Abstract
Background/Objectives: Our objective was to describe the safety and efficacy of enteral droxidopa, a norepinephrine prodrug, for intravenous (IV) vasopressor weaning in intensive care unit (ICU) patients. Methods: This was a single-center, retrospective descriptive study of adult ICU patients. Patients who received ≥ 4 consecutive doses of droxidopa for IV vasopressor weaning were included. The cessation of the IV vasopressor without re-initiation within 72 h of droxidopa initiation was the primary outcome. The adverse events assessed included hypotension, hypertension, and arrhythmias. Results: Forty-six patients were included, with a median age of 61. Forty-two patients (91%) were on midodrine at the time of droxidopa initiation. The median daily midodrine dose was 80 mg. The median time from ICU admission to droxidopa initiation was 17 days. Patients were on a median of one IV vasopressor at the time of droxidopa initiation, with norepinephrine as the most common agent (50%). The median initial daily droxidopa dose was 300 mg, with a median maximum daily dose of 900 mg. Vasopressor support was discontinued within 72 h of droxidopa initiation in 46% of patients, with a median time to IV vasopressor cessation of 3.3 days. There were no incidences of hypotension, hypertension, arrhythmias, or ICU readmissions related to droxidopa. Droxidopa was continued upon discharge in 29% of patients. Conclusions: Droxidopa may be a safe and effective option to facilitate the weaning of IV vasopressor support in patients who are refractory or intolerant to midodrine. Larger prospective studies are needed to confirm these findings.
1. Introduction
Continuous low-dose intravenous (IV) vasopressor needs for meeting blood pressure targets after the resolution of critical illness may be a barrier to intensive care unit (ICU) discharge. Oral vasoactive agents such as midodrine, an oral alpha-1 agonist, have been used off-label to wean IV vasopressors to facilitate ICU discharge [1]. However, studies have shown mixed results regarding its efficacy, and its use may be limited by adverse events such as bradycardia [2,3,4,5,6].
Droxidopa, an oral prodrug of norepinephrine (an alpha/beta agonist), may be an alternative for patients who are not responsive to, or unable to tolerate, midodrine [7]. Droxidopa has a labeled indication for neurogenic orthostatic hypotension. Studies regarding the use of droxidopa off-label for IV vasopressor weaning in critically ill patients are limited [8,9,10,11]. One retrospective study observed that 70.3% (19/30) of patients were weaned off of vasopressors within 7 days of droxidopa initiation, while another retrospective study comparing droxidopa to atomoxetine (a norepinephrine reuptake inhibitor) or combination therapy (droxidopa and atomoxetine) found no difference in discontinuing IV vasopressors in cardiothoracic ICU patients refractory to midodrine [10,11]. The preliminary results of this study presented as an abstract showed that vasopressors were discontinued within 72 h of droxidopa initiation in 40% of patients (11/33) who were refractory or unable to tolerate midodrine [12].
At Stanford Health Care (SHC), midodrine is often used first-line for IV vasopressor weaning in ICU patients with prolonged vasopressor requirements, no longer thought to be in shock. In patients unable to wean IV vasopressors despite increasing midodrine to 20–30 mg q6h, or in patients experiencing adverse events, droxidopa is often trialed either in addition to midodrine or substituted for midodrine. The use of midodrine and/or droxidopa is at the discretion of the treatment team.
The objective of this study is to describe the safety and efficacy of droxidopa for IV vasopressor weaning in ICU patients.
2. Materials and Methods
This was a retrospective descriptive study at SHC, a 613-bed academic medical center. Patients were included if they were ≥18 years of age, admitted to the ICU, and received ≥4 consecutive doses of droxidopa for IV vasopressor weaning from January 2016 to March 2025. Patients were excluded for the following reasons: they were on droxidopa prior to admission, clinically deteriorated after droxidopa initiation, requiring vasopressor escalation for ≥12 h, or transitioned to comfort care within 48 h. Clinical deterioration was defined as acute bleeding, sepsis associated with new antibiotics, or acute cardiogenic shock. Clinical deterioration was determined through a chart review of daily progress notes and medication administration records. These patients would no longer be good candidates for oral vasoactive agents for IV vasopressor weaning. Patients were initially identified for inclusion using an institutional data repository and chart review was used to screen for exclusion(s). Data were further extracted by a review of the electronic medical record (EPIC) for all included patients.
Cessation of IV vasopressor without re-initiation within 72 h of droxidopa initiation was the primary outcome. The cut off of 72 h was chosen to give a pragmatic outcome, as weaning of IV vasopressors shortly after droxidopa initiation would be desired in most clinical scenarios where IV vasopressor use is the only barrier to ICU discharge. Adverse events assessed included hypotension, hypertension, and arrhythmias, as documented in progress notes within the electronic medical record. Additional data collected included patient demographics, pertinent past medical history, reason for ICU admission, IV vasopressor agent and number of IV vasopressors infusing at the time of droxidopa initiation, vasopressor dose in norepinephrine equivalents at time of droxidopa initiation, receipt of mechanical ventilation, dialysis modality, if any, midodrine dose at time of droxidopa initiation, time from ICU admission to droxidopa initiation, mean arterial pressure and systolic blood pressure goal at time of droxidopa initiation, time to IV vasopressor cessation, ICU readmission related to droxidopa, ICU and hospital length of stay, and if the patient was discharged on droxidopa. Norepinephrine equivalents were calculated with the following equation: epinephrine (mcg/kg/min) × 1 + phenylephrine (mcg/kg/min) × 0.06 + dopamine (mcg/kg/min) × 0.01 + vasopressin (units/min) × 2.5 [13]. This study protocol was approved by the Stanford Institutional Review Board and consent was waived. This study was conducted in accordance with the Helsinki Declaration of 1975 and with the ethical standards of the Stanford IRB.
Descriptive statistics were used to summarize results. Normality was assessed using the Shapiro–Wilk test. Parametric continuous variables were reported as mean and standard deviation, while nonparametric continuous variables were reported as median and interquartile range. The effects of the number of vasopressors, dialysis modality, midodrine dose, blood pressure goal, concomitant use of corticosteroids, and ICU type on the primary outcome were examined as exploratory analysis (results in Supplemental Materials). JASP Team (2024). JASP (Version 0.19.3, Amsterdam, The Netherlands) was used to perform all statistical analyses.
3. Results
A total of 112 patients were screened for inclusion, and 46 were included in the final analysis. The most common reason for exclusion was the use of droxidopa prior to admission (N = 25), followed by clinical deterioration (N = 24) (Figure 1). The median time from ICU admission to droxidopa initiation was 17 days. Forty-two patients (91%) were on midodrine at the time of droxidopa initiation. The median total daily midodrine dose was 80 mg. Intravenous vasopressors were infusing in 80% (n = 37) of patients at the time of droxidopa initiation. Patients were on a median of 1 IV vasopressor at the time of droxidopa initiation, with norepinephrine as the most common agent (50%, n = 23). The median IV vasopressor dose (in norepinephrine equivalents) at the time of droxidopa initiation was 0.05 mcg/kg/min (IQR 0.02–0.09). The most common reason for ICU admission was surgery (35%), followed by transplant (30%). Thirty patients were on dialysis at the time of droxidopa initiation, with 45% on intermittent hemodialysis (iHD) and 22% on continuous renal replacement therapy (CRRT). The median mean arterial pressure (MAP) and systolic blood pressure (SBP) goals at the time of droxidopa initiation were >60 and >100, respectively. All baseline characteristics are shown in Table 1.
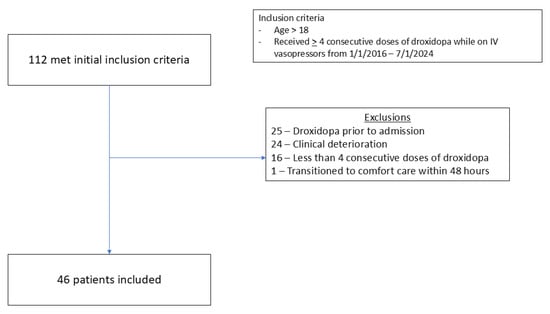
Figure 1.
Consort diagram for inclusion and exclusions.

Table 1.
Baseline characteristics.
The median initial daily dose of droxidopa was 300 mg, with a median max daily dose of 900 mg and median duration of 9 days. Nine (19%) patients received concomitant sedatives and twenty-eight (60%) patients received concomitant opioids at the time of droxidopa initiation. Concomitant steroids were used in 26 (57%) patients. Medication dosing and usage data are shown in Table 2.

Table 2.
Medication dosing and use.
Vasopressor(s) were discontinued within 72 h of droxidopa initiation in 46% (n = 18/39) of patients on IV vasopressors at the time of droxidopa initiation, with a median time to IV vasopressor cessation of 3.3 days. Intravenous vasopressors were restarted in the ICU for >24 h in 11% (n = 5) of patients. Vasopressor(s) were discontinued within 72 h of droxidopa initiation in 58% (15/26) of patients who were on one vasopressor at the time of droxidopa initiation compared to 27% (3/11) of patients who were on two or three vasopressors. The median time to vasopressor discontinuation was 3.2 days in patients who were on one vasopressor at the time of droxidopa initiation compared to 4.7 days in patients who were on two or more vasopressors (Supplemental Table S1). Vasopressors were discontinued within 72 h of droxidopa initiation in 70% (9/13) of patients on no dialysis at the time of droxidopa initiation compared to 50% (8/16) of patients on iHD and 10% (1/10) of patients on CRRT. The median time to vasopressor cessation was 2.3, 3.3, and 4.4 days in patients on no dialysis, iHD, and CRRT at the time of droxidopa initiation, respectively (Supplemental Table S2). There were no incidences of hypotension, hypertension, arrhythmias, or ICU readmissions related to droxidopa. Droxidopa was continued upon discharge in 29% (n = 11) of patients. All outcomes are shown in Table 3. Exploratory analyses are shown in Supplemental Tables S1–S3.

Table 3.
Outcomes.
4. Discussion
In this study including ICU patients receiving droxidopa for vasopressor weaning, 46% were successfully weaned off of IV vasopressor support within 72 h of droxidopa initiation with no observed adverse effects. The majority of patients received concomitant midodrine and had lower than normal MAP and/or SBP goals (usual MAP goal in ICU > 65). The median droxidopa duration was 9 days, and 29% of patients were discharged on droxidopa. A taper should be planned to reduce unnecessary therapy, adverse events, and polypharmacy. This study shows the potential use of droxidopa for IV vasopressor weaning in patients who are refractory or intolerant to midodrine.
The findings of this study are consistent with those of prior studies. Webb et al. conducted a retrospective observational study including 30 ICU patients and found that 70% of patients were weaned off of vasopressors within 7 days of droxidopa initiation, with a median time to vasopressor discontinuation of about 3 days [10]. Similarly, in this study, 46% of patients were weaned off of vasopressors within 3 days, and 79% (31/39) were weaned off within 7 days, with a median time to vasopressor discontinuation of 3.3 days. Lessing et al. conducted a retrospective cohort study comparing droxidopa vs. atomoxetine vs. atomoxetine and droxidopa for vasopressor weaning in cardiothoracic ICU patients [11]. Seventeen patients received droxidopa only, with a median time to IV vasopressor discontinuation of 8 days, which is longer than the 3.3 days observed in this study. This difference may be due to a difference in patient population, severity of illness, and adjunct medications used. Altogether, there is still limited data regarding the use of droxidopa for IV vasopressor weaning, with less than 100 total patients included across these studies, including the ones presented here.
At SHC, midodrine is often the first-line oral agent used for IV vasopressor weaning. Aside from initiating oral vasoactive agents, reducing the blood pressure goal (MAP > 60 vs. 65) may be another strategy used to facilitate IV vasopressor weaning or reduce vasopressor exposure, as demonstrated by Lamontagne et al., which was observed in this study [14]. In this study, 91% of patients were on midodrine at the time of droxidopa initiation. The median daily midodrine dose was 80 mg, with a max of 120 mg per day. Similarly, 90% and 100% of patients were on midodrine at the time of or 24 h prior to droxidopa initiation in the studies conducted by Webb et al. and Lesser et al., respectively. The average wholesale price of midodrine is USD 4.84–9.72 per 10 mg tablet, while droxidopa is typically more expensive, at USD 0.59–36.78 per 100 mg tablet, which may be one factor as to why midodrine is first-line at SHC and at the institutions included in prior studies [15,16]. The use of midodrine may be limited by bradycardia due to the autonomic reflex from unopposed alpha agonism, while droxidopa’s downstream mechanism is alpha-1/beta-1 agonism and has not been associated with bradycardia.
Corticosteroids may also be an alternative oral agent for refractory hypotension, specifically in the setting of adrenal insufficiency. Fludrocortisone, a mineralocorticoid with a labeled indication for orthostatic hypotension and wholesale price of USD 0.75–1.20 per 0.1 mg tablet, may serve as an additional oral option [17]. The use of a pure mineralocorticoid minimizes the risk of glucocorticoid side effects that may be potentially observed with corticosteroids such as hydrocortisone and prednisone. However, there is also limited evidence for the use of corticosteroids for vasopressor weaning. The majority of the literature regarding corticosteroids in the ICU revolves around the treatment of refractory septic shock [18]. In this study, 57% of patients received concomitant corticosteroids as an adjunct to augment blood pressure. In the study conducted by Webb et al., 43% of patients received adjunct fludrocortisone during their ICU admission, and 83% of patients in the study conducted by Lessing et al. received adjunct corticosteroids or fludrocortisone [10,11]. At SHC, fludrocortisone is often trialed in addition to midodrine in the absence of contraindications such as fluid overload or electrolyte abnormalities, which critically ill patients often have.
The optimal dose of droxidopa in this setting is not well described. The typical starting dose for its approved indication, neurogenic orthostatic hypotension, is 100 mg three times daily (TID), and may be titrated every 24–48 h to a maximum of 600 mg TID (6,12). In 89% (n = 41) of the patients, droxidopa 100 mg TID was the initial dose, and of those 70% (n = 29) required dose increase(s). Prior studies have also reported that 100 mg TID was the most frequent starting dose, and the median droxidopa dose at the time of vasopressor discontinuation was 600 mg/day [10,11]. Based on this data, it may be reasonable to start at an initial dose of 100–200 mg TID and titrate by increments of 100 mg TID every 24 h based on hemodynamic response.
There is limited guidance regarding the ideal patient population for oral vasopressor transition. In this study, patients on a single IV vasopressor at the time of droxidopa initiation had a shorter time to vasopressor cessation compared to patients on two or more IV vasopressors. The median vasopressor dose in norepinephrine equivalents at the time of droxidopa initiation was 0.05 mcg/kg/min, which is comparable to 0.06 mcg/kg/min observed in Webb et al. [10]. Anecdotally, patients on a single IV vasopressor at a low dose (norepinephrine dose < 0.05 mcg/kg/min) and with a resolved etiology of shock are ideal candidates for oral vasopressor transition. In this study, 65% of patients were on hemodialysis at the time of droxidopa initiation, which can cause hemodynamic instability with fluid shifts. Labile and/or low blood pressure is a common indication for CRRT in patients requiring dialysis, and droxidopa may facilitate the transition to iHD and subsequently out of the ICU. In this study of patients on CRRT, 10% (1/10) were weaned off vasopressors within 72 h of droxidopa initiation and 60% (6/10) within 7 days, with a median time of 6.2 days. Continuous renal replacement therapy at the time of droxidopa initiation reduced the likelihood of weaning off vasopressors at 72 h and had an increased time to vasopressor cessation compared to patients not receiving dialysis. The use of oral vasopressors may be a strategy to treat intradialytic hypotension and avoid the use of IV vasopressors [19]. The half-life of droxidopa is 2.5 h, with a time to steady state of approximately 24–48 h, while commonly used vasopressors such as norepinephrine have a half-life of <5 min [20].
There were several limitations to our study. The single-centered retrospective descriptive nature invites potential confounders inherent to such a design. During the study period, droxidopa initiation and dosing were at the discretion of the treating team, making it difficult to determine whether the addition of droxidopa was necessary, as midodrine doses may not have been optimized prior to initiation. Nine (20%) patients were weaned off of IV vasopressors prior to droxidopa initiation but were not meeting blood pressure goals on midodrine alone. In these cases, droxidopa was initiated rather than re-initiating IV vasopressors, which may reflect a real-world decision point. Data regarding other medications that may affect hemodynamics, such as concomitant sedatives, opioids, and/or corticosteroids, were collected; however, these were not exhaustive. Sedatives and opioids may potentially reduce blood pressure, while corticosteroids may increase blood pressure. Data regarding the indication for vasopressor initiation was not collected, making it difficult to assess if droxidopa is more or less effective in certain etiologies of prior shock. Blood pressure goals were also at the discretion of the primary team, although patients often had reduced goals to facilitate vasopressor weaning. Data regarding post-discharge outcomes were not collected, making it difficult to assess possible adverse events in patients discharged on droxidopa. The patients included in this study had long ICU and hospital stays (median 28 and 52 days), and these observations may not be generalizable to less acutely ill patients. Patients in the ICU also have many confounding factors that may impact blood pressure and clinical endpoints.
5. Conclusions
In patients who are refractory or intolerant to midodrine, enteral droxidopa may be a safe and effective option to facilitate weaning IV vasopressors. Given the retrospective design of this study, larger prospective and comparative studies are warranted to confirm these findings and to provide more guidance on identifying the ideal patient population, optimal dosing, and place in therapy.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/therapeutics2040020/s1. Table S1: vasopressor outcomes by number of vasopressors at time of droxidopa initiation; Table S2: vasopressor outcomes by dialysis modality for patients on vasopressors at time of droxidopa initiation; Table S3: primary outcome stratified by midodrine dose, steroid exposure, blood pressure targets, and ICU type.
Author Contributions
Conceptualization, C.D.; methodology, C.D., D.V., and A.K.; formal analysis, C.D.; investigation, C.D. and D.V., data curation, C.D. and D.V.; writing—original draft preparation, C.D. and D.V.; writing—review and editing, C.D., D.V. and A.K.; project administration, C.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study protocol was approved by the Stanford Institutional Review Board (IRB # 73566, approval date 1 August 2024). This study was conducted in accordance with the ethical standards of the Stanford IRB and with the Helsinki Declaration of 1975.
Informed Consent Statement
Informed consent was not obtained as this was a retrospective chart review.
Data Availability Statement
The de-identified data presented in this study are available on request from the corresponding author.
Acknowledgments
This article is a revised and expanded version of an abstract entitled “Use of droxidopa to facilitate intravenous vasopressor weaning in critically ill patients”, which was presented at The Society of Critical Care Congress in Orlando, Florida, USA, on 23 February 2025.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- ProAmatine (Midodrine) Prescribing Information; Shire US Inc.: Lexington, MA, USA, 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019815s010lbl.pdf (accessed on 6 January 2025).
- Levine, A.R.; Meyer, M.J.; Bittner, E.A.; Berg, S.; Kalman, R.; Stanislaus, A.B.; Ryan, C.; Ball, S.A.; Eikermann, M. Oral midodrine treatment accelerates the liberation of intensive care unit patients from intravenous vasopressor infusions. J. Crit. Care 2013, 28, 756–762. [Google Scholar] [CrossRef]
- Whitson, M.R.; Mo, E.; Nabi, T.; Healy, L.; Koenig, S.; Narasimhan, M.; Mayo, P.H. Feasibility, Utility, and Safety of Midodrine During Recovery Phase from Septic Shock. Chest 2016, 149, 1380–1383. [Google Scholar] [CrossRef]
- Santer, P.; Anstey, M.H.; Patrocínio, M.D.; Wibrow, B.; Teja, B.; Shay, D.; Shaefi, S.; Parsons, C.S.; Houle, T.T.; Eikermann, M.; et al. Effect of midodrine versus placebo on time to vasopressor discontinuation in patients with persistent hypotension in the intensive care unit (MIDAS): An international randomised clinical trial. Intensive Care Med. 2020, 46, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pinto, R.; Yong, Z.T.; Yanase, F.; Young, C.; Brown, A.; Udy, A.; Young, P.J.; Eastwood, G.; Bellomo, R. A pilot, feasibility, randomised controlled trial of midodrine as adjunctive vasopressor for low-dose vasopressor-dependent hypotension in intensive care patients: The MAVERIC study. J. Crit. Care 2022, 67, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.J.; Rauniyar, R.; Jacques, A.; Palmer, R.N.; Wibrow, B.; Anstey, M.H. Oral midodrine does not expedite liberation from protracted vasopressor infusions: A case-control study. Anaesth. Intensive Care 2023, 51, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Northera (Droxidopa) Prescribing Information; Chelsea Therapeutics Inc.: Charlotte, NC, USA, 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/203202lbl.pdf (accessed on 6 January 2025).
- Ammar, A.; Hong, C.; Koo, A.; Elsamadicy, A.; Ammar, M.; Laurans, M.; Landreneau, M. 904: Combination of droxidopa and midodrine for hemodynamic augmentation in acute spinal cord injury. Crit. Care Med. 2022, 50, 448. [Google Scholar] [CrossRef]
- Hong, C.S.; Effendi, M.K.; Ammar, A.A.; Owusu, K.A.; Ammar, M.A.; Koo, A.B.; Lamsam, L.A.; Elsamadicy, A.A.; Kuzmik, G.A.; Laurans, M.; et al. Use of droxidopa for blood pressure augmentation after acute spinal cord injury: Case reports. Acute Crit. Care 2025, 40, 138–143. [Google Scholar] [CrossRef]
- Webb, A.J.; Casal, G.L.; Newman, K.A.; Culshaw, J.R.; Northam, K.A.; Solomon, E.J.; Beargie, S.M.; Johnson, R.B.; Lopez, N.D.; Hayes, B.D.; et al. Droxidopa for Vasopressor Weaning in Critically Ill Patients with Persistent Hypotension: A Multicenter, Retrospective, Single-Arm Observational Study. J. Intensive Care Med. 2025, 40, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Lessing, J.K.; Kram, S.J.; Levy, J.H.; Grecu, L.M.; Katz, J.N. Droxidopa or Atomoxetine for Refractory Hypotension in Critically Ill Cardiothoracic Surgery Patients. J. Cardiothorac. Vasc. Anesth. 2024, 38, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Diep, C.; Veloria, D.; Koessel, D.; Kloosterboer, A. 914: Use of droxidopa to facilitate intravenous vasopressor weaning in critically ill patients. Crit. Care Med. 2025, 53, 1. [Google Scholar] [CrossRef]
- Kotani, Y.; Di Gioia, A.; Landoni, G.; Belletti, A.; Khanna, A.K. An updated “norepinephrine equivalent” score in intensive care as a marker of shock severity. Crit. Care 2023, 27, 29, Erratum in Crit. Care 2025, 29, 104. https://doi.org/10.1186/s13054-025-05250-9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamontagne, F.; Richards-Belle, A.; Thomas, K.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Camsooksai, J.; Darnell, R.; Gordon, A.C.; Henry, D.; et al. Effect of Reduced Exposure to Vasopressors on 90-Day Mortality in Older Critically Ill Patients with Vasodilatory Hypotension: A Randomized Clinical Trial. JAMA 2020, 323, 938–949. [Google Scholar] [CrossRef]
- Midodrine. In: Lexi-Drugs [Database on the Internet] Wolters Kluwer Waltham, M.A. 2025. Available online: https://online.lexi.com (accessed on 9 April 2025).
- Droxidopa. In: Lexi-Drugs [Database on the Internet] Wolters Kluwer Waltham, M.A. 2025. Available online: https://online.lexi.com (accessed on 9 April 2025).
- Fludrocortisone. In: Lexi-Drugs [Database on the Internet] Wolters Kluwer Waltham, M.A. 2025. Available online: https://online.lexi.com (accessed on 9 April 2025).
- Liang, H.; Song, H.; Zhai, R.; Song, G.; Li, H.; Ding, X.; Kan, Q.; Sun, T. Corticosteroids for Treating Sepsis in Adult Patients: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 709155. [Google Scholar] [CrossRef] [PubMed]
- Husarek, K.; Brunelli, S.M. Midodrine Is an Effective Therapy for Resistant Intradialytic Hypotension: CON. Kidney360 2023, 4, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Norepinephrine. In: Lexi-Drugs [Database on the Internet] Wolters Kluwer Waltham, M.A. 2025. Available online: https://online.lexi.com (accessed on 15 October 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).