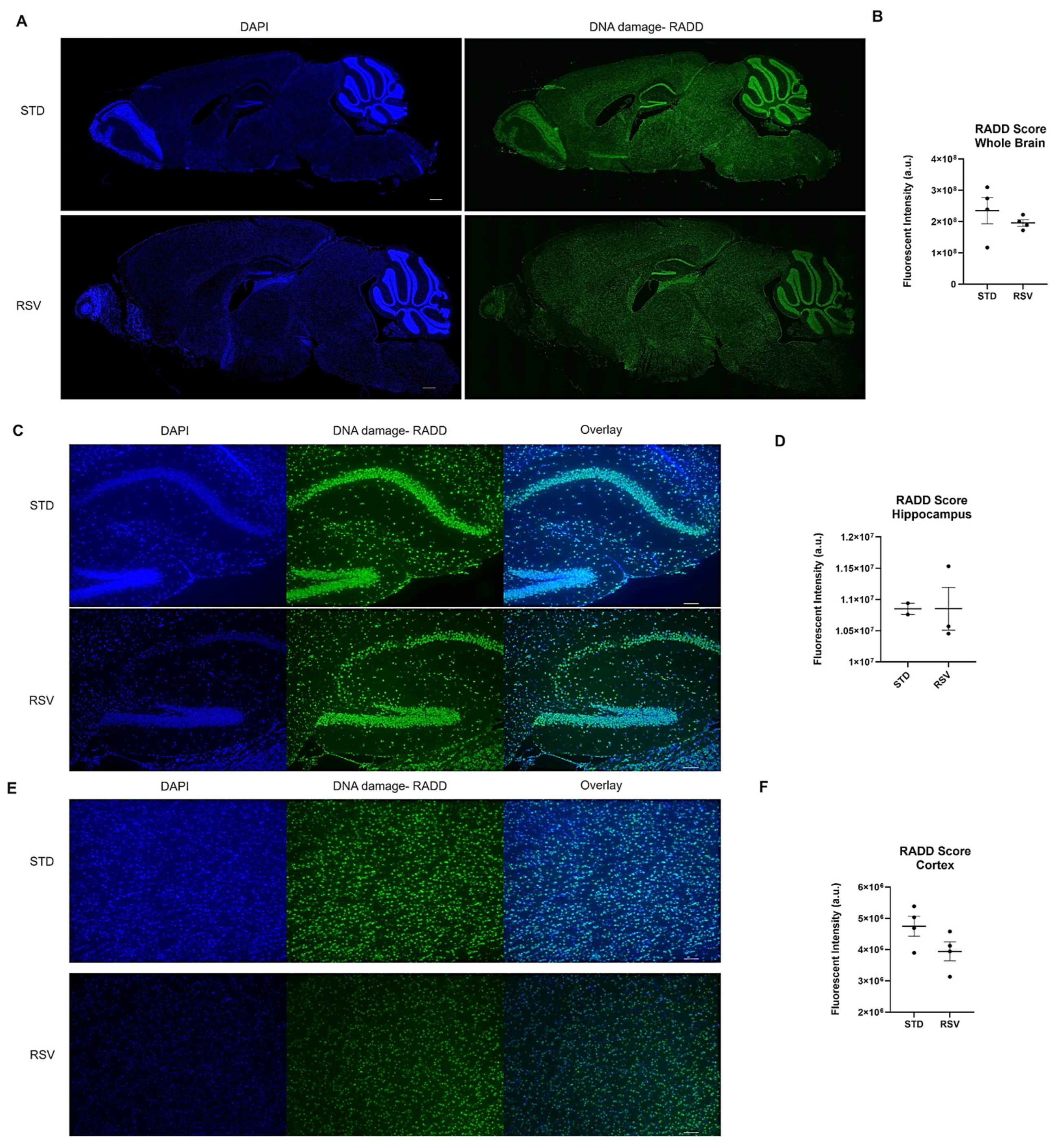
2.5. Enzyme-Mediated DNA Damage Detection Assay
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Madabhushi, R.; Gao, F.; Pfenning, A.R.; Pan, L.; Yamakawa, S.; Seo, J.; Rueda, R.; Phan, T.X.; Yamakawa, H.; Pao, P.C.; et al. Activity-Induced DNA Breaks Govern the Expression of Neuronal Early-Response Genes. Cell 2015, 161, 1592–1605. [Google Scholar] [CrossRef]
- Stott, R.T.; Kritsky, O.; Tsai, L.H. Profiling DNA break sites and transcriptional changes in response to contextual fear learning. PLoS ONE 2021, 16, e0249691. [Google Scholar] [CrossRef]
- Suberbielle, E.; Sanchez, P.E.; Kravitz, A.V.; Wang, X.; Ho, K.; Eilertson, K.; Devidze, N.; Kreitzer, A.C.; Mucke, L. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-beta. Nat. Neurosci. 2013, 16, 613–621. [Google Scholar] [CrossRef]
- Suberbielle, E.; Djukic, B.; Evans, M.; Kim, D.H.; Taneja, P.; Wang, X.; Finucane, M.; Knox, J.; Ho, K.; Devidze, N.; et al. DNA repair factor BRCA1 depletion occurs in Alzheimer brains and impairs cognitive function in mice. Nat. Commun. 2015, 6, 8897. [Google Scholar] [CrossRef]
- Weber Boutros, S.; Unni, V.K.; Raber, J. An Adaptive Role for DNA Double-Strand Breaks in Hippocampus-Dependent Learning and Memory. Int. J. Mol. Sci. 2022, 23, 8352. [Google Scholar] [CrossRef]
- Hylin, M.J.; Orsi, S.A.; Moore, A.N.; Dash, P.K. Disruption of the perineuronal net in the hippocampus or medial prefrontal cortex impairs fear conditioning. Learn. Mem. 2013, 20, 267–273. [Google Scholar] [CrossRef]
- Slaker, M.; Churchill, L.; Todd, R.P.; Blacktop, J.M.; Zuloaga, D.G.; Raber, J.; Darling, R.A.; Brown, T.E.; Sorg, B.A. Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory. J. Neurosci. 2015, 35, 4190–4202. [Google Scholar] [CrossRef]
- Welch, G.; Tsai, L.H. Mechanisms of DNA damage-mediated neurotoxicity in neurodegenerative disease. EMBO Rep. 2022, 23, e54217. [Google Scholar] [CrossRef]
- Maynard, S.; Fang, E.F.; Scheibye-Knudsen, M.; Croteau, D.L.; Bohr, V.A. DNA Damage, DNA Repair, Aging, and Neurodegeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a025130. [Google Scholar] [CrossRef]
- Brobbey, C.; Liu, L.; Yin, S.; Gan, W. The Role of Protein Arginine Methyltransferases in DNA Damage Response. Int. J. Mol. Sci. 2022, 23, 9780. [Google Scholar] [CrossRef]
- Lukas, J.; Lukas, C.; Bartek, J. More than just a focus: The chromatin response to DNA damage and its role in genome integrity maintenance. Nat. Cell Biol. 2011, 13, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Miller, K.M. Histone methylation and the DNA damage response. Mutat. Res. Rev. Mutat. Res. 2019, 780, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Lee, S.Y.; Miller, K.M. Preserving genome integrity and function: The DNA damage response and histone modifications. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 208–241. [Google Scholar] [CrossRef]
- Tubbs, A.; Nussenzweig, A. Endogenous DNA Damage as a Source of Genomic Instability in Cancer. Cell 2017, 168, 644–656. [Google Scholar] [CrossRef]
- McKinnon, P.J. Genome integrity and disease prevention in the nervous system. Genes Dev. 2017, 31, 1180–1194. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.K.; Vemuganti, R. DNA damage and repair following traumatic brain injury. Neurobiol. Dis. 2021, 147, 105143. [Google Scholar] [CrossRef] [PubMed]
- Schwab, N.; Leung, E.; Hazrati, L.N. Cellular Senescence in Traumatic Brain Injury: Evidence and Perspectives. Front. Aging Neurosci. 2021, 13, 742632. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hu, X.; Gan, Y.; Gao, Y.; Liang, W.; Chen, J. Mechanistic insight into DNA damage and repair in ischemic stroke: Exploiting the base excision repair pathway as a model of neuroprotection. Antioxid. Redox Signal. 2011, 14, 1905–1918. [Google Scholar] [CrossRef] [PubMed]
- Collaborators, G.B.D.N. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar]
- Zhang, R.; Liu, H.; Pu, L.; Zhao, T.; Zhang, S.; Han, K.; Han, L. Global Burden of Ischemic Stroke in Young Adults in 204 Countries and Territories. Neurology 2023, 100, e422–e434. [Google Scholar] [CrossRef]
- Ding, C.; Wu, Y.; Chen, X.; Chen, Y.; Wu, Z.; Lin, Z.; Kang, D.; Fang, W.; Chen, F. Global, regional, and national burden and attributable risk factors of neurological disorders: The Global Burden of Disease study 1990–2019. Front. Public Health 2022, 10, 952161. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Neven, J.; Issayama, L.K.; Dewachter, I.; Wilson, D.M., 3rd. Genomic stress and impaired DNA repair in Alzheimer disease. DNA Repair 2024, 139, 103678. [Google Scholar] [CrossRef] [PubMed]
- Shreeya, T.; Ansari, M.S.; Kumar, P.; Saifi, M.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I. Senescence: A DNA damage response and its role in aging and Neurodegenerative Diseases. Front. Aging 2023, 4, 1292053. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Li, Y.L.; Pu, J.L.; Zhang, B.R. DNA Damage-Mediated Neurotoxicity in Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 6313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, H.S.; Wang, X.; Dumont, A.S.; Liu, Q. Cellular senescence, DNA damage, and neuroinflammation in the aging brain. Trends Neurosci. 2024, 47, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Huang, M.; Gunewardena, S.; Haeri, M.; Swerdlow, R.H.; Wang, N. Landscape of Double-Stranded DNA Breaks in Postmortem Brains from Alzheimer’s Disease and Non-Demented Individuals. J. Alzheimers Dis. 2023, 94, 519–535. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.U.; Tufan, T.; Wang, Y.; Hill, C.; Zhu, M.Y. DNA Damage in Major Psychiatric Diseases. Neurotox. Res. 2016, 30, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Shiwaku, H.; Okazawa, H. Impaired DNA damage repair as a common feature of neurodegenerative diseases and psychiatric disorders. Curr. Mol. Med. 2015, 15, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Shishido, R.; Kunii, Y.; Hino, M.; Izumi, R.; Nagaoka, A.; Hayashi, H.; Kakita, A.; Tomita, H.; Yabe, H. Evidence for increased DNA damage repair in the postmortem brain of the high stress-response group of schizophrenia. Front. Psychiatry 2023, 14, 1183696. [Google Scholar] [CrossRef]
- Mueller, F.S.; Amport, R.; Notter, T.; Schalbetter, S.M.; Lin, H.Y.; Garajova, Z.; Amini, P.; Weber-Stadlbauer, U.; Markkanen, E. Deficient DNA base-excision repair in the forebrain leads to a sex-specific anxiety-like phenotype in mice. BMC Biol. 2022, 20, 170. [Google Scholar] [CrossRef] [PubMed]
- Goh, X.X.; Tang, P.Y.; Tee, S.F. 8-Hydroxy-2′-Deoxyguanosine and Reactive Oxygen Species as Biomarkers of Oxidative Stress in Mental Illnesses: A Meta-Analysis. Psychiatry Investig. 2021, 18, 603–618. [Google Scholar] [CrossRef]
- Czarny, P.; Bialek, K.; Ziolkowska, S.; Strycharz, J.; Sliwinski, T. DNA damage and repair in neuropsychiatric disorders. What do we know and what are the future perspectives? Mutagenesis 2020, 35, 79–106. [Google Scholar] [CrossRef] [PubMed]
- Weber Boutros, S.; Krenik, D.; Holden, S.; Unni, V.; Raberj, J. Common cancer treatments targeting DNA double strand breaks affect long-term memory and relate to immediate early gene expression in a sex-dependent manner. Oncotarget 2022, 13, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Weber Boutros, S.; Zimmerman, B.; Nagy, S.; Lee, J.; Perez, R.; Raber, J. Amifostine (WR-2721) Mitigates Cognitive Injury Induced by Heavy Ion Radiation in Male Mice and Alters Behavior and Brain Connectivity. Front. Physiol. 2021, 12, 770502. [Google Scholar]
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J.H.J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Sedelnikova, O.A.; Horikawa, I.; Zimonjic, D.B.; Popescu, N.C.; Bonner, W.M.; Barrett, J.C. Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat. Cell Biol. 2004, 6, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Thadathil, N.; Delotterie, D.F.; Xiao, J.; Hori, R.; McDonald, M.P.; Khan, M.M. DNA Double-Strand Break Accumulation in Alzheimer’s Disease: Evidence from Experimental Models and Postmortem Human Brains. Mol. Neurobiol. 2021, 58, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, N.M.; Evans, M.D.; Mao, W.; Nana, A.L.; Seeley, W.W.; Adame, A.; Rissman, R.A.; Masliah, E.; Mucke, L. Early neuronal accumulation of DNA double strand breaks in Alzheimer’s disease. Acta Neuropathol. Commun. 2019, 7, 77. [Google Scholar] [CrossRef]
- Myung, N.H.; Zhu, X.; Kruman, I.I.; Castellani, R.J.; Petersen, R.B.; Siedlak, S.L.; Perry, G.; Smith, M.A.; Lee, H.G. Evidence of DNA damage in Alzheimer disease: Phosphorylation of histone H2AX in astrocytes. Age 2008, 30, 209–215. [Google Scholar] [CrossRef]
- Milanese, C.; Cerri, S.; Ulusoy, A.; Gornati, S.V.; Plat, A.; Gabriels, S.; Blandini, F.; Di Monte, D.A.; Hoeijmakers, J.H.; Mastroberardino, P.G. Activation of the DNA damage response in vivo in synucleinopathy models of Parkinson’s disease. Cell Death Dis. 2018, 9, 818. [Google Scholar] [CrossRef] [PubMed]
- Camins, A.; Pizarro, J.G.; Alvira, D.; Gutierrez-Cuesta, J.; de la Torre, A.V.; Folch, J.; Sureda, F.X.; Verdaguer, E.; Junyent, F.; Jordán, J.; et al. Activation of ataxia telangiectasia muted under experimental models and human Parkinson’s disease. Cell. Mol. Life Sci. 2010, 67, 3865–3882. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.H.; Mattis, V.B.; Wang, N.; Al-Ramahi, I.; van den Berg, N.; Fratantoni, S.A.; Waldvogel, H.; Greiner, E.; Osmand, A.; Elzein, K.; et al. Targeting ATM ameliorates mutant Huntingtin toxicity in cell and animal models of Huntington’s disease. Sci. Transl. Med. 2014, 6, 268ra178. [Google Scholar] [CrossRef] [PubMed]
- Jeon, G.S.; Kim, K.Y.; Hwang, Y.J.; Jung, M.K.; An, S.; Ouchi, M.; Ouchi, T.; Kowall, N.; Lee, J.; Ryu, H. Deregulation of BRCA1 leads to impaired spatiotemporal dynamics of gamma-H2AX and DNA damage responses in Huntington’s disease. Mol. Neurobiol. 2012, 45, 550–563. [Google Scholar] [CrossRef]
- Crowe, S.L.; Movsesyan, V.A.; Jorgensen, T.J.; Kondratyev, A. Rapid phosphorylation of histone H2A.X following ionotropic glutamate receptor activation. Eur. J. Neurosci. 2006, 23, 2351–2361. [Google Scholar] [CrossRef] [PubMed]
- Crowe, S.L.; Tsukerman, S.; Gale, K.; Jorgensen, T.J.; Kondratyev, A.D. Phosphorylation of histone H2A.X as an early marker of neuronal endangerment following seizures in the adult rat brain. J. Neurosci. 2011, 31, 7648–7656. [Google Scholar] [CrossRef] [PubMed]
- Castaldo, I.; De Rosa, M.; Romano, A.; Zuchegna, C.; Squitieri, F.; Mechelli, R.; Peluso, S.; Borrelli, C.; Del Mondo, A.; Salvatore, E.; et al. DNA damage signatures in peripheral blood cells as biomarkers in prodromal huntington disease. Ann. Neurol. 2019, 85, 296–301. [Google Scholar] [CrossRef]
- Yu, H.; Harrison, F.E.; Xia, F. Altered DNA repair; an early pathogenic pathway in Alzheimer’s disease and obesity. Sci. Rep. 2018, 8, 5600. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cohen, M.L.; Lerner, A.J.; Yang, Y.; Herrup, K. DNA damage and cell cycle events implicate cerebellar dentate nucleus neurons as targets of Alzheimer’s disease. Mol. Neurodegener. 2010, 5, 60. [Google Scholar] [CrossRef]
- Mao, G.; Pan, X.; Zhu, B.B.; Zhang, Y.; Yuan, F.; Huang, J.; Lovell, M.A.; Lee, M.P.; Markesbery, W.R.; Li, G.M.; et al. Identification and characterization of OGG1 mutations in patients with Alzheimer’s disease. Nucleic Acids Res. 2007, 35, 2759–2766. [Google Scholar] [CrossRef]
- Lovell, M.A.; Xie, C.; Markesbery, W.R. Decreased base excision repair and increased helicase activity in Alzheimer’s disease brain. Brain Res. 2000, 855, 116–123. [Google Scholar] [CrossRef]
- Hegde, M.L.; Gupta, V.B.; Anitha, M.; Harikrishna, T.; Shankar, S.K.; Muthane, U.; Subba Rao, K.; Jagannatha Rao, K.S. Studies on genomic DNA topology and stability in brain regions of Parkinson’s disease. Arch. Biochem. Biophys. 2006, 449, 143–156. [Google Scholar] [CrossRef]
- Shackelford, D.A. DNA end joining activity is reduced in Alzheimer’s disease. Neurobiol. Aging 2006, 27, 596–605. [Google Scholar] [CrossRef]
- Kass, E.M.; Helgadottir, H.R.; Chen, C.C.; Barbera, M.; Wang, R.; Westermark, U.K.; Ludwig, T.; Moynahan, M.E.; Jasin, M. Double-strand break repair by homologous recombination in primary mouse somatic cells requires BRCA1 but not the ATM kinase. Proc. Natl. Acad. Sci. USA 2013, 110, 5564–5569. [Google Scholar] [CrossRef] [PubMed]
- Kass, E.M.; Lim, P.X.; Helgadottir, H.R.; Moynahan, M.E.; Jasin, M. Robust homology-directed repair within mouse mammary tissue is not specifically affected by Brca2 mutation. Nat. Commun. 2016, 7, 13241. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Mao, Z.; Tian, X.; Spencer, B.; Seluanov, A.; Gorbunova, V. Knock-in reporter mice demonstrate that DNA repair by non-homologous end joining declines with age. PLoS Genet. 2014, 10, e1004511. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, Z.; Chen, Z.; Jiang, Y.; Mao, Z. IDDoR: A novel reporter mouse system for simultaneous and quantitative in vivo analysis of both DNA double-strand break repair pathways. Protein Cell 2023, 14, 369–375. [Google Scholar] [CrossRef]
- El-Saadi, M.W.; Tian, X.; Grames, M.; Ren, M.; Keys, K.; Li, H.; Knott, E.; Yin, H.; Huang, S.; Lu, X.H. Tracing brain genotoxic stress in Parkinson’s disease with a novel single-cell genetic sensor. Sci. Adv. 2022, 8, eabd1700. [Google Scholar] [CrossRef]
- Bazzani, V.; Equisoain Redin, M.; McHale, J.; Perrone, L.; Vascotto, C. Mitochondrial DNA Repair in Neurodegenerative Diseases and Ageing. Int. J. Mol. Sci. 2022, 23, 11391. [Google Scholar] [CrossRef] [PubMed]
- Hudson, E.K.; Hogue, B.A.; Souza-Pinto, N.C.; Croteau, D.L.; Anson, R.M.; Bohr, V.A.; Hansford, R.G. Age-associated change in mitochondrial DNA damage. Free Radical Res. 1998, 29, 573–579. [Google Scholar] [CrossRef]
- Sanders, L.H.; McCoy, J.; Hu, X.; Mastroberardino, P.G.; Dickinson, B.C.; Chang, C.J.; Chu, C.T.; Van Houten, B.; Greenamyre, J.T. Mitochondrial DNA damage: Molecular marker of vulnerable nigral neurons in Parkinson’s disease. Neurobiol. Dis. 2014, 70, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.; Krishnan, K.J.; Morris, C.M.; Taylor, G.A.; Reeve, A.K.; Perry, R.H.; Jaros, E.; Hersheson, J.S.; Betts, J.; Klopstock, T.; et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 2006, 38, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Becanovic, K.; Asghar, M.; Gadawska, I.; Sachdeva, S.; Walker, D.; Lazarowski, E.R.; Franciosi, S.; Park, K.H.J.; Cote, H.C.F.; Leavitt, B.R. Age-related mitochondrial alterations in brain and skeletal muscle of the YAC128 model of Huntington disease. NPJ Aging Mech. Dis. 2021, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Bulstrode, H.; Nicoll, J.A.; Hudson, G.; Chinnery, P.F.; Di Pietro, V.; Belli, A. Mitochondrial DNA and traumatic brain injury. Ann. Neurol. 2014, 75, 186–195. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.P.; Horsburgh, K.J.; Graham, D.I.; Nicoll, J.A. Mitochondrial DNA deletions in acute brain injury. Neuroreport 1999, 10, 1875–1878. [Google Scholar] [CrossRef] [PubMed]
- Gureev, A.P.; Shaforostova, E.A.; Starkov, A.A.; Popov, V.N. Simplified qPCR method for detecting excessive mtDNA damage induced by exogenous factors. Toxicology 2017, 382, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Delbarba, A.; Abate, G.; Prandelli, C.; Marziano, M.; Buizza, L.; Arce Varas, N.; Novelli, A.; Cuetos, F.; Martinez, C.; Lanni, C.; et al. Mitochondrial Alterations in Peripheral Mononuclear Blood Cells from Alzheimer’s Disease and Mild Cognitive Impairment Patients. Oxid. Med. Cell Longev. 2016, 2016, 5923938. [Google Scholar] [CrossRef]
- Reid, D.M.; Barber, R.C.; Jones, H.P.; Thorpe, R.J.; Sun, J.; Zhou, Z.; Phillips, N.R. Integrative blood-based characterization of oxidative mitochondrial DNA damage variants implicates Mexican American’s metabolic risk for developing Alzheimer’s disease. Sci. Rep. 2023, 13, 14765. [Google Scholar] [CrossRef] [PubMed]
- Kilbaugh, T.J.; Lvova, M.; Karlsson, M.; Zhang, Z.; Leipzig, J.; Wallace, D.C.; Margulies, S.S. Peripheral Blood Mitochondrial DNA as a Biomarker of Cerebral Mitochondrial Dysfunction following Traumatic Brain Injury in a Porcine Model. PLoS ONE 2015, 10, e0130927. [Google Scholar] [CrossRef]
- Al-Kafaji, G.; Bakheit, H.F.; Alharbi, M.A.; Farahat, A.A.; Jailani, M.; Ebrahin, B.H.; Bakhiet, M. Mitochondrial DNA Copy Number in Peripheral Blood as a Potential Non-invasive Biomarker for Multiple Sclerosis. Neuromol. Med. 2020, 22, 304–313. [Google Scholar] [CrossRef]
- Qi, R.; Sammler, E.; Gonzalez-Hunt, C.P.; Barraza, I.; Pena, N.; Rouanet, J.P.; Naaldijk, Y.; Goodson, S.; Fuzzati, M.; Blandini, F.; et al. A blood-based marker of mitochondrial DNA damage in Parkinson’s disease. Sci. Transl. Med. 2023, 15, eabo1557. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.W.; Jeong, Y.E.; Wong, M.; Martin, L.J. DNA damage accumulates and responses are engaged in human ALS brain and spinal motor neurons and DNA repair is activatable in iPSC-derived motor neurons with SOD1 mutations. Acta Neuropathol. Commun. 2020, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Li, W.; Zhang, F.; Sun, F.Y.; Nagayama, T.; O’Horo, C.; Chen, J. Inducible repair of oxidative DNA lesions in the rat brain after transient focal ischemia and reperfusion. J. Cereb. Blood Flow Metab. 2003, 23, 1324–1339. [Google Scholar] [CrossRef] [PubMed]
- Pao, P.C.; Patnaik, D.; Watson, L.A.; Gao, F.; Pan, L.; Wang, J.; Adaikkan, C.; Penney, J.; Cam, H.P.; Huang, W.C.; et al. HDAC1 modulates OGG1-initiated oxidative DNA damage repair in the aging brain and Alzheimer’s disease. Nat. Commun. 2020, 11, 2484. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.; Gan, W.; Shi, F.; Hu, G.X.; Chen, L.G.; Hayakawa, H.; Sekiguchi, M.; Cai, J.P. Age-dependent accumulation of 8-oxoguanine in the DNA and RNA in various rat tissues. Oxid. Med. Cell Longev. 2013, 2013, 303181. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Løhr, M.; Folkmann, J.K.; Mikkelsen, L.; Loft, S. Aging and oxidatively damaged nuclear DNA in animal organs. Free Radic. Biol. Med. 2010, 48, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Leon, J.; Sakumi, K.; Abolhassani, N.; Sheng, Z.; Tsuchimoto, D.; LaFerla, F.M.; Nakabeppu, Y. MTH1 and OGG1 maintain a low level of 8-oxoguanine in Alzheimer’s brain, and prevent the progression of Alzheimer’s pathogenesis. Sci. Rep. 2021, 11, 5819. [Google Scholar] [CrossRef]
- Gabbita, S.P.; Lovell, M.A.; Markesbery, W.R. Increased nuclear DNA oxidation in the brain in Alzheimer’s disease. J. Neurochem. 1998, 71, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Shigenaga, M.K.; Gimeno, C.J.; Ames, B.N. Urinary 8-hydroxy-2′-deoxyguanosine as a biological marker of in vivo oxidative DNA damage. Proc. Natl. Acad. Sci. USA 1989, 86, 9697–9701. [Google Scholar] [CrossRef]
- Zabel, M.; Nackenoff, A.; Kirsch, W.M.; Harrison, F.E.; Perry, G.; Schrag, M. Markers of oxidative damage to lipids, nucleic acids and proteins and antioxidant enzymes activities in Alzheimer’s disease brain: A meta-analysis in human pathological specimens. Free Radic. Biol. Med. 2018, 115, 351–360. [Google Scholar] [CrossRef]
- Shimura-Miura, H.; Hattori, N.; Kang, D.; Miyako, K.-I.; Nakabeppu, Y.; Mizuno, Y. Increased 8-oxo-dGTPase in the mitochondria of substantia nigral neurons in Parkinson’s disease. Ann. Neurol. 1999, 46, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Perry, G.; Smith, M.A.; Robertson, D.; Olson, S.J.; Graham, D.G.; Montine, T.J. Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am. J. Pathol. 1999, 154, 1423–1429. [Google Scholar] [CrossRef] [PubMed]
- Shirendeb, U.; Reddy, A.P.; Manczak, M.; Calkins, M.J.; Mao, P.; Tagle, D.A.; Hemachandra Reddy, P. Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington’s disease: Implications for selective neuronal damage. Hum. Mol. Genet. 2011, 20, 1438–1455. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, R.O.; Sandouka, S.; Saadi, A.; Kovac, S.; Shekh-Ahmad, T. Sestrin 3 promotes oxidative stress primarily in neurons following epileptic seizures in rats. Neuropharmacology 2023, 238, 109670. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Gabbita, S.P.; Markesbery, W.R. Increased DNA oxidation and decreased levels of repair products in Alzheimer’s disease ventricular CSF. J. Neurochem. 1999, 72, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, E.; Sardas, S.; Aslan, S.; Isik, E.; Esat Karakaya, A. Detection of oxidative DNA damage in lymphocytes of patients with Alzheimer’s disease. Biomarkers 2004, 9, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim Fouad, G.; Ahmed, K.A. Neuroprotective Potential of Berberine Against Doxorubicin-Induced Toxicity in Rat’s Brain. Neurochem. Res. 2021, 46, 3247–3263. [Google Scholar] [CrossRef]
- Krynetskiy, E.; Krynetskaia, N.; Rihawi, D.; Wieczerzak, K.; Ciummo, V.; Walker, E. Establishing a model for assessing DNA damage in murine brain cells as a molecular marker of chemotherapy-associated cognitive impairment. Life Sci. 2013, 93, 605–610. [Google Scholar] [CrossRef][Green Version]
- Gilardoni, M.; Léonço, D.; Caffin, F.; Gros-Désormeaux, F.; Eldin, C.; Béal, D.; Ouzia, S.; Junot, C.; Fenaille, F.; Piérard, C.; et al. Evidence for the systemic diffusion of (2-chloroethyl)-ethyl-sulfide, a sulfur mustard analog, and its deleterious effects in brain. Toxicology 2021, 462, 152950. [Google Scholar] [CrossRef]
- Kisby, G.E.; Fry, R.C.; Lasarev, M.R.; Bammler, T.K.; Beyer, R.P.; Churchwell, M.; Doerge, D.R.; Meira, L.B.; Palmer, V.S.; Ramos-Crawford, A.L.; et al. The cycad genotoxin MAM modulates brain cellular pathways involved in neurodegenerative disease and cancer in a DNA damage-linked manner. PLoS ONE 2011, 6, e20911. [Google Scholar] [CrossRef]
- Cemeli, E.; Smith, I.F.; Peers, C.; Urenjak, J.; Godukhin, O.V.; Obrenovitch, T.P.; Anderson, D. Oxygen-induced DNA damage in freshly isolated brain cells compared with cultured astrocytes in the Comet assay. Teratog. Carcinog. Mutagen. 2003, 23 (Suppl. S2), 43–52. [Google Scholar] [CrossRef]
- Lorenzo-López, L.; Lema-Arranz, C.; Fernández-Bertólez, N.; Costa, S.; Costa, C.; Teixeira, J.P.; Pásaro, E.; Valdiglesias, V.; Laffon, B. Relationship between DNA damage measured by the comet-assay and cognitive function. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2022, 883–884, 503557. [Google Scholar] [CrossRef]
- Swain, U.; Subba Rao, K. Study of DNA damage via the comet assay and base excision repair activities in rat brain neurons and astrocytes during aging. Mech. Ageing Dev. 2011, 132, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Cordelli, E.; Bignami, M.; Pacchierotti, F. Comet assay: A versatile but complex tool in genotoxicity testing. Toxicol. Res. 2021, 10, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Migliore, L.; Fontana, I.; Trippi, F.; Colognato, R.; Coppede, F.; Tognoni, G.; Nucciarone, B.; Siciliano, G. Oxidative DNA damage in peripheral leukocytes of mild cognitive impairment and AD patients. Neurobiol. Aging 2005, 26, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Migliore, L.; Petrozzi, L.; Lucetti, C.; Gambaccini, G.; Bernardini, S.; Scarpato, R.; Trippi, F.; Barale, R.; Frenzilli, G.; Rodilla, V.; et al. Oxidative damage and cytogenetic analysis in leukocytes of Parkinson’s disease patients. Neurology 2002, 58, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; MacGarvey, U.; Beal, M.F. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Ann. Neurol. 1994, 36, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Markesbery, W.R. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic Acids Res. 2007, 35, 7497–7504. [Google Scholar] [CrossRef] [PubMed]
- Lyras, L.; Cairns, N.J.; Jenner, A.; Jenner, P.; Halliwell, B. An assessment of oxidative damage to proteins, lipids, and DNA in brain from patients with Alzheimer’s disease. J. Neurochem. 1997, 68, 2061–2069. [Google Scholar] [CrossRef]
- Wu, W.; Hill, S.E.; Nathan, W.J.; Paiano, J.; Callen, E.; Wang, D.; Shinoda, K.; van Wietmarschen, N.; Colon-Mercado, J.M.; Zong, D.; et al. Neuronal enhancers are hotspots for DNA single-strand break repair. Nature 2021, 593, 440–444. [Google Scholar] [CrossRef]
- Gilat, N.; Fridman, D.; Sharim, H.; Margalit, S.; Gassman, N.R.; Michaeli, Y.; Ebenstein, Y. From single-molecule to genome-wide mapping of DNA lesions: Repair-assisted damage detection sequencing. Biophys. Rep. 2021, 1, 100017. [Google Scholar] [CrossRef]
- Mingard, C.; Wu, J.; McKeague, M.; Sturla, S.J. Next-generation DNA damage sequencing. Chem. Soc. Rev. 2020, 49, 7354–7377. [Google Scholar] [CrossRef]
- Bradley-Whitman, M.A.; Timmons, M.D.; Beckett, T.L.; Murphy, M.P.; Lynn, B.C.; Lovell, M.A. Nucleic acid oxidation: An early feature of Alzheimer’s disease. J. Neurochem. 2014, 128, 294–304. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Yasuhara, T.; Agari, T.; Kondo, A.; Kuramoto, S.; Kameda, M.; Kadota, T.; Baba, T.; Tajiri, N.; Wang, F.; et al. Urinary 8-OHdG elevations in a partial lesion rat model of Parkinson’s disease correlate with behavioral symptoms and nigrostriatal dopaminergic depletion. J. Cell Physiol. 2011, 226, 1390–1398. [Google Scholar] [CrossRef]
- Wang, J.; Markesbery, W.R.; Lovell, M.A. Increased oxidative damage in nuclear and mitochondrial DNA in mild cognitive impairment. J. Neurochem. 2006, 96, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Balbo, S.; Turesky, R.J.; Villalta, P.W. DNA adductomics. Chem. Res. Toxicol. 2014, 27, 356–366. [Google Scholar] [CrossRef]
- Guo, J.; Turesky, R.J. Emerging Technologies in Mass Spectrometry-Based DNA Adductomics. High Throughput 2019, 8, 13. [Google Scholar] [CrossRef]
- Collins, A.; Moller, P.; Gajski, G.; Vodenkova, S.; Abdulwahed, A.; Anderson, D.; Bankoglu, E.E.; Bonassi, S.; Boutet-Robinet, E.; Brunborg, G.; et al. Measuring DNA modifications with the comet assay: A compendium of protocols. Nat. Protoc. 2023, 18, 929–989. [Google Scholar] [CrossRef]
- Touat, M.; Li, Y.Y.; Boynton, A.N.; Spurr, L.F.; Iorgulescu, J.B.; Bohrson, C.L.; Cortes-Ciriano, I.; Birzu, C.; Geduldig, J.E.; Pelton, K.; et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature 2020, 580, 517–523. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Borresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef]
- Ropert, B.; Gallrein, C.; Schumacher, B. DNA repair deficiencies and neurodegeneration. DNA Repair 2024, 138, 103679. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; You, P.; SenGupta, T.; Nilsen, H.; Sharma, K. Crosstalk between Different DNA Repair Pathways Contributes to Neurodegenerative Diseases. Biology 2021, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.; Sun, W.; Dehkordi, S.K.; Zare, H.; Fongang, B.; Bieniek, K.F.; Frost, B. Nanopore-based DNA long-read sequencing analysis of the aged human brain. bioRxiv 2024. [Google Scholar] [CrossRef]
- Verheijen, B.M.; Chung, C.; Thompson, B.; Kim, H.; Nakahara, A.; Anink, J.J.; Mills, J.D.; Phatnani, H.; Kwan, J.; Sareen, D.; et al. The cycad genotoxin methylazoxymethanol, linked to Guam ALS/PDC, induces transcriptional mutagenesis. Acta Neuropathol. Commun. 2024, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Jovasevic, V.; Wood, E.M.; Cicvaric, A.; Zhang, H.; Petrovic, Z.; Carboncino, A.; Parker, K.K.; Bassett, T.E.; Moltesen, M.; Yamawaki, N. Formation of memory assemblies through the DNA-sensing TLR9 pathway. Nature 2024, 628, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.M.; Kamel, F.; Ross, G.W.; Hoppin, J.A.; Goldman, S.M.; Korell, M.; Marras, C.; Bhudhikanok, G.S.; Kasten, M.; Chade, A.R.; et al. Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 2011, 119, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Chaim, I.A.; Nagel, Z.D.; Jordan, J.J.; Mazzucato, P.; Ngo, L.P.; Samson, L.D. In vivo measurements of interindividual differences in DNA glycosylases and APE1 activities. Proc. Natl. Acad. Sci. USA 2017, 114, E10379–E10388. [Google Scholar] [CrossRef] [PubMed]
- Nagel, Z.D.; Margulies, C.M.; Chaim, I.A.; McRee, S.K.; Mazzucato, P.; Ahmad, A.; Abo, R.P.; Butty, V.L.; Forget, A.L.; Samson, L.D. Multiplexed DNA repair assays for multiple lesions and multiple doses via transcription inhibition and transcriptional mutagenesis. Proc. Natl. Acad. Sci. USA 2014, 111, E1823–E1832. [Google Scholar] [CrossRef] [PubMed]
- Pierce, A.J.; Johnson, R.D.; Thompson, L.H.; Jasin, M. XRCC3 promotes homology-directed repair of DNA damage in mammalian cells. Genes Dev. 1999, 13, 2633–2638. [Google Scholar] [CrossRef]
- Butler, M.; Pongor, L.; Su, Y.T.; Xi, L.; Raffeld, M.; Quezado, M.; Trepel, J.; Aldape, K.; Pommier, Y.; Wu, J. MGMT Status as a Clinical Biomarker in Glioblastoma. Trends Cancer 2020, 6, 380–391. [Google Scholar] [CrossRef]
- Wang, C.; Tang, H.; Geng, A.; Dai, B.; Zhang, H.; Sun, X.; Chen, Y.; Qiao, Z.; Zhu, H.; Yang, J.; et al. Rational combination therapy for hepatocellular carcinoma with PARP1 and DNA-PK inhibitors. Proc. Natl. Acad. Sci. USA 2020, 117, 26356–26365. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.W.; Cerniglia, G.J.; Giandomenico, A.R.; Koch, C.J. DNA damaging agents improve stable gene transfer efficiency in mammalian cells. Radiat. Oncol. Investig. 1998, 6, 1–9. [Google Scholar] [CrossRef]
- Alexander, I.E.; Russell, D.W.; Miller, A.D. DNA-damaging agents greatly increase the transduction of nondividing cells by adeno-associated virus vectors. J. Virol. 1994, 68, 8282–8287. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.H.; Yang, X.W. Genetically-directed Sparse Neuronal Labeling in BAC Transgenic Mice through Mononucleotide Repeat Frameshift. Sci. Rep. 2017, 7, 43915. [Google Scholar] [CrossRef] [PubMed]
- Wegner, W.; Ilgen, P.; Gregor, C.; van Dort, J.; Mott, A.C.; Steffens, H.; Willig, K.I. In vivo mouse and live cell STED microscopy of neuronal actin plasticity using far-red emitting fluorescent proteins. Sci. Rep. 2017, 7, 11781. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Sauer, M.; Geis, C. Super-resolving Microscopy in Neuroscience. Chem. Rev. 2021, 121, 11971–12015. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, M.; Gockel, N.; Arizono, M.; Dembitskaya, Y.; Nagerl, U.V.; Pennacchietti, F.; Damenti, M.; Testa, I.; Willig, K.I. Super-Resolution Microscopy Opens New Doors to Life at the Nanoscale. J. Neurosci. 2022, 42, 8488–8497. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.T.B.; Whelan, D.R.; Rozario, A.M. Chapter 13—Visualizing DNA damage and repair using single molecule super resolution microscopy. In Methods in Cell Biology; Zierhut, C., Galluzzi, L., Eds.; Academic Press: Cambridge, MA, USA, 2024; Volume 182, pp. 237–245. [Google Scholar]
- Qian, H.; Margaretha Plat, A.; Jonker, A.; Hoebe, R.A.; Krawczyk, P. Super-resolution GSDIM microscopy unveils distinct nanoscale characteristics of DNA repair foci under diverse genotoxic stress. DNA Repair 2024, 134, 103626. [Google Scholar] [CrossRef] [PubMed]
- Varga, D.; Majoros, H.; Ujfaludi, Z.; Erdelyi, M.; Pankotai, T. Quantification of DNA damage induced repair focus formation via super-resolution dSTORM localization microscopy. Nanoscale 2019, 11, 14226–14236. [Google Scholar] [CrossRef]
- Ayala-Torres, S.; Chen, Y.; Svoboda, T.; Rosenblatt, J.; Van Houten, B. Analysis of gene-specific DNA damage and repair using quantitative polymerase chain reaction. Methods 2000, 22, 135–147. [Google Scholar] [CrossRef]
- Santos, J.H.; Meyer, J.N.; Mandavilli, B.S.; Van Houten, B. Quantitative PCR-based measurement of nuclear and mitochondrial DNA damage and repair in mammalian cells. Methods Mol. Biol. 2006, 314, 183–199. [Google Scholar] [PubMed]
- Dannenmann, B.; Lehle, S.; Lorscheid, S.; Huber, S.M.; Essmann, F.; Schulze-Osthoff, K. Simultaneous quantification of DNA damage and mitochondrial copy number by long-run DNA-damage quantification (LORD-Q). Oncotarget 2017, 8, 112417–112425. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, H.S.; Lim, S.; Jo, K. Visualization of UV-induced damage on single DNA molecules. Chem. Commun. 2013, 49, 4740–4742. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, Y.; Lim, S.; Jo, K. Single-molecule visualization of ROS-induced DNA damage in large DNA molecules. Analyst 2016, 141, 847–852. [Google Scholar] [CrossRef]
- Singh, V.; Johansson, P.; Lin, Y.L.; Hammarsten, O.; Westerlund, F. Shining light on single-strand lesions caused by the chemotherapy drug bleomycin. DNA Repair 2021, 105, 103153. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Johansson, P.; Torchinsky, D.; Lin, Y.L.; Oz, R.; Ebenstein, Y.; Hammarsten, O.; Westerlund, F. Quantifying DNA damage induced by ionizing radiation and hyperthermia using single DNA molecule imaging. Transl. Oncol. 2020, 13, 100822. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Lee, J.; Kim, J.; Oh, Y.; Kim, D.; Lee, J.; Lim, S.; Jo, K. Analysis of alcohol-induced DNA damage in Escherichia coli by visualizing single genomic DNA molecules. Analyst 2016, 141, 4326–4331. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Piett, C.; Andrews, J.; Mann, E.; Nagel, Z.; Gassman, N. Defective base excision repair in the response to DNA damaging agents in triple negative breast cancer. PLoS ONE 2019, 14, e0223725. [Google Scholar] [CrossRef]
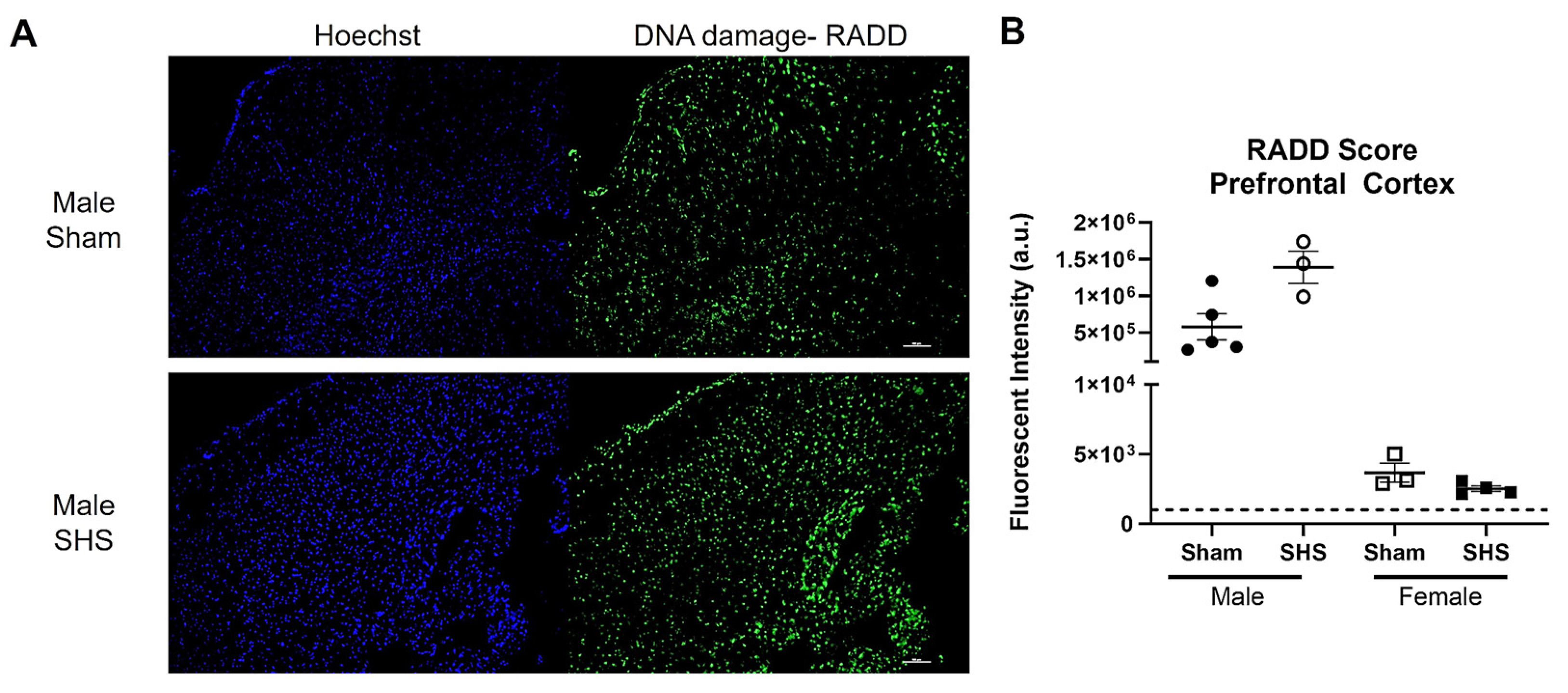
- Lee, K.J.; Mann, E.; da Silva, L.M.; Scalici, J.; Gassman, N.R. DNA damage measurements within tissue samples with Repair Assisted Damage Detection (RADD). Curr. Res. Biotechnol. 2019, 1, 78–86. [Google Scholar] [CrossRef]
- Holton, N.W.; Ebenstein, Y.; Gassman, N.R. Broad spectrum detection of DNA damage by Repair Assisted Damage Detection (RADD). DNA Repair 2018, 66–67, 42–49. [Google Scholar] [CrossRef]
- Krieger, K.L.; Gohlke, J.H.; Lee, K.J.; Piyarathna, D.W.B.; Castro, P.D.; Jones, J.A.; Ittmann, M.M.; Gassman, N.R.; Sreekumar, A. Repair-Assisted Damage Detection Reveals Biological Disparities in Prostate Cancer between African Americans and European Americans. Cancers 2022, 14, 1012. [Google Scholar] [CrossRef]
- Mann, E.K.; Lee, K.J.; Chen, D.; da Silva, L.M.; Dal Zotto, V.L.; Scalici, J.; Gassman, N.R. Associations between DNA Damage and PD-L1 Expression in Ovarian Cancer, a Potential Biomarker for Clinical Response. Biology 2021, 10, 385. [Google Scholar] [CrossRef]
- Matsuno, Y.; Atsumi, Y.; Alauddin, M.; Rana, M.M.; Fujimori, H.; Hyodo, M.; Shimizu, A.; Ikuta, T.; Tani, H.; Torigoe, H.; et al. Resveratrol and its Related Polyphenols Contribute to the Maintenance of Genome Stability. Sci. Rep. 2020, 10, 5388. [Google Scholar] [CrossRef]
- Mulgrave, V.E.; Alsayegh, A.A.; Jaldi, A.; Omire-Mayor, D.T.; James, N.; Ntekim, O.; Walters, E.; Akala, E.O.; Allard, J.S. Exercise modulates APOE expression in brain cortex of female APOE3 and APOE4 targeted replacement mice. Neuropeptides 2023, 97, 102307. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Q.; Wu, M. Antioxidant and neuroprotective actions of resveratrol in cerebrovascular diseases. Front. Pharmacol. 2022, 13, 948889. [Google Scholar] [CrossRef]
- Rahman, M.H.; Akter, R.; Bhattacharya, T.; Abdel-Daim, M.M.; Alkahtani, S.; Arafah, M.W.; Al-Johani, N.S.; Alhoshani, N.M.; Alkeraishan, N.; Alhenaky, A.; et al. Resveratrol and Neuroprotection: Impact and Its Therapeutic Potential in Alzheimer’s Disease. Front. Pharmacol. 2020, 11, 619024. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Izquierdo, V.; Corpas, R.; Roig-Soriano, J.; Chillón, M.; Andres-Lacueva, C.; Somogyvári, M.; Sőti, C.; Sanfeliu, C.; et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021, 67, 101271. [Google Scholar] [CrossRef]
- Raber, J.; Perez, R.; Torres, E.R.S.; Krenik, D.; Boutros, S.; Patel, E.; Chlebowski, A.C.; Torres, E.R.; Perveen, Z.; Penn, A.; et al. Effects of Chronic Secondhand Smoke (SHS) Exposure on Cognitive Performance and Metabolic Pathways in the Hippocampus of Wild-Type and Human Tau Mice. Environ. Health Perspect. 2021, 129, 57009. [Google Scholar] [CrossRef]
- Lopes, L.A.; Davenport, C.; Ramos Torres, E.; Chlebowski, A.; Mikami, A.; Raber, J.; Ruth Torres, E.; Kisby, G. Neuropathological Examination of Mice Chronically Exposed to Secondhand Smoke. Mil. Med. 2023, 188 (Suppl. S6), 575–583. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Stagaman, K.; Kasschau, K.D.; Davenport, C.; Lopes, L.; Nguyen, D.; Torres, E.R.; Sharpton, T.J.; Kisby, G. Behavioral and Cognitive Performance Following Exposure to Second-Hand Smoke (SHS) from Tobacco Products Associated with Oxidative-Stress-Induced DNA Damage and Repair and Disruption of the Gut Microbiome. Genes 2023, 14, 1702. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Mann, E.; Wright, G.; Piett, C.G.; Nagel, Z.D.; Gassman, N.R. Exploiting DNA repair defects in triple negative breast cancer to improve cell killing. Ther. Adv. Med. Oncol. 2020, 12, 1758835920958354. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hedlich-Dwyer, J.; Allard, J.S.; Mulgrave, V.E.; Kisby, G.E.; Raber, J.; Gassman, N.R. Novel Techniques for Mapping DNA Damage and Repair in the Brain. Int. J. Mol. Sci. 2024, 25, 7021. https://doi.org/10.3390/ijms25137021
Hedlich-Dwyer J, Allard JS, Mulgrave VE, Kisby GE, Raber J, Gassman NR. Novel Techniques for Mapping DNA Damage and Repair in the Brain. International Journal of Molecular Sciences. 2024; 25(13):7021. https://doi.org/10.3390/ijms25137021
Chicago/Turabian StyleHedlich-Dwyer, Jenna, Joanne S. Allard, Veronica E. Mulgrave, Glen E. Kisby, Jacob Raber, and Natalie R. Gassman. 2024. "Novel Techniques for Mapping DNA Damage and Repair in the Brain" International Journal of Molecular Sciences 25, no. 13: 7021. https://doi.org/10.3390/ijms25137021
APA StyleHedlich-Dwyer, J., Allard, J. S., Mulgrave, V. E., Kisby, G. E., Raber, J., & Gassman, N. R. (2024). Novel Techniques for Mapping DNA Damage and Repair in the Brain. International Journal of Molecular Sciences, 25(13), 7021. https://doi.org/10.3390/ijms25137021







