The Golden Hamster: A Valuable Model for Designing Cancer Therapies
Abstract
1. Introduction
2. The Necessity for a Reliable Animal Model
2.1. Limitations
2.2. Current Alternatives
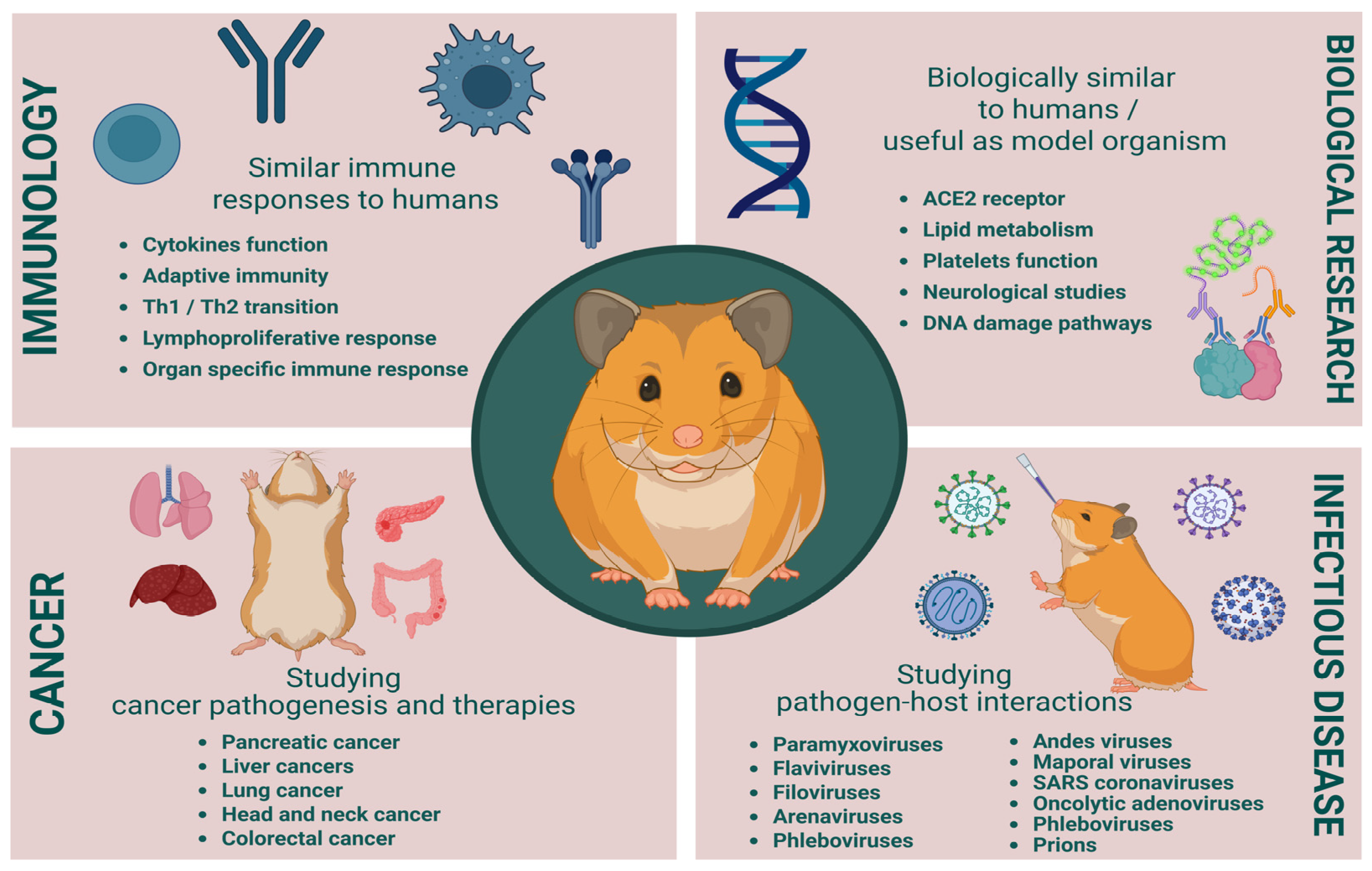
3. Unique Biological Similarity Between Hamsters and Humans
3.1. Anatomical and Physiological Advantages of the Syrian Hamster
3.2. Lung: Similarities and Differences
3.3. Pancreas: Similarities and Differences
3.4. Liver: Similarities and Differences
3.5. Gastrointestinal Tract: Similarities and Differences
4. Golden Syrian Hamsters in Cancer Research
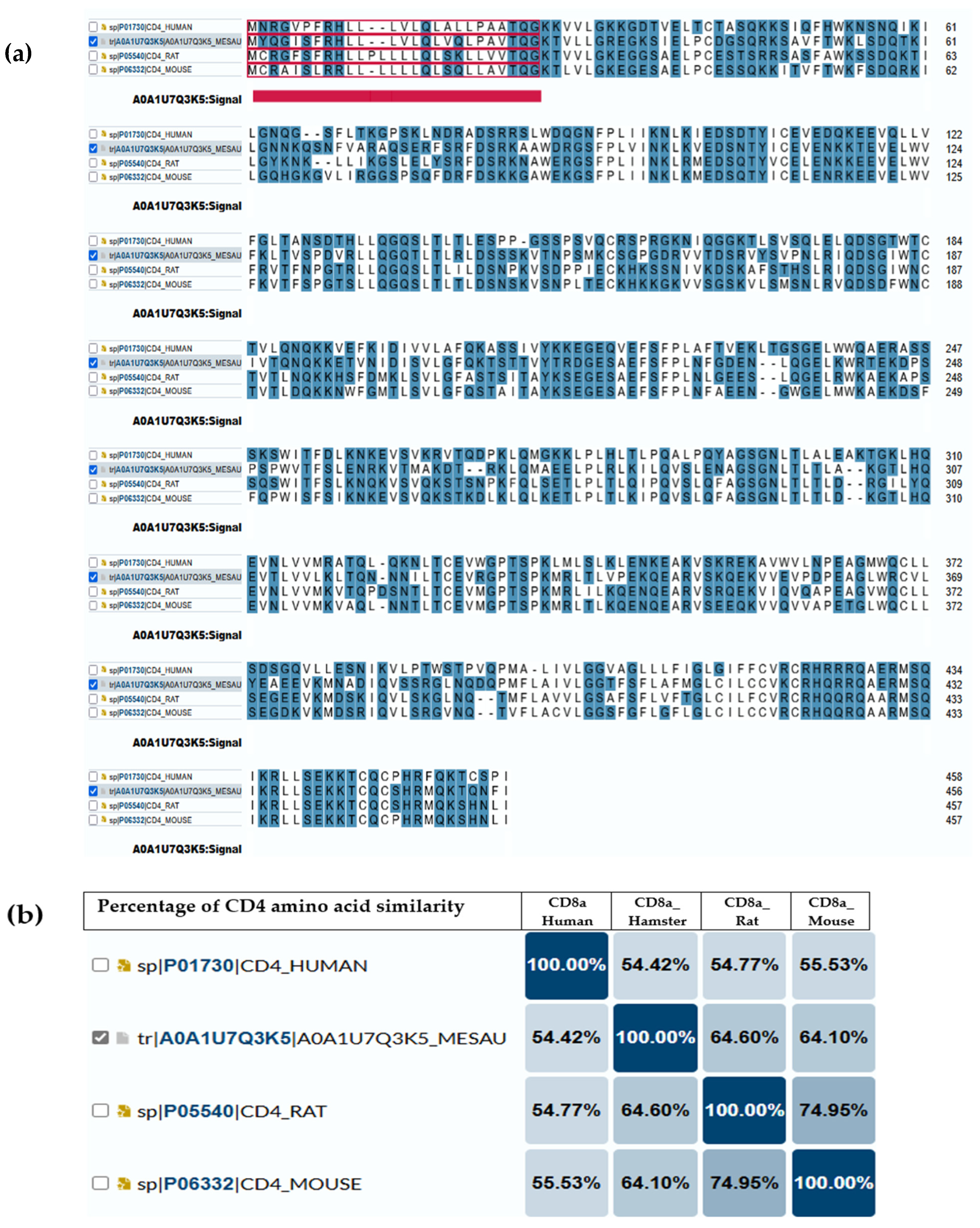
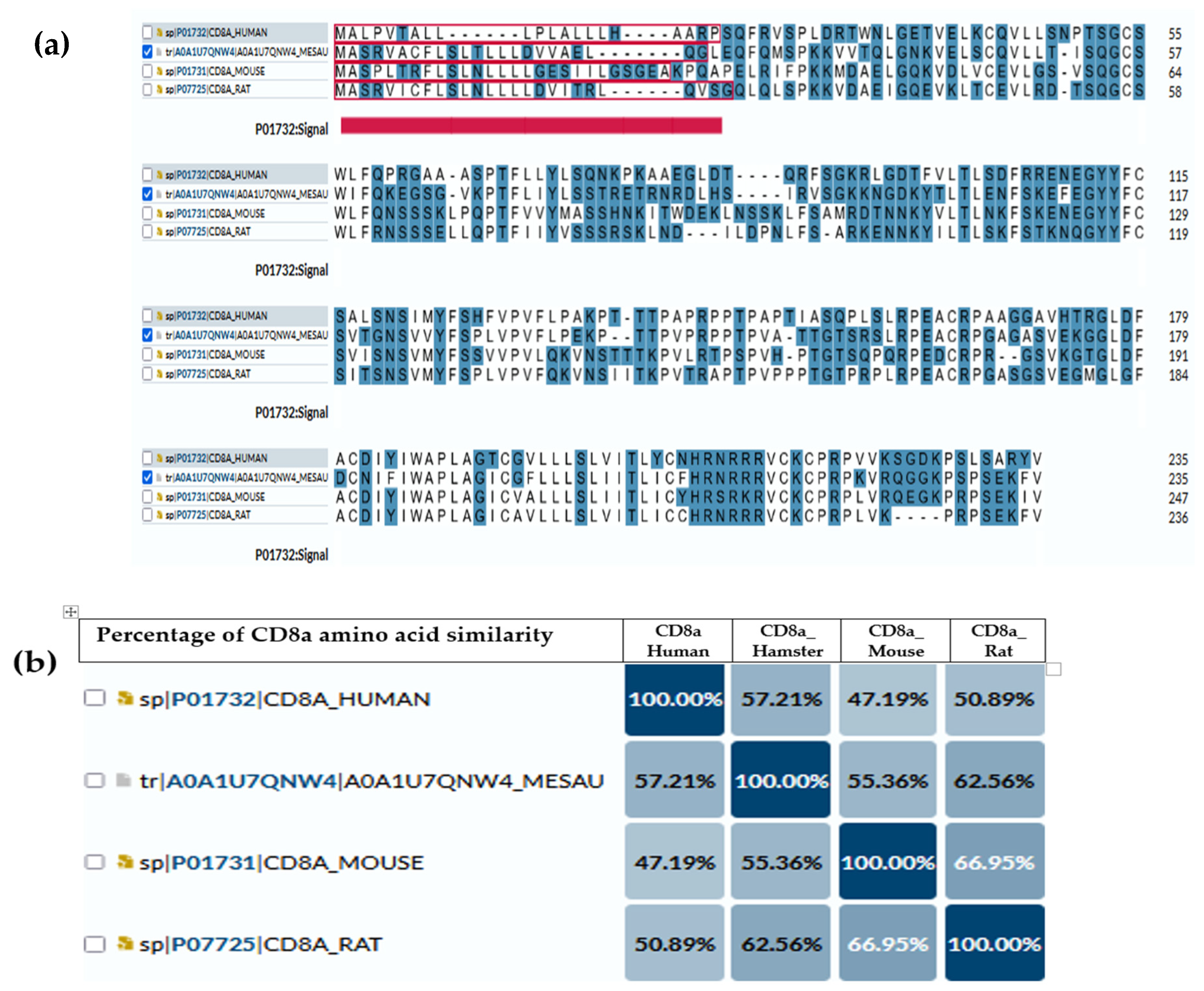
5. Immunological Connections Between Hamsters and Human
Immune System Parallels and Divergences
6. Limitations with Hamster Research
- (a)
- Limited Immunological Tools and Reagents: Compared to mice and rats, fewer antibodies and immunological assays are commercially available for hamsters because of limited studies determining the functionality and expression of these markers, thus hampering detailed immune response analyses [20].
- (b)
- Incomplete Genome Annotation: Hamster genome sequencing has been achieved, but functional annotation of immune-related genes remains incomplete, restricting genetic manipulation capabilities. This limitation may be addressed firstly by employing long-read sequencing to improve the contiguity and accuracy of the genome assembly [141]. Second, the application of RNA-Seq across multiple tissues and developmental time points enables a detailed characterization of the transcriptome and enhances gene annotation through the identification of a wide range of transcripts. The third objective is to use comparative genomics to predict gene structures by aligning hamster sequences against the well-annotated genomes of related species like mice and rats. This method improves annotation accuracy through the utilization of conserved genomic features [141]. Fourthly, bioinformatics tools will be utilized to functionally annotate predicted genes and elucidate gene roles and interactions based on domain content and homology [139]. Finally, experimental validation of gene models by CRISPR/cas9 knockouts and RT-PCR will be required to confirm the functions, and structure will be needed.
- (c)
- Uncharacterized Immune System Components: Certain immune mechanisms, such as cytokine interactions and T-cell responses, are not fully understood in hamsters, making it difficult to extrapolate findings directly to humans. Comparative analysis of immune responses in simplified experimental models (human and murine) and corresponding disease states may elucidate cytokine/chemokine secretion patterns. Analysis of diverse trials across various cancers and infectious disease models, stratified by disease severity, will elucidate the patterns of immune mediator secretion, including source, target cells, concentrations, and biological effects.
- (d)
- Unknown ability to correlate with human outcomes: Usually, nine out of ten drugs that appear promising in animal studies fail in human clinical trials. By characterizing the immune response and performing genomic annotation, we can better understand the disparities between human and murine models, thereby improving the translation of hamster study results to human outcomes.
- (e)
- Lack of Standardization: The disease outcomes can be influenced by variables such as inoculation dose and volume, requiring careful experimental design for reproducibility and comparison across studies [142]. Once fundamental biological data from hamsters, including immune mechanisms, available immunological CD markers, cytokine/chemokine secretion, genomic annotation, and metabolic processes, are established, subsequent cancer research will benefit from enhanced standardization and reproducibility.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dominguez-Oliva, A.; Hernandez-Avalos, I.; Martinez-Burnes, J.; Olmos-Hernandez, A.; Verduzco-Mendoza, A.; Mota-Rojas, D. The Importance of Animal Models in Biomedical Research: Current Insights and Applications. Animals 2023, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Soufizadeh, P.; Mansouri, V.; Ahmadbeigi, N. A review of animal models utilized in preclinical studies of approved gene therapy products: Trends and insights. Lab. Anim. Res. 2024, 40, 17. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.J.; Grieder, F.B. The continued importance of animals in biomedical research. Lab Anim. 2024, 53, 295–297. [Google Scholar] [CrossRef]
- Zhao, X.; Bhattacharyya, A. Human Models Are Needed for Studying Human Neurodevelopmental Disorders. Am. J. Hum. Genet. 2018, 103, 829–857. [Google Scholar] [CrossRef] [PubMed]
- Bjornson-Hooper, Z.B.; Fragiadakis, G.K.; Spitzer, M.H.; Chen, H.; Madhireddy, D.; Hu, K.; Lundsten, K.; McIlwain, D.R.; Nolan, G.P. A Comprehensive Atlas of Immunological Differences Between Humans, Mice, and Non-Human Primates. Front. Immunol. 2022, 13, 867015. [Google Scholar] [CrossRef]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Part 2: Potential Alternatives to the Use of Animals in Preclinical Trials. JACC Basic Transl. Sci. 2020, 5, 387–397. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, S.; Li, X.J. Genetically modified large animal models for investigating neurodegenerative diseases. Cell Biosci. 2021, 11, 218. [Google Scholar] [CrossRef]
- MacDougall, G.; Brown, L.Y.; Kantor, B.; Chiba-Falek, O. The Path to Progress Preclinical Studies of Age-Related Neurodegenerative Diseases: A Perspective on Rodent and hiPSC-Derived Models. Mol. Ther. 2021, 29, 949–972. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: A review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef]
- Bracken, M.B. Why animal studies are often poor predictors of human reactions to exposure. J. R. Soc. Med. 2009, 102, 120–122. [Google Scholar] [CrossRef]
- Monteggia, L.M.; Heimer, H.; Nestler, E.J. Meeting Report: Can We Make Animal Models of Human Mental Illness? Biol. Psychiatry 2018, 84, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Hylander, B.L.; Repasky, E.A.; Sexton, S. Using Mice to Model Human Disease: Understanding the Roles of Baseline Housing-Induced and Experimentally Imposed Stresses in Animal Welfare and Experimental Reproducibility. Animals 2022, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Nishikawa, M.; Kutsuzawa, N.; Tokito, F.; Kobayashi, T.; Kurniawan, D.A.; Shioda, H.; Cao, W.; Shinha, K.; Nakamura, H.; et al. Advancements in Microphysiological systems: Exploring organoids and organ-on-a-chip technologies in drug development focus on pharmacokinetics related organs. Drug Metab. Pharmacokinet. 2025, 60, 101046. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Li, C.; Cao, J.; Cui, Z.; Du, J.; Fu, Z.; Yang, H.; Chen, P. Organ-on-a-chip meets artificial intelligence in drug evaluation. Theranostics 2023, 13, 4526–4558. [Google Scholar] [CrossRef]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Wang, Z.; Cormier, R.T. Golden Syrian Hamster Models for Cancer Research. Cells 2022, 11, 2395. [Google Scholar] [CrossRef]
- Miao, J.; Chard, L.S.; Wang, Z.; Wang, Y. Syrian Hamster as an Animal Model for the Study on Infectious Diseases. Front. Immunol. 2019, 10, 2329. [Google Scholar] [CrossRef]
- Warner, B.M.; Safronetz, D.; Kobinger, G.P. Syrian Hamsters as a Small Animal Model for Emerging Infectious Diseases: Advances in Immunologic Methods. Adv. Exp. Med. Biol. 2017, 972, 87–101. [Google Scholar] [CrossRef]
- Saini, S.; Rai, A.K. Hamster, a close model for visceral leishmaniasis: Opportunities and challenges. Parasite Immunol. 2020, 42, e12768. [Google Scholar] [CrossRef] [PubMed]
- Miedel, E.L.; Hankenson, F.C. Chapter 5—Biology and Diseases of Hamsters. In Laboratory Animal Medicine, 3rd ed.; Fox, J.G., Anderson, L.C., Otto, G.M., Pritchett-Corning, K.R., Whary, M.T., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 209–245. [Google Scholar]
- Kliman, R.M.; Lynch, G.R. Evidence for independence of circadian characters and extent of photoresponsiveness in the Djungarian hamster, Phodopus sungorus. J. Biol. Rhythm 1991, 6, 159–166. [Google Scholar] [CrossRef]
- Hankenson, F.C.; Van Hoosier, G.L. Chapter 5—Biology and Diseases of Hamsters. In Laboratory Animal Medicine, 2nd ed.; Fox, J.G., Anderson, L.C., Loew, F.M., Quimby, F.W., Eds.; Academic Press: Burlington, MA, USA, 2002; pp. 167–202. [Google Scholar]
- Martin, B.; Ji, S.; Maudsley, S.; Mattson, M.P. “Control” laboratory rodents are metabolically morbid: Why it matters. Proc. Natl. Acad. Sci. USA 2010, 107, 6127–6133. [Google Scholar] [CrossRef]
- Dedoni, S.; Scherma, M.; Camoglio, C.; Siddi, C.; Dazzi, L.; Puliga, R.; Frau, J.; Cocco, E.; Fadda, P. An overall view of the most common experimental models for multiple sclerosis. Neurobiol. Dis. 2023, 184, 106230. [Google Scholar] [CrossRef]
- Masopust, D.; Sivula, C.P.; Jameson, S.C. Of Mice, Dirty Mice, and Men: Using Mice To Understand Human Immunology. J. Immunol. 2017, 199, 383–388. [Google Scholar] [CrossRef]
- Lai, F.; Chen, Q. Humanized Mouse Models for the Study of Infection and Pathogenesis of Human Viruses. Viruses 2018, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Coers, J.; Starnbach, M.N.; Howard, J.C. Modeling infectious disease in mice: Co-adaptation and the role of host-specific IFNgamma responses. PLoS Pathog. 2009, 5, e1000333. [Google Scholar] [CrossRef]
- Ottaiano, A.; Ianniello, M.; Santorsola, M.; Ruggiero, R.; Sirica, R.; Sabbatino, F.; Perri, F.; Cascella, M.; Di Marzo, M.; Berretta, M.; et al. From Chaos to Opportunity: Decoding Cancer Heterogeneity for Enhanced Treatment Strategies. Biology 2023, 12, 1183. [Google Scholar] [CrossRef] [PubMed]
- Walrath, J.C.; Hawes, J.J.; Van Dyke, T.; Reilly, K.M. Genetically engineered mouse models in cancer research. Adv. Cancer Res. 2010, 106, 113–164. [Google Scholar] [CrossRef]
- Sarkar, S.; Heise, M.T. Mouse Models as Resources for Studying Infectious Diseases. Clin. Ther. 2019, 41, 1912–1922. [Google Scholar] [CrossRef]
- Avdoshina, D.V.; Kondrashova, A.S.; Belikova, M.G.; Bayurova, E.O. Murine Models of Chronic Viral Infections and Associated Cancers. Mol. Biol. 2022, 56, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.K.; Pheby, D.; Henehan, G.; Brown, R.; Sieving, P.; Sykora, P.; Marks, R.; Falsini, B.; Capodicasa, N.; Miertus, S.; et al. Ethical considerations regarding animal experimentation. J. Prev. Med. Hyg. 2022, 63, E255–E266. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A. The flaws and human harms of animal experimentation. Camb. Q. Healthc. Ethics 2015, 24, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Cui, W.; Zhang, B.; Fonseca, P.; Zhao, Q.; Zhang, P.; Xu, B.; Zhang, Q.; Li, Z.; Seashore-Ludlow, B.; et al. Patient-derived organoids in precision cancer medicine. Med 2024, 5, 1351–1377. [Google Scholar] [CrossRef]
- Azar, J.; Bahmad, H.F.; Daher, D.; Moubarak, M.M.; Hadadeh, O.; Monzer, A.; Al Bitar, S.; Jamal, M.; Al-Sayegh, M.; Abou-Kheir, W. The Use of Stem Cell-Derived Organoids in Disease Modeling: An Update. Int. J. Mol. Sci. 2021, 22, 7667. [Google Scholar] [CrossRef]
- Wang, H.; Brown, P.C.; Chow, E.C.Y.; Ewart, L.; Ferguson, S.S.; Fitzpatrick, S.; Freedman, B.S.; Guo, G.L.; Hedrich, W.; Heyward, S.; et al. 3D cell culture models: Drug pharmacokinetics, safety assessment, and regulatory consideration. Clin. Transl. Sci. 2021, 14, 1659–1680. [Google Scholar] [CrossRef]
- Kondo, J.; Inoue, M. Application of Cancer Organoid Model for Drug Screening and Personalized Therapy. Cells 2019, 8, 470. [Google Scholar] [CrossRef]
- Bose, S.; Clevers, H.; Shen, X. Promises and Challenges of Organoid-Guided Precision Medicine. Med 2021, 2, 1011–1026. [Google Scholar] [CrossRef]
- El Harane, S.; Zidi, B.; El Harane, N.; Krause, K.H.; Matthes, T.; Preynat-Seauve, O. Cancer Spheroids and Organoids as Novel Tools for Research and Therapy: State of the Art and Challenges to Guide Precision Medicine. Cells 2023, 12, 1001. [Google Scholar] [CrossRef]
- Rudroff, T. Artificial Intelligence as a Replacement for Animal Experiments in Neurology: Potential, Progress, and Challenges. Neurol. Int. 2024, 16, 805–820. [Google Scholar] [CrossRef]
- Ezanno, P.; Picault, S.; Beaunee, G.; Bailly, X.; Munoz, F.; Duboz, R.; Monod, H.; Guegan, J.F. Research perspectives on animal health in the era of artificial intelligence. Vet. Res. 2021, 52, 40. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.C.; Enoch, S.J.; Paini, A.; Cronin, M.T.D. A Review of In Silico Tools as Alternatives to Animal Testing: Principles, Resources and Applications. Altern. Lab. Anim. 2020, 48, 146–172. [Google Scholar] [CrossRef]
- Barre-Sinoussi, F.; Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Future Sci. OA 2015, 1, FSO63. [Google Scholar] [CrossRef]
- VandeBerg, J.L.; Williams-Blangero, S. Advantages and limitations of nonhuman primates as animal models in genetic research on complex diseases. J. Med. Primatol. 1997, 26, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Estes, J.D.; Wong, S.W.; Brenchley, J.M. Nonhuman primate models of human viral infections. Nat. Rev. Immunol. 2018, 18, 390–404. [Google Scholar] [CrossRef]
- Proetzel, G.; Wiles, M.V.; Roopenian, D.C. Genetically engineered humanized mouse models for preclinical antibody studies. BioDrugs 2014, 28, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Legrand, N.; Ploss, A.; Balling, R.; Becker, P.D.; Borsotti, C.; Brezillon, N.; Debarry, J.; de Jong, Y.; Deng, H.; Di Santo, J.P.; et al. Humanized mice for modeling human infectious disease: Challenges, progress, and outlook. Cell Host Microbe 2009, 6, 5–9. [Google Scholar] [CrossRef]
- Ma, M.; Ge, J.Y.; Nie, Y.Z.; Li, Y.M.; Zheng, Y.W. Developing Humanized Animal Models with Transplantable Human iPSC-Derived Cells. Front. Biosci. 2024, 29, 34. [Google Scholar] [CrossRef]
- Allen, T.M.; Brehm, M.A.; Bridges, S.; Ferguson, S.; Kumar, P.; Mirochnitchenko, O.; Palucka, K.; Pelanda, R.; Sanders-Beer, B.; Shultz, L.D.; et al. Humanized immune system mouse models: Progress, challenges and opportunities. Nat. Immunol. 2019, 20, 770–774. [Google Scholar] [CrossRef]
- Zhou, Q.; Facciponte, J.; Jin, M.; Shen, Q.; Lin, Q. Humanized NOD-SCID IL2rg−/− mice as a preclinical model for cancer research and its potential use for individualized cancer therapies. Cancer Lett. 2014, 344, 13–19. [Google Scholar] [CrossRef]
- Tillman, H.; Janke, L.J.; Funk, A.; Vogel, P.; Rehg, J.E. Morphologic and Immunohistochemical Characterization of Spontaneous Lymphoma/Leukemia in NSG Mice. Vet. Pathol. 2020, 57, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Vaeteewoottacharn, K.; Kariya, R. Application of Highly Immunocompromised Mice for the Establishment of Patient-Derived Xenograft (PDX) Models. Cells 2019, 8, 889. [Google Scholar] [CrossRef]
- de Arruda, M.S.; Montenegro, M.R. The hamster cheek pouch: An immunologically privileged site suitable to the study of granulomatous infections. Rev. Inst. Med. Trop. Sao Paulo 1995, 37, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.A. Chapter 27—Anatomy, Physiology, and Behavior. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Suckow, M.A., Stevens, K.A., Wilson, R.P., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 753–763. [Google Scholar]
- Williams, D.E.; Evans, D.M.; Blamey, R.W. The primary implantation of human tumours to the hamster cheek pouch. Br. J. Cancer 1971, 25, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.M.; Li, S.; Gumin, J.; Daou, M.; Ledbetter, D.; Yang, J.; Singh, S.; Parker Kerrigan, B.C.; Hossain, A.; Yuan, Y.; et al. An immune-competent, replication-permissive Syrian Hamster glioma model for evaluating Delta-24-RGD oncolytic adenovirus. Neuro-Oncology 2021, 23, 1911–1921. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Y.; Dunmall, L.S.C.; Lemoine, N.R.; Wang, P.; Wang, Y. Syrian hamster as an ideal animal model for evaluation of cancer immunotherapy. Front. Immunol. 2023, 14, 1126969. [Google Scholar] [CrossRef]
- Phelps, C.; Huey, D.D.; Niewiesk, S. Production of Humanized Mice through Stem Cell Transfer. Curr. Protoc. 2023, 3, e800. [Google Scholar] [CrossRef]
- Puchalapalli, M.; Zeng, X.; Mu, L.; Anderson, A.; Hix Glickman, L.; Zhang, M.; Sayyad, M.R.; Mosticone Wangensteen, S.; Clevenger, C.V.; Koblinski, J.E. NSG Mice Provide a Better Spontaneous Model of Breast Cancer Metastasis than Athymic (Nude) Mice. PLoS ONE 2016, 11, e0163521. [Google Scholar] [CrossRef]
- Kaur, K.; Jewett, A. Differences in Tumor Growth and Differentiation in NSG and Humanized-BLT Mice; Analysis of Human vs. Humanized-BLT-Derived NK Expansion and Functions. Cancers 2022, 15, 112. [Google Scholar] [CrossRef]
- Hanazawa, A.; Ito, R.; Katano, I.; Kawai, K.; Goto, M.; Suemizu, H.; Kawakami, Y.; Ito, M.; Takahashi, T. Generation of Human Immunosuppressive Myeloid Cell Populations in Human Interleukin-6 Transgenic NOG Mice. Front. Immunol. 2018, 9, 152. [Google Scholar] [CrossRef]
- Barker, C.F.; Billingham, R.E. The lymphatic status of hamster cheek pouch tissue in relation to its properties as a graft and as a graft site. J. Exp. Med. 1971, 133, 620–639. [Google Scholar] [CrossRef]
- Banerjee, J.; Papu John, A.M.; Al-Wadei, M.H.; Schuller, H.M. Prevention of pancreatic cancer in a hamster model by cAMP decrease. Oncotarget 2016, 7, 44430–44441. [Google Scholar] [CrossRef]
- Wiblin, C.N.; MacPherson, I.A. The transformation of BHK 21 hamster cells by simian virus 40. Int. J. Cancer 1972, 10, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.M.; Keir, H.M. Excretion of polyamines from baby hamster kidney cells (BHK-21/C13: Effect of infection with Herpes Simplex Virus Type 1. J. Gen. Virol. 1981, 56, 251–258. [Google Scholar] [CrossRef]
- Taylor, H.C.; Fallon, M.D.; Velasco, M.E. Oncogenic osteomalacia and inappropriate antidiuretic hormone secretion due to oat-cell carcinoma. Ann. Intern. Med. 1984, 101, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Sandham, J.S. Health Promotion Authority for Wales. Br. Dent. J. 1989, 167, 370–371. [Google Scholar] [CrossRef]
- Fossati, C. [Collateral effects of an anti-arrhythmic agent: Amiodarone]. Clin. Ter. 1985, 114, 509–514. [Google Scholar] [PubMed]
- Young, B.A.; Spencer, J.F.; Ying, B.; Tollefson, A.E.; Toth, K.; Wold, W.S. The role of cyclophosphamide in enhancing antitumor efficacy of an adenovirus oncolytic vector in subcutaneous Syrian hamster tumors. Cancer Gene Ther. 2013, 20, 521–530. [Google Scholar] [CrossRef]
- Urmanova, M.A.; Tsareva, A.A. [The cytogenetic study of established Syrian hamster cell lines. II. A comparative analysis of the karyotypes of cell lines HaK, CER and BHK-21 (C-13)]. Tsitologiia 1996, 38, 639–645. [Google Scholar]
- Suklabaidya, S.; Das, B.; Ali, S.A.; Jain, S.; Swaminathan, S.; Mohanty, A.K.; Panda, S.K.; Dash, P.; Chakraborty, S.; Batra, S.K.; et al. Characterization and use of HapT1-derived homologous tumors as a preclinical model to evaluate therapeutic efficacy of drugs against pancreatic tumor desmoplasia. Oncotarget 2016, 7, 41825–41842. [Google Scholar] [CrossRef]
- Abraham, A.T.; Shah, S.R.; Davidson, B.R. The HaP-T1 Syrian golden hamster pancreatic cancer model: Cell implantation is better than tissue implantation. Pancreas 2004, 29, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Vanathi, M.; Azimeera, S.; Gupta, N.; Tandon, R. Study on change in corneal biomechanics and effect of percent tissue altered in myopic laser-assisted in situ keratomileusis. Indian J. Ophthalmol. 2020, 68, 2964–2974. [Google Scholar] [CrossRef]
- Maistro, E.L.; Carvalho, J.C.; Mantovani, M.S. Evaluation of the genotoxic potential of the Casearia sylvestris extract on HTC and V79 cells by the comet assay. Toxicol. In Vitro 2004, 18, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Kitsak, V.; Mikhailova, G.R.; Tsareva, A.A.; Gushchina, E.A.; Novokhatskii, A.S. [Morphological, cytogenetic and proliferative characteristics of Syrian hamster HTC-2 and HTC-1 cells and of the HTCT tumor cell line transformed by herpes simplex type 2 virus]. Vopr. Virusol. 1982, 27, 415–418. [Google Scholar] [PubMed]
- Silva Gunawardene, Y.I.; Bendena, W.G.; Tobe, S.S.; Chan, S.M. Comparative immunohistochemistry and cellular distribution of farnesoic acid O-methyltransferase in the shrimp and the crayfish. Peptides 2003, 24, 1591–1597. [Google Scholar] [CrossRef]
- Oswiecimska, J.; Brus, R.; Szkilnik, R.; Nowak, P.; Kostrzewa, R.M. 7-OH-DPAT, unlike quinpirole, does not prime a yawning response in rats. Pharmacol. Biochem. Behav. 2000, 67, 11–15. [Google Scholar] [CrossRef]
- Englebienne, P. The serum steroid transport proteins: Biochemistry and clinical significance. Mol. Asp. Med. 1984, 7, 313–396. [Google Scholar] [CrossRef]
- Byers, T.L.; Bitonti, A.J.; McCann, P.P. bis(benzyl)polyamine analogues are substrates for a mammalian cell-transport system which is distinct from the polyamine-transport system. Biochem. J. 1990, 269, 35–40. [Google Scholar] [CrossRef]
- Jeon, M.J.; Ko, H.; Shin, S.J.; Kim, M. Cytotoxicity and genotoxicity of various types of endodontic sealers in Chinese hamster ovary (CHO-K1) cells. Dent. Mater. J. 2023, 42, 774–779. [Google Scholar] [CrossRef]
- Moore, G.J.; Bebchuk, J.M.; Hasanat, K.; Chen, G.; Seraji-Bozorgzad, N.; Wilds, I.B.; Faulk, M.W.; Koch, S.; Glitz, D.A.; Jolkovsky, L.; et al. Lithium increases N-acetyl-aspartate in the human brain: In vivo evidence in support of bcl-2’s neurotrophic effects? Biol. Psychiatry 2000, 48, 1–8. [Google Scholar] [CrossRef]
- Mire-Sluis, A.R.; Hoffbrand, A.V.; Wickremasinghe, R.G. Evidence that guanine-nucleotide binding regulatory proteins couple cell-surface receptors to the breakdown of inositol-containing lipids during T-lymphocyte mitogenesis. Biochem. Biophys. Res. Commun. 1987, 148, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- De, S. Bronchus-associated Lymphoid Tissue Lymphoma. J. Bronchol. Interv. Pulmonol. 2011, 18, 295–296. [Google Scholar] [CrossRef]
- Braxton, A.M.; Creisher, P.S.; Ruiz-Bedoya, C.A.; Mulka, K.R.; Dhakal, S.; Ordonez, A.A.; Beck, S.E.; Jain, S.K.; Villano, J.S. Hamsters as a Model of Severe Acute Respiratory Syndrome Coronavirus-2. Comp. Med. 2021, 71, 398–410. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Desrosiers, A.; Terzaghi, M.; Little, J.B. Morphometric and histological analysis of the lungs of Syrian golden hamsters. J. Anat. 1978, 125, 527–553. [Google Scholar]
- Imai, M.; Iwatsuki-Horimoto, K.; Hatta, M.; Loeber, S.; Halfmann, P.J.; Nakajima, N.; Watanabe, T.; Ujie, M.; Takahashi, K.; Ito, M.; et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl. Acad. Sci. USA 2020, 117, 16587–16595. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.R.; Little, J.B. Respiratory System Differences Relevant to Lung Carcinogenesis between Syrian Hamsters and Other Species. In Progress in Tumor Research, Proceedings of the Symposium on the Syrian Hamster in Toxicology and Carcinogenesis Research, Boston, MA, USA, 30 November–2 December 1977; Homburger, F., Ed.; Karger International: Basel, Switzerland, 1979; Volume 24, pp. 302–314. [Google Scholar]
- Kennedy, A.R.; McGandy, R.B.; Little, J.B. Morphologic and histochemical characteristics of cell lines derived from hamster peripheral lung tumors. Eur. J. Cancer 1977, 13, 1341–1350. [Google Scholar] [CrossRef]
- Hammond, W.G.; Teplitz, R.L.; Benfield, J.R. Lung cancer model for study of the metastatic process. Ann. Thorac. Surg. 1991, 52, 732–736; discussion 737. [Google Scholar] [CrossRef] [PubMed]
- Tsuchitani, M.; Sato, J.; Kokoshima, H. A comparison of the anatomical structure of the pancreas in experimental animals. J. Toxicol. Pathol. 2016, 29, 147–154. [Google Scholar] [CrossRef]
- Wang, Y.; Kayoumu, A.; Lu, G.; Xu, P.; Qiu, X.; Chen, L.; Qi, R.; Huang, S.; Li, W.; Wang, Y.; et al. Experimental Models in Syrian Golden Hamster Replicate Human Acute Pancreatitis. Sci. Rep. 2016, 6, 28014. [Google Scholar] [CrossRef]
- Takahashi, M.; Pour, P.; Althoff, J.; Donnelly, T. The pancreas of the Syrian hamster (Mesocricetus auratus). I Anatomical study. Lab. Anim. Sci. 1977, 27, 336–342. [Google Scholar]
- Jewell, H.A.; Charipper, H.A. The morphology of the pancreas of the golden hamster, Cricetus auratus, with special reference to the histology and cytology of the islets of Langerhans. Anat. Rec. 1951, 111, 401–415. [Google Scholar] [CrossRef]
- Takahashi, M.; Runge, R.; Donnelly, T.; Pour, P. The morphologic and biologic patterns of chemically induced pancreatic adenocarcinoma in Syrian golden hamsters after homologous transplantation. Cancer Lett. 1979, 7, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Egami, H.; Tomioka, T.; Tempero, M.; Kay, D.; Pour, P.M. Development of intrapancreatic transplantable model of pancreatic duct adenocarcinoma in Syrian golden hamsters. Am. J. Pathol. 1991, 138, 557–561. [Google Scholar] [PubMed]
- Schneider, M.B.; Matsuzaki, H.; Haorah, J.; Ulrich, A.; Standop, J.; Ding, X.Z.; Adrian, T.E.; Pour, P.M. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology 2001, 120, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Nistal-Villan, E.; Bunuales, M.; Poutou, J.; Gonzalez-Aparicio, M.; Bravo-Perez, C.; Quetglas, J.I.; Carte, B.; Gonzalez-Aseguinolaza, G.; Prieto, J.; Larrea, E.; et al. Enhanced therapeutic effect using sequential administration of antigenically distinct oncolytic viruses expressing oncostatin M in a Syrian hamster orthotopic pancreatic cancer model. Mol. Cancer 2015, 14, 210. [Google Scholar] [CrossRef]
- Bortolanza, S.; Bunuales, M.; Otano, I.; Gonzalez-Aseguinolaza, G.; Ortiz-de-Solorzano, C.; Perez, D.; Prieto, J.; Hernandez-Alcoceba, R. Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in Syrian hamsters. Mol. Ther. 2009, 17, 614–622. [Google Scholar] [CrossRef]
- Yanagi, K.; Onda, M.; Uchida, E. Effect of angiostatin on liver metastasis of pancreatic cancer in hamsters. Jpn. J. Cancer Res. 2000, 91, 723–730. [Google Scholar] [CrossRef]
- Valentine, H.; Daugherity, E.K.; Singh, B.; Maurer, K.J. The Experimental Use of Syrian Hamsters. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Academic Press: Cambridge, MA, USA, 2012; pp. 875–906. [Google Scholar]
- Hickman, D.L.; Johnson, J.; Vemulapalli, T.H.; Crisler, J.R.; Shepherd, R. Commonly Used Animal Models. In Principles of Animal Research for Graduate and Undergraduate Students; Academic Press: Cambridge, MA, USA, 2017; pp. 117–175. [Google Scholar] [CrossRef]
- Souza, A.J.S.; Souza Filho, A.F.; Zimpel, C.K.; Ayupe, M.C.; Araujo, M.V.; Machado, R.R.G.; Salles, E.; Salgado, C.L.; Tavares, M.S.; Silva-Pereira, T.T.; et al. Hepatic endotheliitis in Golden Syrian hamsters (Mesocricetus auratus) experimentally infected with SARS-CoV-2. Rev. Inst. Med. Trop. Sao Paulo 2024, 66, e44. [Google Scholar] [CrossRef]
- Bhamarapravati, N.; Thammavit, W.; Vajrasthira, S. Liver changes in hamsters infected with a liver fluke of man, Opisthorchis viverrini. Am. J. Trop. Med. Hyg. 1978, 27, 787–794. [Google Scholar] [CrossRef]
- Russell, P.S.; Hong, J.; Windsor, J.A.; Itkin, M.; Phillips, A.R.J. Renal Lymphatics: Anatomy, Physiology, and Clinical Implications. Front. Physiol. 2019, 10, 251. [Google Scholar] [CrossRef]
- Kruepunga, N.; Hakvoort, T.B.M.; Hikspoors, J.; Kohler, S.E.; Lamers, W.H. Anatomy of rodent and human livers: What are the differences? Biochim. Biophys. Acta—Mol. Basis Dis. 2019, 1865, 869–878. [Google Scholar] [CrossRef]
- Zhao, Y.; Qu, H.; Wang, Y.; Xiao, W.; Zhang, Y.; Shi, D. Small rodent models of atherosclerosis. Biomed. Pharmacother. 2020, 129, 110426. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lai, P.; Guo, J.; Wang, Y.; Xian, X. Genetically-engineered hamster models: Applications and perspective in dyslipidemia and atherosclerosis-related cardiovascular disease. Med. Rev. 2021, 1, 92–110. [Google Scholar] [CrossRef] [PubMed]
- Dillard, A.; Matthan, N.R.; Lichtenstein, A.H. Use of hamster as a model to study diet-induced atherosclerosis. Nutr. Metab. 2010, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Horn, P.S.; Heubi, J.E.; Woollett, L.A. The liver plays a key role in whole body sterol accretion of the neonatal Golden Syrian hamster. Biochim. Biophys. Acta 2007, 1771, 550–557. [Google Scholar] [CrossRef]
- Chen, G.; Dai, Z.K.; Liang, R.G.; Xiao, S.J.; He, S.Q.; Zhao, H.L.; Xu, Q. Characterization of diethylnitrosamine-induced liver carcinogenesis in Syrian golden hamsters. Exp. Ther. Med. 2012, 3, 285–292. [Google Scholar] [CrossRef]
- Martinez, I.; Wallace, G.; Zhang, C.; Legge, R.; Benson, A.K.; Carr, T.P.; Moriyama, E.N.; Walter, J. Diet-induced metabolic improvements in a hamster model of hypercholesterolemia are strongly linked to alterations of the gut microbiota. Appl. Environ. Microbiol. 2009, 75, 4175–4184. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; Nilaweera, K.; Ross, P.R.; Shanahan, F.; O’Toole, P.W.; Cotter, P.D. The gut microbiota and its relationship to diet and obesity: New insights. Gut Microbes 2012, 3, 186–202. [Google Scholar] [CrossRef]
- Wan, S.; You, P.; Shi, Q.; Hu, H.; Zhang, L.; Chen, L.; Wu, Z.; Lin, S.; Song, X.; Luo, Y.; et al. Gut microbiome changes in mouse, Mongolian gerbil, and hamster models following Clostridioides difficile challenge. Front. Microbiol. 2024, 15, 1368194. [Google Scholar] [CrossRef]
- Boswald, L.F.; Popper, B.; Matzek, D.; Neuhaus, K.; Wenderlein, J. Characterization of the gastrointestinal microbiome of the Syrian hamster (Mesocricetus auratus) and comparison to data from mice. FEBS Open Bio 2024, 14, 1701–1717. [Google Scholar] [CrossRef]
- Xiao, J.; Metzler-Zebeli, B.U.; Zebeli, Q. Gut Function-Enhancing Properties and Metabolic Effects of Dietary Indigestible Sugars in Rodents and Rabbits. Nutrients 2015, 7, 8348–8365. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Zheng, Y.; Xue, H.; Xu, J.; Wu, M.; Chen, L.; Xu, L. Different gut microbial types were found in captive striped hamsters. PeerJ 2023, 11, e16365. [Google Scholar] [CrossRef] [PubMed]
- Takiyama, Y.; Egami, H.; Pour, P.M. Expression of human tumor-associated antigens in pancreatic cancer induced in Syrian hamsters. Am. J. Pathol. 1990, 136, 707–715. [Google Scholar]
- Nambiar, P.R.; Kirchain, S.; Fox, J.G. Gastritis-associated Adenocarcinoma and Intestinal Metaplasia in a Syrian Hamster Naturally Infected with Helicobacter Species. Vet. Pathol. 2005, 42, 386–390. [Google Scholar] [CrossRef]
- Yapijakis, C.; Kalogera, S.; Papakosta, V.; Vassiliou, S. The Hamster Model of Sequential Oral Carcinogenesis: An Update. In Vivo 2019, 33, 1751–1755. [Google Scholar] [CrossRef]
- Doetschman, T.; Georgieva, T. Gene Editing with CRISPR/Cas9 RNA-Directed Nuclease. Circ. Res. 2017, 120, 876–894. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.A.; Spencer, J.F.; La Regina, M.C.; Dhar, D.; Tollefson, A.E.; Toth, K.; Wold, W.S. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006, 66, 1270–1276. [Google Scholar] [CrossRef]
- Nelles, M.J.; Duncan, W.R.; Streilein, J.W. Immune response to acute virus infection in the Syrian hamster. II. Studies on the identity of virus-induced cytotoxic effector cells. J. Immunol. 1981, 126, 214–218. [Google Scholar] [CrossRef]
- Avula, L.R.; Hagerty, B.; Alewine, C. Molecular mediators of peritoneal metastasis in pancreatic cancer. Cancer Metastasis Rev. 2020, 39, 1223–1243. [Google Scholar] [CrossRef]
- Zivcec, M.; Safronetz, D.; Haddock, E.; Feldmann, H.; Ebihara, H. Validation of assays to monitor immune responses in the Syrian golden hamster (Mesocricetus auratus). J. Immunol. Methods 2011, 368, 24–35. [Google Scholar] [CrossRef]
- Samant, M.; Sahu, U.; Pandey, S.C.; Khare, P. Role of Cytokines in Experimental and Human Visceral Leishmaniasis. Front. Cell. Infect. Microbiol. 2021, 11, 624009. [Google Scholar] [CrossRef] [PubMed]
- Poutou, J.; Bunuales, M.; Gonzalez-Aparicio, M.; Garcia-Aragoncillo, E.; Quetglas, J.I.; Casado, R.; Bravo-Perez, C.; Alzuguren, P.; Hernandez-Alcoceba, R. Safety and antitumor effect of oncolytic and helper-dependent adenoviruses expressing interleukin-12 variants in a hamster pancreatic cancer model. Gene Ther. 2015, 22, 696–706. [Google Scholar] [CrossRef]
- Loukas, A.; Prociv, P. Immune responses in hookworm infections. Clin. Microbiol. Rev. 2001, 14, 689–703, table of contents. [Google Scholar] [CrossRef]
- Francis, M.E.; Goncin, U.; Kroeker, A.; Swan, C.; Ralph, R.; Lu, Y.; Etzioni, A.L.; Falzarano, D.; Gerdts, V.; Machtaler, S.; et al. SARS-CoV-2 infection in the Syrian hamster model causes inflammation as well as type I interferon dysregulation in both respiratory and non-respiratory tissues including the heart and kidney. PLoS Pathog. 2021, 17, e1009705. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, Y.; Zhu, Z.; Lu, C.; Zhang, C.; Zeng, L.; Xie, F.; Zhang, L.; Zhou, F. Mucosal immune response in biology, disease prevention and treatment. Signal Transduct. Target. Ther. 2025, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Siurala, M.; Vaha-Koskela, M.; Havunen, R.; Tahtinen, S.; Bramante, S.; Parviainen, S.; Mathis, J.M.; Kanerva, A.; Hemminki, A. Syngeneic syrian hamster tumors feature tumor-infiltrating lymphocytes allowing adoptive cell therapy enhanced by oncolytic adenovirus in a replication permissive setting. OncoImmunology 2016, 5, e1136046. [Google Scholar] [CrossRef]
- Mendez, S.; Valenzuela, J.G.; Wu, W.; Hotez, P.J. Host cytokine production, lymphoproliferation, and antibody responses during the course of Ancylostoma ceylanicum infection in the Golden Syrian hamster. Infect. Immun. 2005, 73, 3402–3407. [Google Scholar] [CrossRef]
- Thomas, M.A.; Spencer, J.F.; Toth, K.; Sagartz, J.E.; Phillips, N.J.; Wold, W.S. Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol. Ther. 2008, 16, 1665–1673. [Google Scholar] [CrossRef]
- Prakash, S.; Dhanushkodi, N.R.; Singer, M.; Quadiri, A.; Zayou, L.; Vahed, H.; Coulon, P.-G.; Ibraim, I.C.; Tafoya, C.; Hitchcock, L. A Broad-Spectrum Multi-Antigen mRNA/LNP-Based Pan-Coronavirus Vaccine Induced Potent Cross-Protective Immunity Against Infection and Disease Caused by Highly Pathogenic and Heavily Spike-Mutated SARS-CoV-2 Variants of Concern in the Syrian Hamster Model. bioRxiv 2024. [Google Scholar] [CrossRef]
- Dhanushkodi, N.R.; Prakash, S.; Quadiri, A.; Zayou, L.; Srivastava, R.; Shaik, A.M.; Suzer, B.; Ibraim, I.C.; Landucci, G.; Tifrea, D.F. Antiviral and anti-inflammatory therapeutic effect of RAGE-Ig protein against multiple SARS-CoV-2 variants of concern demonstrated in K18-hACE2 mouse and Syrian golden hamster models. J. Immunol. 2024, 212, 576–585. [Google Scholar] [CrossRef]
- Zabelina, D.S.; Osipov, I.D.; Maslov, D.E.; Kovner, A.V.; Vasikhovskaia, V.A.; Demina, D.S.; Romanov, S.E.; Shishkina, E.V.; Davydova, J.; Netesov, S.V.; et al. Ad6-Based GM-CSF Expressing Vector Displays Oncolytic and Immunostimulatory Effects in an Immunocompetent Syrian Hamster Model of Cholangiocarcinoma. Viruses 2025, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Jiao, Y.; Gao, J.; Wang, T.; Xia, Y.; Li, K.; Yang, Y.; Zhang, J.; Bao, H.; Wang, L.; et al. Enhancement of immune responses to classical swine fever virus E2 in mice by fusion or mixture with the porcine IL-28B. Appl. Microbiol. Biotechnol. 2025, 109, 44. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Hamdy, R.; Ghonaim, J.H.; Husseiny, M.I. Metabolic Imbalance in Immune Cells in Relation to Metabolic Disorders, Cancer, and Infections. In Metabolic Dynamics in Host-Microbe Interaction; Springer Nature: Singapore, 2025; pp. 187–218. [Google Scholar]
- Singer, M.; Hamdy, R.; Elsayed, T.M.; Husseiny, M.I. The Mechanisms and Therapeutic Implications of Metabolic Communication in the Tumor-Immune Microenvironment. In Metabolic Dynamics in Host-Microbe Interaction; Springer Nature: Singapore, 2025; pp. 291–315. [Google Scholar]
- Wang, C.; Cheng, Z.; Miao, J.; Xue, X.; Dong, Y.; Zhao, L.; Guo, H.; Wang, J.; Wang, Z.; Lu, S.; et al. Genomic-transcriptomic analysis identifies the Syrian hamster as a superior animal model for human diseases. BMC Genom. 2025, 26, 286. [Google Scholar] [CrossRef] [PubMed]
- Handley, A.; Ryan, K.A.; Davies, E.R.; Bewley, K.R.; Carnell, O.T.; Challis, A.; Coombes, N.S.; Fotheringham, S.A.; Gooch, K.E.; Charlton, M.; et al. SARS-CoV-2 Disease Severity in the Golden Syrian Hamster Model of Infection Is Related to the Volume of Intranasal Inoculum. Viruses 2023, 15, 748. [Google Scholar] [CrossRef]
| Feature | Hamster Cheek Pouch | NSG Mice | Humanized Mice |
|---|---|---|---|
| Immune system | Local immune privilege | Fully immunodeficient | Engrafted human immune cells |
| Imaging access | Excellent | Limited | Limited |
| Tumour-immune interaction | Minimal | Absent | Human-like |
| Cost and simplicity | Low and simple | Moderate | High and complex |
| Immunotherapy research | No | No | Yes |
| Tumour microenvironment study | Appropriate for local interaction and angiogenesis | Moderate | Appropriate with immune aspect |
| Standardization | Less standard | High | Moderate (but variable) |
| Cell Line | Origin/Tissue | Species | Application and Similarity to Human Cancer | Ref. |
|---|---|---|---|---|
| BHK-21 | Baby Hamster Kidney fibroblasts | Syrian hamster |
| [66,67,68,69,70] |
| HaK | Kidney carcinoma | Syrian hamster | Transformation studies, tumorigenesis, anchorage-independent growth, alteration in cell cycle checkpoint and response to DNA damaging agents. | [71,72] |
| HapT1 | Pancreatic adenocarcinoma | Syrian hamster |
| [73,74,75] |
| HTC | Hepatoma tissue culture | Rat (Rattus norvegicus) |
| [76,77,78,79,80] |
| CHO-K1 | Ovary epithelial line | Chinese hamster |
| [81,82,83,84,85] |
| Feature | Hamster | Human |
|---|---|---|
| Major microbiota phyla | Firmicutes, Bacteroidetes (Firmicutes dominant) | Firmicutes, Bacteroidetes (more balanced) |
| Main fermentation site | Cecum | Colon |
| Stomach structure | Compartmentalized (nonglandular + glandular) | Simple glandular |
| Cecum | Large, functional | Rudimentary |
| Shared bacterial genera | Yes (e.g., Ruminococcus, Lactobacillus) | Yes |
| Feature | Hamster Model Advantage |
|---|---|
| Immune competence | Permits study of intact immune responses to tumors and therapies [123]. |
| Viral permissiveness | Supports human AdV/VV replication, unlike mice [123]. |
| Pathological fidelity | Pancreatic/oral carcinogenesis closely mimics human genetics and histology [18,59,121] |
| Therapeutic window | Larger body size allows repeated sampling and imaging [18]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singer, M.; Imagawa, D.K.; Alexander, M.; Abi-Jaoudeh, N. The Golden Hamster: A Valuable Model for Designing Cancer Therapies. Therapeutics 2025, 2, 10. https://doi.org/10.3390/therapeutics2030010
Singer M, Imagawa DK, Alexander M, Abi-Jaoudeh N. The Golden Hamster: A Valuable Model for Designing Cancer Therapies. Therapeutics. 2025; 2(3):10. https://doi.org/10.3390/therapeutics2030010
Chicago/Turabian StyleSinger, Mahmoud, David K. Imagawa, Michael Alexander, and Nadine Abi-Jaoudeh. 2025. "The Golden Hamster: A Valuable Model for Designing Cancer Therapies" Therapeutics 2, no. 3: 10. https://doi.org/10.3390/therapeutics2030010
APA StyleSinger, M., Imagawa, D. K., Alexander, M., & Abi-Jaoudeh, N. (2025). The Golden Hamster: A Valuable Model for Designing Cancer Therapies. Therapeutics, 2(3), 10. https://doi.org/10.3390/therapeutics2030010







