Abstract
Background: Combination therapy for Enterococcus faecalis was established in the 1940s due to high rates of treatment failure, especially for infective endocarditis (IE). However, during this period antimicrobials were limited, optimal dosing was unknown, and development of resistance was rapid. Today, nearly 80 years later, combination therapy is still the standard practice for IE caused by E. faecalis despite improvements in antimicrobial availability, activity, and evidence-based, optimized antimicrobial dosing. These treatment decisions are guided by in vitro synergy principles and are frequently extrapolated to E. faecalis bloodstream infections (BSI) without IE. The paucity of clinical data to support this practice, paired with the known risks from unnecessary antibiotic exposure, makes further research and clinical guidance necessary. Methods: This single-center retrospective observational study of hospitalized adult patients with E. faecalis BSI treated with combination therapy aimed to describe treatment approaches and outcome data. Results: Between 1 January 2017, and 30 September 2024, 358 patients were screened, and 54 met study inclusion criteria. IE was present in 53.7% of patients, and 25.9% met the composite outcome (30-day mortality, 60-day hospital readmission, and/or 60-day recurrence). Adverse events were noted in 5.6% of patients. Conclusions: Observational data from this review supports the hypothesis that guideline recommendations for the use of combination therapy in E. faecalis IE are occasionally extrapolated to patients without IE. Given the in vitro and dated observational data used as the basis for these recommendations and the risks associated with unnecessary antibiotic exposure, more extensive, prospective, interventional studies are needed to address this dogmatic practice surrounding a high-morbidity, high-mortality disease state.
1. Introduction
Enterococcus faecalis is a Gram-positive facultative anaerobe, commonly found within the human intestinal microbiota, and is a common cause of bloodstream infections (BSI). Depending on the source of infection and presence of metastatic sites, E. faecalis BSI can have varying levels of severity, carrying significant morbidity and mortality risks [1]. As such, timely and appropriate antimicrobial treatment is essential for improved patient outcomes. Currently, no dedicated consensus guidelines exist for the management of E. faecalis BSI, often leaving practitioners to extrapolate data from other clinical scenarios such as infective endocarditis (IE) or BSI caused by other pathogens. This has led some practitioners to consider combination antibiotic therapy for the treatment of E. faecalis BSI, even in the absence of IE [2]. However, the recommendation for combination therapy in IE is largely based on dogma and relies predominantly on historical in vitro data demonstrating synergy but lacks compelling clinical outcomes data in the setting of modern antibiotics. While data supporting the clinical benefit of combination therapy is lacking, the deleterious effects of unnecessary antimicrobial use have been well documented.
In 1899, Thiercelin shared his observations of a saprophytic microbe that lives within the human gastrointestinal tract. Around the same time, MacCallum and Hastings, unknowingly, published one of the first cases of E. faecalis IE nearly thirty years prior to the discovery of penicillin in 1928 by Alexander Fleming. Therefore, data supporting the use of penicillin for IE caused by E. faecalis did not emerge until the 1940s. Notably, in 1944, Loewe et al. demonstrated successful treatment of IE caused by Streptococcal spp. using only 200,000 units of penicillin per day, a much lower dose than that used in current clinical practice [3]. Subsequently, penicillin use increased, and resistance quickly followed. In 1948, resistance rates were theorized to be secondary to inadequate dosing of penicillin, as Enterococci demonstrated the need for higher doses, roughly 10 to 20 million units per day, for growth inhibition. As a result, Enterococci were deemed “more difficult to treat” than other streptococcal species. There was also concern that due to the high inoculum of IE and the propensity of E. faecalis to form biofilms, monotherapy, including beta-lactams, would fail to demonstrate bactericidal activity [2]. Due to concerns about using bacteriostatic agents in a high bacterial burden infection, researchers began experimenting with multiple treatment modalities, including combination therapy, namely with penicillin and streptomycin, to achieve synergistic effects as demonstrated in in vitro studies [4,5,6].
As more active, safer antimicrobials, such as ampicillin, were discovered, penicillin and streptomycin use for E. faecalis IE decreased, but the use of combination therapy has persisted. As a result, ampicillin monotherapy has never been adequately studied as a treatment option for E. faecalis IE, despite its enhanced activity and high barrier of resistance compared to penicillin. Instead, ampicillin was originally used in combination with gentamicin, another aminoglycoside. However, the increased and prolonged use of gentamicin led to the development of resistance and safety concerns. This ushered in the desire to seek new synergistic combinations. Eventually, ampicillin plus ceftriaxone became a widely preferred treatment, as it was shown to be as effective and safer than an aminoglycoside combination regimen [7].
Given the current quality of evidence used for the recommendation of combination therapy for E. faecalis IE, new insights on the lack of clinical benefit associated with the use of bactericidal versus bacteriostatic antibiotics, and enhanced performance of antibiotics used to treat E. faecalis in current practice, there is a need to further assess the risks and benefits of combination therapy [8]. While the data is lacking, there is no shortage of clinical debate on this topic, indicating there is a desire to further explore the use of combination therapy for E. faecalis BSI with or without IE [9,10]. To our knowledge, no study has been published comparing monotherapy and combination therapy for E. faecalis BSI with or without IE, leaving providers to potentially extrapolate a limited evidence practice from IE to non-IE BSI. This manuscript aims to provide observational data evaluating combination therapy use in E. faecalis BSI with or without IE.
2. Materials and Methods
2.1. Study Cohort
This was a single-center, retrospective, observational study of hospitalized adult patients with E. faecalis BSI between 1 January 2017, and 30 September 2024. Electronic medical records were reviewed to confirm subject eligibility, gather baseline characteristics, treatment information, and clinical outcomes. The study was approved by the organization’s Institutional Review Board and was determined to be exempt, as there was no greater than minimal risk present to study subjects.
2.2. Eligibility Criteria
Eligible patients were 18 years of age or older with at least one blood culture positive for E. faecalis. Patients were excluded from the study if they expired within 72 h of index culture, had a polymicrobial BSI, did not receive combination therapy (defined as two or more antimicrobials used after pathogen identification and for greater than 50% of the treatment course where either both agents had activity against E. faecalis or at least one agent had activity and was given with a second agent that has demonstrated in vitro synergy), did not receive antibiotic treatment, and/or had E. faecalis isolated on culture from any site in the previous 60 days [10,11,12,13]. The source of infection was based on positive cultures at other sites, echocardiogram or diagnostic imaging findings, or provider documentation.
2.3. Outcomes Definitions
The primary outcome was a composite of 30-day all-cause mortality, 60-day all-cause hospital readmission, and 60-day recurrence (defined as repeat positive blood cultures 60 days from the end of antimicrobial therapy). Secondary outcomes included each individual component of the composite outcome, persistent BSI (defined as repeat culture positivity > 48 h after initiation of active antimicrobial therapy), hospital length of stay (LOS), duration of therapy, and adverse events (ADEs). ADEs evaluated included hypersensitivity reactions, acute kidney injury (AKI) (defined by serum creatinine (SCr) > 2-fold increase from baseline within a week or an increase in SCr of 0.3 in 48 h), Clostridioidies difficile infection (CDI), diarrhea without CDI (defined as physician-documented diarrhea, physician-ordered CDI diagnostic tests, or receipt of anti-diarrheal agents), and leukopenia (defined as a white blood cell count reduction to <4000/mcL (<4 × 109/L)).
3. Results
3.1. Patients and Demographics
From 1 January 2017 to 30 September 2024, a total of 358 patients were screened, and 54 patients were included in the final analysis (Figure 1). The mean age of the cohort was 63.9 years old, 37.6% were male, and 57.4% were white. Nearly all patients (98.1%) received an echocardiogram. Approximately half (53.7%) of patients were found to have IE. Despite the high observed rate of IE, only 1.9% of patients had repeat blood culture positivity after 48 h of active antimicrobial therapy (Table 1). In accordance with current guideline recommendations for E. faecalis IE, the most common antibiotic combination was ampicillin plus ceftriaxone (90.7%) (Table 2).
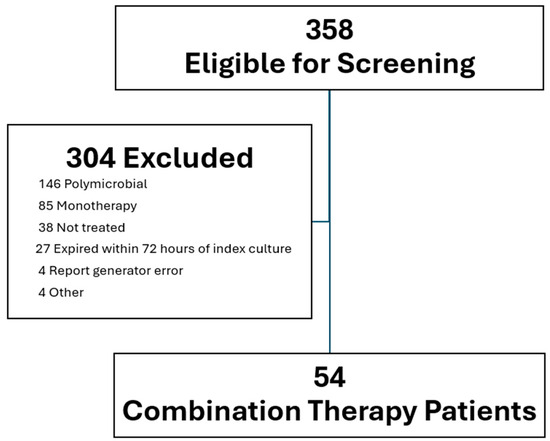
Figure 1.
Flowchart of patients excluded and included.

Table 1.
Patient demographics.

Table 2.
Combination therapy regimens.
3.2. Outcomes Data
Of the 54 patients included, 25.9% met the composite outcome. The rates of 30-day all-cause mortality and 60-day all-cause readmission were 3.7% and 22.2%, respectively. There were no cases of 60-day recurrence (Table 3). The median total duration of therapy and hospital LOS were 38.8 and 15 days, respectively (Table 3). Adverse events occurred in 5.6% of patients (Table 3).

Table 3.
Outcomes data.
4. Discussion
The present study highlights several inconsistencies and areas of investigation that should be addressed to optimize the therapy of E. faecalis BSI with or without IE. The most basic of these is to evaluate the need for combination therapy as well as the need to define frequently used terms pertaining to E. faecalis BSI, namely the distinction of or need for terms such as “complicated” and “uncomplicated” BSI [1]. Additionally, these data demonstrate that 46.3% of patients receiving combination therapy had non-IE E. faecalis BSI, indicating frequent extrapolation from E. faecalis IE guideline recommendations. These guidelines recommend combination therapy of 4 to 6 weeks (28 to 42 days) in the setting of confirmed E. faecalis IE. However, this recommendation is not based on multiple, high-quality, reproducible clinical trial data but rather on lower-quality evidence. Additionally, these guidelines propagate a dated statement on pharmacodynamic principles, noting, “…streptococci usually are killed by monotherapy…whereas enterococci are inhibited, but not killed [2].” This misapplication of the use of bacteriostatic and bactericidal terminology, in vitro designations assigned based on changes in bacterial growth or density on agar plates in opportune conditions, may be definitionally flawed, as multiple studies have demonstrated no inherent advantages for antimicrobials that meet the bactericidal threshold [8,14,15].
As previously mentioned, observed practice is to define bloodstream infections as uncomplicated and complicated, which are terms extrapolated from the Infectious Diseases Society of America Staphylococcus aureus (SA) guidelines [16]. However, notable differences exist between the virulence and pathologic characteristics of SA and E. faecalis. Providers should exercise caution when using recommendations tailored toward SA when treating E. faecalis. While there is no consensus definition of persistent bacteremia, recent studies have demonstrated that patients with SA BSI tend to remain bacteremic for longer: 15.7–23.2% of SA cases compared to 7.4–14.7% of E. faecalis BSIs [17,18]. One such study found 32% of patients had at least one additional positive culture following active antimicrobial therapy with a median duration of bacteremia of 3 days [19]. Highlighting the importance of rapid clearance, investigators found crude mortality increased from 22% to 39% if bacteremia persisted for 2–4 days and was 43% if bacteremia persisted 5–7 days. Reasons for these discrepancies include fundamental differences in virulence factors, nidus of infections, and presence of metastasis in SA BSI. Another difference to consider is SA’s notable fluctuating blood culture positivity known as “skip phenomenon”, which studies show occurring in approximately 4–13% of patients [20,21,22]. Due to these variations in characteristics, the use of and durations of therapy associated with the terms “uncomplicated” and “complicated” in the setting of SA BSI may not be fully applicable to other pathogens, such as E. faecalis.
Additionally, multiple observational studies have demonstrated the efficacy of shorter courses of therapy for Enterococcal BSI [23,24]. Without further data to guide decision making, some concerns include the use of additional antibiotics and prolonged treatment courses, which may put patients at risk for potential adverse effects, such as CDI, antimicrobial resistance, or intravenous (IV) catheter complications for those receiving prolonged IV therapy [25]. Interestingly, in the present study, the median duration of therapy for patients treated with combination therapy was over twice as long as the median hospital LOS. In addition to the need to further elucidate the role of combination therapy in general, the optimal duration of combination therapy and the role of sequential oral therapy should be further clarified. The POET trial evaluated oral therapy in stable patients with left-sided IE and found sequential oral treatment to be non-inferior to definitive IV therapy. However, only 25.4% of patients included in the study had E. faecalis IE. Acceptable combinations consisted of amoxicillin, linezolid, moxifloxacin, and rifampin. Notably, a secondary analysis evaluating pharmacokinetics and pharmacodynamics from subjects in the POET trial demonstrated a high probability of target attainment among patients receiving high-dose amoxicillin [11]. Additionally, Loudermilk et al. evaluated E. faecalis BSI, with variable sources, and found that hospital LOS was nearly halved by opting for sequential oral therapies [26]. By further evaluating the impact of sequential oral therapy in this patient population, treatment may be optimized to minimize exposure of unnecessarily prolonged outpatient IV therapy.
Other notable observations include high utilization rates of echocardiograms and collection of repeat blood cultures (98.1% for both), indicating a high level of surveillance for IE. While Enterococcus species comprise only about 15.5% of all IE cases, one large Danish study found that 16.7% of patients with E. faecalis BSI had IE. These rates surpass even those of SA BSI (10.1%) [27]. The high rates of IE associated with E. faecalis observed in the present study exceed those in the previous literature, likely due to selection bias and confounding by indication as a result of including only patients treated with combination therapy. Additionally, only 1.9% of patients had repeat positive blood cultures after 48 h of active antimicrobial therapy despite relatively high rates of confirmed IE, highlighting the importance of appropriate patient workup balanced with diagnostic stewardship [28]. This paradoxical finding of rapid clearance of blood cultures, despite relatively high rates of IE paired with high treatment success rates, may further suggest that concerns with high bacterial density and the need for a second antimicrobial are unfounded when using more modern therapeutic options for E. faecalis IE.
To our knowledge, this is the largest cohort review to date evaluating the diagnosis, workup, treatment, and outcomes of patients treated with combination therapy for E. faecalis BSI with or without IE. However, the present study is also not without limitations, largely due to its size (n = 54) and retrospective nature, which limits generalizability. One notable limitation is immortal time bias, as patients were required to survive at least 72 h from index culture to be included in the analysis. Therefore, we cannot comment on the impact of combination therapy on early mortality among patients with E. faecalis BSI. Similarly, we could not assess the rationale for using combination therapy in all cases, as there was incomplete documentation regarding Duke Criteria, preexisting IE risk factors such as intravenous drug use, structural cardiac abnormalities, physical exam findings, or the presence of cardiac devices that may have impacted treatment and diagnostic decisions. As a result, there may be prescribing biases present associated with the presenting severity of illness or clinical trajectory. Additionally, we were unable to accurately and consistently track patients after discharge, including changes in outpatient antimicrobial regimens, such as transitions to monotherapy, sequential oral therapy, or treatment duration reduction or extension. We were also unable to reliably track outcomes that occurred outside of the study facility.
5. Conclusions
Combination therapy to treat E. faecalis BSI has relied on in vitro data and early case reports of success with penicillin plus aminoglycosides. Conclusions may have been drawn prematurely about combination therapy as the standard of care for E. faecalis. IE was determined, in part, due to since-addressed factors such as the emergence of penicillin resistance and suboptimal dosing. Nevertheless, many years later, combination therapy remains a guideline recommendation and common practice today. Additionally, combination therapy for E. faecalis BSI without IE is becoming an extrapolated practice based solely on dogma. Prolonging therapy and using additional antimicrobials, in the absence of high-quality reproducible evidence, increases the risks of patient harm through adverse drug events and antimicrobial resistance. Therefore, it is paramount to further elucidate the benefits, if any, of combination therapy for E. faecalis BSI with or without IE. The findings presented here further demonstrate wide practice variability due to a clearly identified gap in the literature in which dogmatic practice and recommendations have gone unchallenged for decades. Future data may demonstrate the superiority of combination therapy compared to monotherapy, but given known risks, it is prudent that the practice be further investigated in high-quality controlled studies.
Author Contributions
Conceptualization, D.T.A. and J.E.; methodology, D.T.A. and J.E.; validation, D.T.A. and J.E.; formal analysis, A.D.F.; investigation, A.D.F.; resources, A.D.F., D.T.A. and J.E.; data curation, A.D.F.; writing—original draft preparation, A.D.F.; writing—review and editing, D.T.A. and J.E.; supervision, D.T.A.; project administration, D.T.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Augusta University (2236153-2, 16 December 2024).
Informed Consent Statement
Patient consent was waived as this was a non-interventional, observational, and retrospective chart review study. Researchers had no interaction with the subjects.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author on reasonable request. The data are not publicly available to ensure protection of patient privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BSI | Bloodstream infection |
| IE | Infective endocarditis |
| ADEs | Adverse events |
| LOS | Length of stay |
| SD | Standard deviation |
| kg | Kilogram |
| IQR | Interquartile range |
| SA | Staphylococcus aureus |
| IV | Intravenous |
| TEE | Transesophageal echocardiograms |
| TTE | Transthoracic echocardiograms |
| CDI | Clostridioidies difficile infection |
| SCr | Serum creatinine |
References
- Rosselli Del Turco, E.; Bartoletti, M.; Dahl, A.; Cervera, C.; Pericàs, J.M. How do I manage a patient with enterococcal bacteraemia? Clin. Microbiol. Infect. 2021, 27, 364–371. [Google Scholar] [CrossRef]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals from the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Loewe, L.; Rosenblatt, P.; Greene, H.J.; Russell, M. Combined penicillin and heparin therapy of subacute bacterial endocarditis: Report of seven consecutive successfully treated patients. J. Am. Med. Assoc. 1944, 124, 144–149. [Google Scholar] [CrossRef]
- Clark, W.H.; Bryner, S.; Rantz, L.A. Penicillin-resistant non-hemolytic streptococcal subacute bacterial endocarditis. Am. J. Med. 1948, 4, 671–689. [Google Scholar] [CrossRef]
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef]
- Moellering, R.C., Jr.; Wennersten, C.; Weinberg, A.N. Studies on antibiotic synergism against enterococci. Bacteriologic studies. J. Lab. Clin. Med. 1971, 77, 821–828. [Google Scholar]
- Fernandez-Hidalgo, N.; Almirante, B.; Gavalda, J.; Gurgui, M.; Peña, C.; de Alarcon, A.; Ruiz, J.; Vilacosta, I.; Montejo, M.; Vallejo, N.; et al. Ampicillin plus ceftriaxone is as effective as ampicillin plus gentamicin for treating Enterococcus faecalis infective endocarditis. Clin. Infect. Dis. 2013, 56, 1261–1268. [Google Scholar] [CrossRef]
- Spellberg, B.; Wald-Dickler, N.; Holtom, P.; Meyer-Sautter, P.; Camp, A.; Diaz, A.D.; Buhamad, R.; Vazquez, A.S.M.; Aguirre-Garcia, G.M.; Stanton, M.; et al. Static vs. cidal: It’s not complex; it’s simply incorrect. Antimicrob. Agents Chemother. 2025, 69, e00513-25. [Google Scholar] [CrossRef]
- Prosty, C.; Lee, T.C.; McDonald, E.G. Is more always better? Rethinking monotherapy for Enterococcus faecalis infective endocarditis. Clin. Infect. Dis. 2025, 80, 1169–1170. [Google Scholar] [CrossRef] [PubMed]
- Beganovic, M.; Luther, M.K.; Rice, L.B.; Arias, C.A.; Rybak, M.J.; LaPlante, K.L. A review of combination antimicrobial therapy for Enterococcus faecalis bloodstream infections and infective endocarditis. Clin. Infect. Dis. 2018, 67, 303–309. [Google Scholar] [CrossRef]
- Bock, M.; Theut, A.M.; van Hasselt, J.G.C.; Wang, H.; Fuursted, K.; Hoiby, N.; Lerche, C.J.; Ihlemann, N.; Gill, S.; Christiansen, U.; et al. Attainment of target antibiotic levels by oral treatment of left-sided infective endocarditis: A POET substudy. Clin. Infect. Dis. 2023, 77, 242–251. [Google Scholar] [CrossRef]
- Desbiolles, N.; Piroth, L.; Lequeu, C.; Neuwirth, C.; Portier, H.; Chavanet, P. Fractional maximal effect method for in vitro synergy between amoxicillin and ceftriaxone and between vancomycin and ceftriaxone against Enterococcus faecalis and penicillin-resistant Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 3328–3333. [Google Scholar] [CrossRef]
- Herrera-Hidalgo, L.; Fernández-Rubio, B.; Luque-Márquez, R.; López-Cortés, L.; Gil-Navarro, M.V.; de Alarcón, A. Treatment of Enterococcus faecalis Infective Endocarditis: A Continuing Challenge. Antibiotics 2023, 12, 704. [Google Scholar] [CrossRef]
- Jansson-Lofmark, R.; Hjorth, S.; Gabrielsson, J. Does in vitro potency predict clinically efficacious concentrations? Clin. Pharmacol. Ther. 2020, 108, 298–305. [Google Scholar] [CrossRef]
- Wald-Dickler, N.; Holtom, P.; Spellberg, B. Busting the Myth of “Static vs Cidal”: A Systemic Literature Review. Clin. Infect. Dis. 2018, 66, 1470–1474. [Google Scholar] [CrossRef]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.P.; Park, S.J.; Kim, H.S.; Kim, E.S.; Kim, M.N.; Park, K.H.; Kim, S.H.; Lee, S.O.; Choi, S.H.; Jeong, J.Y.; et al. Persistent Staphylococcus aureus bacteremia: A prospective analysis of risk factors, outcomes, and microbiologic and genotypic characteristics of isolates. Medicine 2013, 92, 98–108. [Google Scholar] [CrossRef]
- Rogers, R.; Rice, L.B. State-of-the-art review: Persistent enterococcal bacteremia. Clin. Infect. Dis. 2024, 78, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, R.; Morata, L.; Boeing, C.; Subirana, I.; Seifert, H.; Rieg, S.; Bin Kim, H.; Kim, E.S.; Liao, C.-H.; Tilley, R.; et al. Defining persistent Staphylococcus aureus bacteraemia: Secondary analysis of a prospective cohort study. Lancet Infect Dis. 2020, 20, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.D.; Graham, M.; Kotsanas, D.; Woolley, I.; Korman, T.M. Intermittent negative blood cultures in Staphylococcus aureus bacteremia; a retrospective study of 1071 episodes. Open Forum Infect. Dis. 2019, 6, ofz494. [Google Scholar] [CrossRef]
- Jang, S.; Jeon, M.; Kim, S.H.; Mun, S.J. Clinical implications of the skip phenomenon in patients with persistent Staphylococcus aureus bacteremia. Microb. Drug Resist. 2025, 31, 21–25. [Google Scholar] [CrossRef]
- Fiala, J.; Palraj, B.R.; Sohail, M.R.; Lahr, B.; Baddour, L.M. Is a single set of negative blood cultures sufficient to ensure clearance of bloodstream infection in patients with Staphylococcus aureus bacteremia? The skip phenomenon. Infection 2019, 47, 1047–1053. [Google Scholar] [CrossRef]
- Rosselli Del Turco, E.; Pasquini, Z.; Scolz, K.; Amedeo, A.; Beci, G.; Giglia, M.; Bussini, L.; Carvalho-Brugger, S.; Gutiérrez, L.; Tedeschi, S.; et al. Treatment duration for central line-associated infection caused by Enterococcus spp.: A retrospective evaluation of a multicenter cohort. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 1203–1206. [Google Scholar] [CrossRef]
- Bahrs, C.; Rieg, S.; Hennigs, A.; Hitzenbichler, F.; Brehm, T.T.; Rose, N.; Jacobi, R.J.; Heine, V.; Hornuss, D.; Huppertz, G.; et al. Short-course versus long-course antibiotic treatment for uncomplicated vancomycin-resistant enterococcal bacteraemia: A retrospective multicentre cohort study. Clin. Microbiol. Infect. 2023, 29, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Loudermilk, C.; Eudy, J.; Albrecht, S.; Slaton, C.N.; Stramel, S.; Tu, P.; Albrecht, B.; Green, S.B.; Bouchard, J.L.; Orvin, A.I.; et al. Evaluation of sequential oral versus intravenous antibiotic treatment of Enterococcus faecalis bloodstream infections. Ann. Pharmacother. 2025, 59, 127–133. [Google Scholar] [CrossRef]
- Ostergaard, L.; Voldstedlund, M.; Bruun, N.E.; Bundgaard, H.; Iversen, K.; Kober, N.; Christensen, J.J.; Rosenvinge, F.S.; Jarløv, J.O.; Moser, C.; et al. Temporal changes, patient characteristics, and mortality, according to microbiological cause of infective endocarditis: A nationwide study. J. Am. Heart Assoc. 2022, 11, e025801. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Hidalgo, N.; Escola-Verge, L. Enterococcus faecalis bacteremia: Consider an echocardiography, but consult an infectious diseases specialist. J. Am. Coll. Cardiol. 2019, 74, 202–204. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).