Abstract
Animal models are indispensable in biomedical research, offering critical insights into disease mechanisms and therapeutic strategies. However, existing models often inadequately replicate human pathophysiology, leading to discrepancies between preclinical and clinical outcomes. Despite their contributions, many models exhibit significant limitations, especially concerning cancer and infectious diseases. Inaccurate modeling of human biological responses can result in failed clinical trials, escalated research costs, and delays in developing effective treatments. The golden hamster (Mesocricetus auratus) has emerged as a viable model, particularly in cancer and infectious disease research. Sharing physiological and immunological profiles similar to humans, the golden hamster offers distinct advantages over other rodent models, such as mice and rats. This review explores the benefits of using golden hamsters in cancer research, highlighting their contributions to scientific advancements while also addressing the limitations due to incomplete immunological and molecular knowledge about this species.
1. Introduction
Animal models are fundamental in biomedical research by helping researchers understand disease mechanisms and contributing to the development as well as perfection of therapeutic interventions [1,2,3]. One of the major challenges in preclinical and clinical research is the use of unsuitable animal models that fail to accurately replicate human physiology, pathology, and drug responses. This can lead to misleading results, ineffective treatments, and even harmful consequences when therapies move to human trials [2]. A major limitation of animal models is their inherent biological differences from humans. Even genetically similar species, such as primates, differ significantly in metabolism, immune system function, and organ structure [4,5]. Rodents, which are widely used in preclinical research, process drugs differently due to variations in liver enzyme activity, leading to inaccurate predictions of drug efficacy and toxicity. Many drugs that show promising results in animal models fail during human clinical trials [4,6,7].
According to studies, approximately 90% of drugs that pass preclinical animal testing fail in human trials because of a lack of efficacy or unexpected toxicity [6]. For example, treatments for neurodegenerative diseases such as Alzheimer’s and Parkinson’s often show success in mouse models but fail in humans because rodent brains do not fully replicate human neurobiology [8,9]. Using inappropriate animal models raises ethical concerns, as it subjects animals to unnecessary suffering while providing limited scientific benefits. Additionally, the high failure rate of animal-tested drugs results in significant financial losses for pharmaceutical companies and research institutions, increasing the cost of drug development [10,11]. Certain diseases, especially those related to human-specific conditions (such as psychiatric disorders, cancer metastasis, and autoimmune diseases), do not have accurate animal equivalents. For example, small animals like mice do not naturally develop many human cancers or replicate the complexity of human immune responses, making them poor models for immunotherapy research [11,12,13].
To address these challenges, alternative models are explored, such as human organoids, microfluidic “organ-on-a-chip” systems, and AI-driven drug discovery methods [14,15]. These innovations aim to provide more accurate, ethical, and cost-effective alternatives to traditional animal testing, potentially improving the reliability of preclinical research [16]. However, relying on mice and rats as preclinical models has shown incompatible results with the complex interactions that are found in humans [16,17].
Golden hamsters provide unique advantages in specific research areas, especially in oncology and infectious diseases. Their susceptibility to human-like tumor growth and a wide range of viral and bacterial pathogens makes them invaluable in translational medicine [18]. There are several strains of hamsters used as animal models in biomedical research, each with unique physiological and genetic characteristics suited for different studies. The Syrian hamster (Mesocricetus auratus) is a valuable animal model for infectious diseases (such as SARS-CoV-2 and leishmaniasis) and cancer research (such as Leukemia [18,19,20,21]. The Chinese hamster (Cricetulus griseus) is widely utilized in genetic and biopharmaceutical research, particularly for producing recombinant proteins in Chinese hamster ovary (CHO) cells [22]. Other strains, such as the Armenian hamster (Cricetulus migratorius) and the Djungarian hamster (Phodopus sungorus), have been employed in circadian rhythm, metabolic, and neurobiological studies [23]. The choice of hamster strain depends on the research focus, as differences in immune response, metabolism, and susceptibility to diseases can significantly impact study outcomes [24]. In our study, we focus only on the Syrian golden hamster (Mesocricetus auratus) as the new optional strain for cancer research.
2. The Necessity for a Reliable Animal Model
2.1. Limitations
The commonly used animal models, such as mice and rats, show marked differences with humans in immune system function, genetic composition, and metabolic processes. These differences manifest in different responses and outcomes during infectious diseases and tumors when compared to humans [25]. Due to these disparities, results are often misleading and do not accurately reflect human experiences, hindering the ability to translate research findings. For instance, mice are naturally resistant to certain human-specific pathogens, such as HIV, Shigella flexneri and Plasmodium falciparum. The resistance is dependent on how the pathogen co-evolves and adapts to the host-selected animal model [26,27,28,29].
The complexity of human cancers, encompassing tumor heterogeneity and microenvironment interactions, poses a significant challenge for animal models [30]. Classic xenograft models lack a complete immune system; in contrast, the mutations in genetically modified mouse models may not perfectly reflect those in human cancers. The existing animal models of tumor angiogenesis or metastasis are not without their limitations and controversies [31]. Some laboratory mice strains are susceptible or resistant to cancer-causing viruses, which requires the search for alternative animal models [32,33]. These models do not reflect the pathophysiology in humans. The principle of 3Rs (replacement, reduction and refinement) causes ethical concerns about animal welfare, which limits the use of highly intrusive experiments on mammals and other species [6,34,35].
2.2. Current Alternatives
Human-derived organoid and 3D culture systems are easy solutions that offer a promising alternative because they closely mimic the structure and diseases of human tissue in a lab setting [36,37]. Implementing these systems enables a sharper analysis of drug efficacy and patient responses, leading to improved drug-testing methods and the creation of truly personalized medicine through detailed data analysis [38,39,40]. However, the accuracy of organoid models in representing pathogen-associated cancers needs improvement; current research aims to enhance their predictive capabilities and better reflect the complexities of these diseases [40,41]. To that end, artificial intelligence (AI) simulations and computer models are being used to complement animal studies, which may lead to a reduction in the number of animals used in research [42,43].
The rapid analytical speed offers a considerable edge over the slower pace of traditional methods. Although in-silico models have advanced, the inherent complexities of living systems necessitate animal experimentation to verify results and account for the countless in vivo variables that can lead to discrepancies [44,45]. The close genetic and physiological kinship between non-human primates and humans makes them invaluable for infectious disease research; subtle physiological similarities allow for more effective testing of new medicines and vaccines. Ethical, logistical, and cost issues limit their use [46,47]. Progress in genetic engineering has resulted in the development of mice with human components, known as humanized mice [48,49]. Although these models improve the relevance of preclinical data, accurately replicating human immune responses remains challenging, especially in immune-related processes [50,51]. Given the aforementioned limitations and existing alternatives, the optimal animal model should ideally exhibit unique characteristics to facilitate translation between preclinical and clinical studies to ensure a smooth transition. These unique characteristics include (1) closer physiology to humans; (2) possessing a closer resemblance to the human immunological mechanism; (3) a tumor microenvironment of similar nature; (4) similar genetic expression and metabolic profiles; (5) logistically feasibility; and (6) availability in large numbers.
3. Unique Biological Similarity Between Hamsters and Humans
3.1. Anatomical and Physiological Advantages of the Syrian Hamster
Although NOD scid gamma (NSG) mice serve as a widely used animal model for human tumor xenografts [52], they are susceptible to infections, leading to inaccurate results when studying cancer and its treatment [53]. Moreover, it has polymorphism in the SIRP-alpha gene which impacts macrophages and immune cells, which influences studying the immune response to tumors [54]. The Syrian hamster’s cheek pouch, an immune-privileged site lacking lymphatic drainage, leaving systemic immune function unaffected, enables long-term engraftment of human tumor xenografts without the need for immunosuppression medications [55]. The cheek pouch’s thin, translucent, and easily accessible structure enables non-invasive in vivo imaging in hamster (such as angiogenesis, tumor progression, and intravital microscopy) studies, which is less invasive than surgical implantation in the internal organs of mice [56,57]. The tumor microenvironment may be manipulated with greater ease, and vascularization and tumor–host interactions can be observed in real time [58,59]. Hamsters exhibit greater robustness compared to NSG mice, necessitating less stringent pathogen-free conditions, potentially resulting in reduced husbandry expenses [60]. Comparative limitations of hamsters versus NSG mice are less genetically tractable (fewer transgenic lines, genetic tools), less standardized with no established protocols nor widespread use, and not as well-characterized for long-term tumor growth and metastasis studies [61,62,63]. Comparisons between the hamster cheek pouch, NSG and humanized mice models are summarized in Table 1.

Table 1.
Comparison between hamster check pouch, NSG and humanized mice models.
This feature has been exploited to study myofibrosarcoma (MFS-l) and melanotic melanoma (ME-l), which retain drug sensitivity profiles mirroring human tumors [55,64]. Unlike murine models, hamster pancreatic ductal adenocarcinomas exhibit 99% homology in sonic hedgehog (SHH) signaling pathways with humans, driving desmoplastic stromal reactions identical to those in clinical specimens [65]. Hamster’s cancer cell lines that can be used in cancer research are summarized in Table 2.

Table 2.
Hamster cell lines used in cancer research.
3.2. Lung: Similarities and Differences
The anatomical similarities between the lungs of golden hamsters and humans make hamsters a good model for respiratory research. Hamsters and humans both have lobed structures, but have different lobe numbers [86]. Like humans, hamsters have a bronchial tree branching from the trachea into progressively smaller bronchi and finally bronchioles, culminating in alveoli where gas exchange occurs [87]. Both species have alveoli lined with epithelial cells and surrounded by capillaries for gas exchange. These structural similarities aid researchers in studying disease processes such as viral infections [88].
In cancer research, hamster lungs exhibit a unique similarity to human lungs not found in other animal models; specifically, their capacity to develop lung tumors that closely resemble the histopathological subtypes and metastatic patterns observed in human non-small cell lung cancer (NSCLC), including adenocarcinoma [87,89,90]. The induction of lung cancer in Syrian hamsters by chemical carcinogens, such as N-nitrosobis(2-oxopropyl)amine (BOP), demonstrates histological and molecular similarities to human NSCLC. Specifically, these models replicate the overexpression of cyclooxygenase-2 (COX-2) and nuclear factor kappa B (NF-κB), key components of human lung cancer pathogenesis [59].
Moreover, the metastatic frequencies and distribution patterns of primary NSCLCs in hamsters closely resemble those observed in human patients, a characteristic not consistently replicated in murine or rat models [91]. Hamsters’ immunological compatibility and similar tumor biology make their lung model suitable for preclinical lung cancer research [59].
3.3. Pancreas: Similarities and Differences
Human and hamster pancreases are anatomically similar. Both species’ pancreases are compact and located in the upper abdomen, near the posterior abdominal wall [92]. Both hamsters and human pancreases have a higher content of adipose tissue at the pancreatic tail [93]. This structural organization is not commonly observed in other rodent models, such as mice and rats, allowing improved comparative studies of localized pancreatic functions and pathologies [93]. Despite similarities, the pancreatic anatomy differs between hamsters and humans. The hamster pancreas comprises three lobes: gastric, splenic, and duodenal, with an average weight of approximately 0.46 g or 0.4-0.5% of total body weight [94]. The human pancreas is divided by head, body, and tail. Hamsters possess a gastric lobe in their pancreas unique to them compared to all other species [92]. Islet cell distribution also varies between species. In hamsters, the islet cell distribution varies across the pancreatic most ubiquitous in the tail [95] unlike the organized structure seen in humans [92].
Human pancreatic cancer’s progression and key genetic mutations (like KRAS and TP53) are closely mirrored in golden Syrian hamsters [18]. The histological presentation of well-differentiated ductal adenocarcinomas resulting from pancreatic cancer induction or transplantation in golden hamsters closely mirrors that observed in human pancreatic cancer. These neoplasms show invasive growth and metastatic potential, specifically to lymph nodes and the liver, thereby replicating the metastatic spread seen in human disease [96,97]. Orthotopic pancreatic cancer cell transplantation in hamsters produces higher metastasis (e.g., 100% in liver and lungs in some models) than in mice, exhibiting human-like ascites, cachexia, and local invasion [59]. Additionally, hamster pancreatic cancer cell lines, such as HaP-T1 which is described in Table 2, exhibit epithelial traits and express a nearly identical (99%) N-terminal sonic hedgehog (SHH) to that of humans, thus inducing desmoplasia—characteristic of human pancreatic cancers [59]. Analysis of pancreatic cancer in hamsters reveals the expression of blood group-related antigens and tumor-associated markers (A, B, H, Le(b), Le(y), Le(x), and TAG-7) in both primary and orthotopic hamster pancreatic cancer cells, mirroring patterns observed in human pancreatic cancers [97]. This antigenic profile shows that the model is useful for studying human tumor biology and the immune system’s response [97]. Hamster models present advantages in the study of acute pancreatitis, exhibiting parallels with human responses to ethanol and fatty acids to induce pancreatitis, and the comparable alterations in lipid metabolism [93]. The usage of metformin was found to protect from harmful metabolic changes and insulin resistance that can subsequently lead to pancreatic cancer [98].
The common similarities between humans and hamsters open an avenue for testing the pancreatic cancer virotherapy. The sequential administration of engineered oncolytic viruses to express the immunostimulatory cytokine oncostatin M or IL-12 produces a significantly enhanced antitumor effect in the Syrian hamster model of pancreatic cancer [99,100]. Also, research using the hamster animal model has explored using therapy (such as Angiostatin) to treat pancreatic cancer that metastasizes to the liver [101].
3.4. Liver: Similarities and Differences
Hamster livers, though small, possess a remarkable functionality relative to their body size, proving advantageous for experimental studies involving tissue biopsies or surgical procedures [102,103]. Conversely, the larger size and more intricate vascular system of human livers present challenges for experimental models yet offer a greater physiological capacity [102,103]. Both golden hamsters and humans share similar hepatic lobular architecture, including sinusoids, bile ducts, and hepatocytes organized to facilitate metabolic and detoxification processes [104,105]. However, the hepatic segments are larger and divided into clear segments as opposed to hamsters [106,107]. Both species exhibit similar lipid metabolism characteristics, including lipoprotein synthesis, processing, and recycling. This characteristic makes hamsters a suitable model for atherosclerosis [108,109,110]. However, newborn hamsters have a significantly faster rate of cholesterol synthesis in their livers than humans, because of the difference in developmental metabolic needs [111].
Golden hamsters, through chemically induced models, show similar histological, molecular, and pathological progression to human liver cancer. Hamsters exposed to hepatocarcinogens such as diethylnitrosamine (DEN) or streptozotocin develop HCC, which is poorly differentiated, exhibits frequent mitosis, and expresses AFP—a biomarker also elevated in human HCC [59]. The development of invasive HCC from pre-neoplastic lesions, such as hyperplastic nodules, fatty metamorphosis, and bile duct proliferation, closely mirrors the stages of human hepatocarcinogenesis, encompassing initial toxic injury, subsequent regenerative hyperplasia, and ultimately, malignant transformation [112]. Recently, chemical carcinogens like N-methyl-N-nitrosourea (MNU) and carbon tetrachloride (CCl4) have been employed to induce liver tumors in hamsters, which has provided insights into the pathological and molecular progression of liver cancer similar to humans [18,59]. The advancements in genetic engineering, such as CRISPR/Cas9, have allowed for the development of gene-targeted knockout hamster models (e.g., TP53, KCNQ1, IL2RG), which can further elucidate the genetic keystones of liver cancer and support translational research for new therapies [18].
3.5. Gastrointestinal Tract: Similarities and Differences
Hamsters and humans, like mice and many other mammals, share a dominant gut microbiota consisting primarily of Firmicutes and Bacteroidetes phyla at the highest taxonomic level [113]. The proportions, however, vary between hamsters and humans. Hamsters show a strong Firmicutes dominance (92.6%), while humans have a more balanced Firmicutes and Bacteroidetes population [114]. Even with this distinction, both humans and hamsters share many of the same key bacterial families and genera, such as Erysipelotrichaceae, Ruminococcaceae, and Lactobacillaceae [115]. While both hamsters and humans rely on pancreatic amylase in their small intestines to digest starch, the process’s effectiveness varies depending on their respective diets and evolutionary adaptations [116] (Table 3). In both hamsters and humans, microbial fermentation of undigested nutrients takes place, but the location differs; in hamsters, it primarily occurs in the cecum pouch of their large intestine, while in humans, with their reduced cecum, fermentation happens mainly in the colon [117]. While both species share a similar gastrointestinal structure, including a stomach, small intestine, cecum, and colon, human stomachs consist of a single glandular pouch, unlike the compartmentalized stomachs of hamsters, which have a non-glandular forestomach and a glandular region. The human cecum is small; the hamster’s is large and essential to fermentation [116]. Similar carbohydrate fermentation and metabolic processes in humans and hamsters are reflected in the presence of shared bacterial genera, including Ruminococcus and Lactobacillus [118].

Table 3.
Shared features between the human and hamster gastrointestinal tracts.
The Syrian hamster exhibits a strong congruence with human gastrointestinal (GI) tract cancers regarding tumorigenesis, histopathology, and progression [18,59]. Both human and hamster GI tract cancers can express similar tumor-associated antigens, such as those with blood group specificities (A, B, H, Leb, Lex, Ley), and markers like CA 125, TAG-72, and 17-1A [119]. Human and hamster cancers, especially oral and gastric, exhibit analogous histological characteristics and pathological mechanisms. For example, hamster models of oral squamous cell carcinoma and gastric adenocarcinoma display similar cellular abnormalities, differentiation patterns, and progression from chronic inflammation or precancerous lesions to invasive cancer, mirroring human observations [59,120].
4. Golden Syrian Hamsters in Cancer Research
The use of hamsters in cancer research is selected over mice, due to their unique biological traits and heightened sensitivity to carcinogens, making them exceptionally useful for evaluating therapies, especially immunotherapies [59].
Hamsters are highly susceptible to chemically induced cancers, facilitating carcinogenesis studies [102]. For example, the DMBA (7,12-dimethylbenz[a]anthracen)-induced cheek pouch model of oral squamous cell carcinoma is similar to those of human cancer, starting with normal cells progressing to hyperplasia, then dysplasia, and finally carcinoma. This immunoprivileged site allows tumor growth without rejection, facilitating studies on several carcinogenic pathways, such as oncogene activation (Egfr, Myc, Hras), loss of tumor suppressor (p53, p16), Wnt/β-catenin pathway dysregulation, mirroring of the human oral cancer and chemopreventive agent testing (e.g., rosmarinic acid, black raspberries) [121].
The application of CRISPR/Cas9 technology has broadened the utility of hamsters through the generation of knockout (KO) models that display human-like cancer characteristics [122], such as the TP53 KO hamster that develops aggressive acute myelogenous leukemia (AML), the KCNQ1 KO hamster that exhibits gastric neoplasia, and IL2RG KO that enables human tumor xenograft studies for immunodeficient studies [59].
In cancer therapy, the Syrian hamster is suitable for testing the oncolytic virotherapy in immunocompetent animals. The viral replication, tumor-specific T-cell infiltration, and prolonged-survival post-human adenovirus (Adv) treatment (e.g., Delta-24-RGD) is promising as an appropriate model for glioma and pancreatic cancer [59,123]. Hamsters can be used in combination therapies for cancers such as the sequential administration of oncolytic adenovirus and vaccinia virus (VV) for kidney cancer models [59]. Immunologically, the immune response of hamster’s natural killer cells to vaccinia virus administration mirrors human responses, aiding in the study of the immune mechanism and optimization of immunotherapy [59,124], (Table 4).

Table 4.
Comparative advantages of the hamster model over the mouse model in cancer research.
The tumor immune microenvironment (TIME), metastasis development and tumor-stroma interactions are akin to humans, facilitating their study [59]. The intraperitoneal injection of SHPC6 cancer cells forms disseminated nodules mimicking advanced human pancreatic cancer metastasis [125].
5. Immunological Connections Between Hamsters and Human
Immune System Parallels and Divergences
Hamsters possess omental milky spots—lymphoid structures rich in macrophages—that mimic human peritoneal immune surveillance and cancer metastasis [59]. However, key immune markers diverge, while ribosomal protein L18 (RPL18) serves as a stable reference gene for qRT-PCR, and assays for cytokines like IFN-γ and IL-6 require species-specific validation [126].
The immunology of golden hamsters shows remarkable similarities to that of humans, especially concerning cytokine function and adaptive immunity [20,127]. Hamsters exhibit biological responsiveness to human cytokines like GM-CSF, IL-12, IL-21, and IL-2, thus facilitating direct evaluation of cytokine therapies without species-specific adjustments [128]. The administration of oncolytic viruses expressing human IL-12 or IL-2 in hamster models resulted in significant anti-tumor immune responses, evidenced by interferon-γ production and CD8+ T-cell infiltration, thus corroborating observations from human clinical trials [59,128]. Furthermore, hamsters demonstrate Th1/Th2 immune polarization comparable to humans when dealing with infections [127]. In hamsters with hookworm infections, the initial inflammatory response (Th1, IFN-γ) gives way to a Th2-driven response (IL-4, IL-10) as the infection develops, a pattern consistent with human parasitic diseases [129]. Their adaptability is key to studying chronic infection immune modulation. The hamster’s immune system provides a close analog to human responses to viral pathogens. Hamsters infected with SARS-CoV-2 experience cytokine storms with high levels of IFN-γ, IL-6, and IL-17A—inflammatory mediators also seen in humans—and maintain memory B and T cells for weeks afterward [130,131]. Similar to human immunity, these memory cells protect against cancer and reinfection. The cytokine functionality in cancers is similar in hamsters as in humans, such as in GM-CSF, IL-2, IL-21 and IL-2, with shared cytokine pathways (IFN-α and TNF-α) in colorectal cancer specifically [59]. In pancreatic cancer, Syrian hamsters showed similar CD4+ and CD8+ T cell infiltration in the human tumor microenvironment, and local delivery of IL-2 via oncolytic adenoviruses [132]. Furthermore, hamsters exhibit human-like lymphoproliferative responses and organ-specific immune activation, such as splenomegaly and lung mononuclear cell infiltration during viral clearance [133]. The ability to support adenovirus replication in liver and human tumor xenografts in immunocompetent settings further validates their translational applicability [58,134].
6. Limitations with Hamster Research
Despite their advantages, golden hamsters are not a perfect animal model because of gaps in immunological understanding:
- (a)
- Limited Immunological Tools and Reagents: Compared to mice and rats, fewer antibodies and immunological assays are commercially available for hamsters because of limited studies determining the functionality and expression of these markers, thus hampering detailed immune response analyses [20].
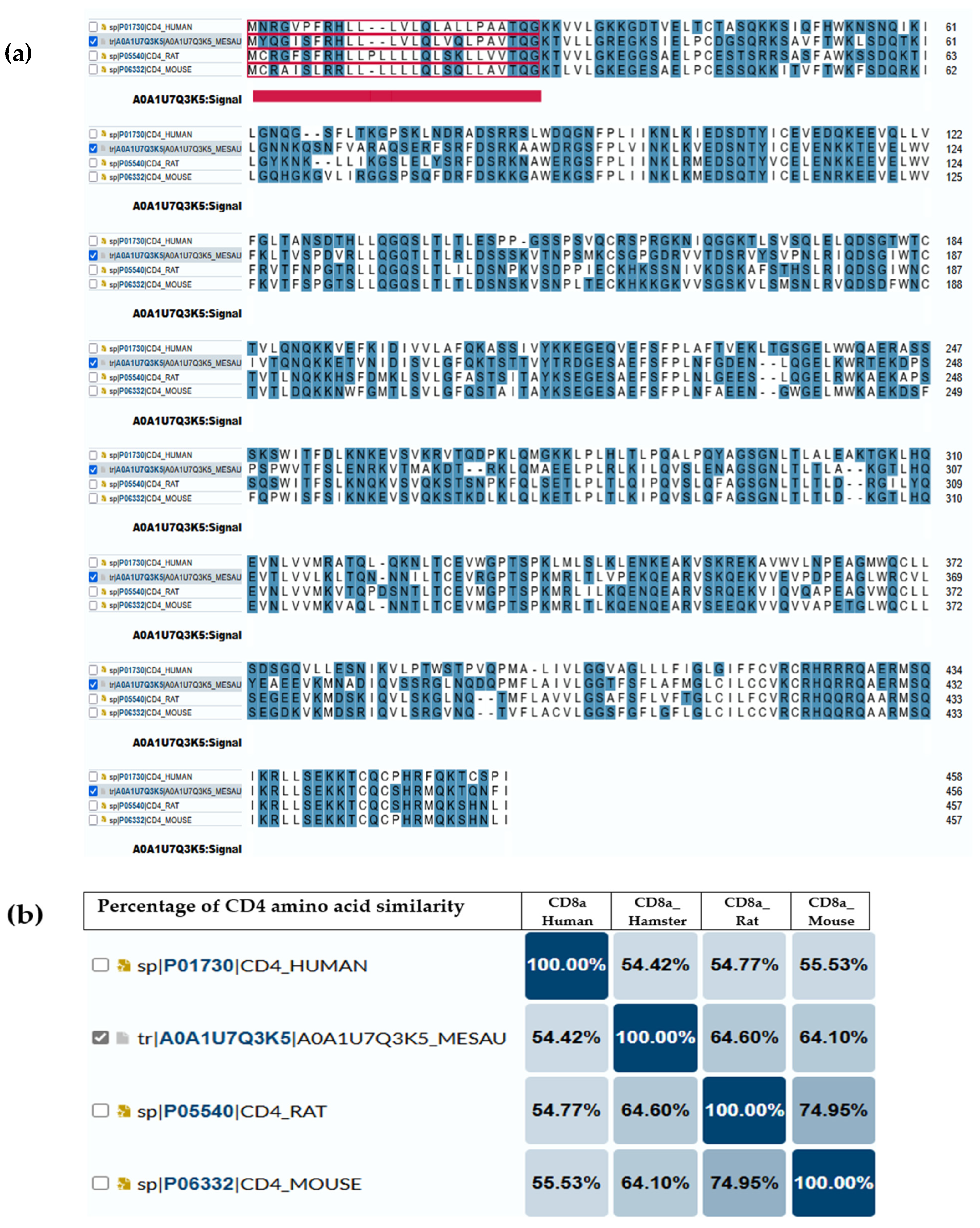
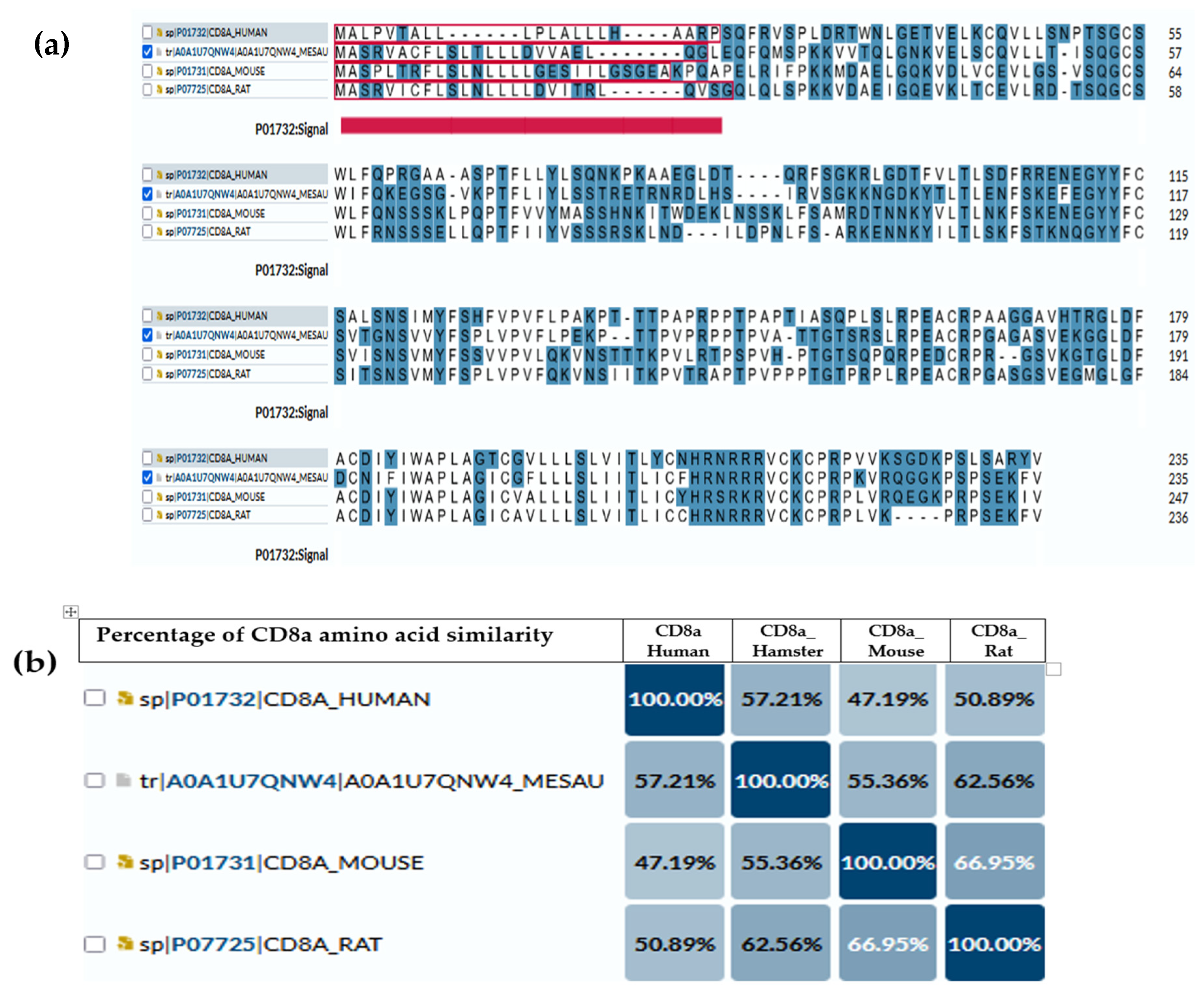
Several studies have used anti-mouse or anti-rat CD markers to investigate the immune cell infiltration in the target organ of hamsters [135,136,137,138]. Similarly, while the immune metabolome has been extensively studied in humans and mice, it remains distinct and relatively unexplored in hamsters [139,140]. For that reason, we illustrate the comparisons of amino acid compositions of CD4 (Figure 1) and CD8a (Figure 2) antigens between hamsters and different species (humans, mice and rats) based on amino acid polarity, to prove that the expected antigenic similarity is not enough to rely on the cross-reactive antibodies or similar immune reaction to build the mechanism and understand disease pathogenesis. The amino acid sequence of CD4 and CD8a showed a low abundance of conserved continuous sequences, making it unexpected to have a common antigenic determinant for finding multi-species antibodies.

Figure 1.
CD4 antigen sequence across species. (a) Amino acid comparison between golden hamsters versus humans, mice and rats; the highlighted blue are the amino acids that have charge. The underlined red part is the intercellular domain responsible for signal transduction inside the cells, while the rest (non-underline) is the transmembrane domain (exposed part). (b) The percentage of similarity between them, where the highest similarity (64.6%) is between a hamster’s CD4 and rat’s CD4. Darker color means more similarity between each 2 animal models.
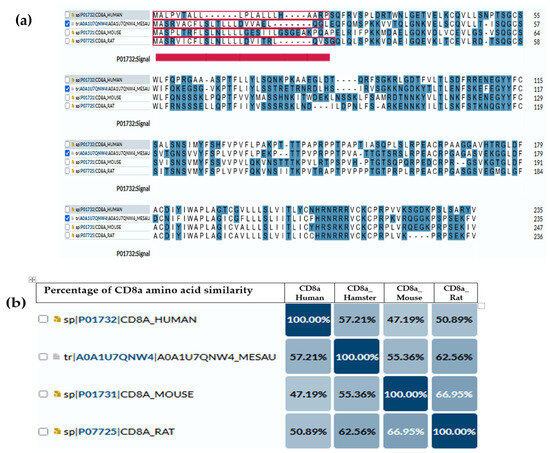
Figure 2.
CD8a antigen sequence across species. (a) Amino acid comparison between golden hamsters versus humans, mice and rats; the highlighted blue are the amino acids that have charge. The underlined red part is the intercellular domain responsible for signal transduction inside the cells, while the rest (non-underline) is the transmembrane domain (exposed part). (b) Percentage of similarity between them, where the highest similarity (62.65%) is between a hamster’s CD4 and rat’s CD8a.
To overcome this limitation, studies should be addressed to identify the unique epitopes in surface antigens expressed on hamsters’ immune cells, such as CD3, CD4, and CD8a. From these effective epitopes, we can identify suitable antibody paratopes to be designed and manufactured later on. A confirmational functional assay will be needed to ensure the antigenic functionality in terms of expression; for example, CD69 expression in hamsters is related to early T-cell activation, which is similar to humans and mice.
Understanding immune mechanisms in hamsters could enhance their use in preclinical research, potentially offering greater benefits for studying diseases compared to other commonly used animal models.
- (b)
- Incomplete Genome Annotation: Hamster genome sequencing has been achieved, but functional annotation of immune-related genes remains incomplete, restricting genetic manipulation capabilities. This limitation may be addressed firstly by employing long-read sequencing to improve the contiguity and accuracy of the genome assembly [141]. Second, the application of RNA-Seq across multiple tissues and developmental time points enables a detailed characterization of the transcriptome and enhances gene annotation through the identification of a wide range of transcripts. The third objective is to use comparative genomics to predict gene structures by aligning hamster sequences against the well-annotated genomes of related species like mice and rats. This method improves annotation accuracy through the utilization of conserved genomic features [141]. Fourthly, bioinformatics tools will be utilized to functionally annotate predicted genes and elucidate gene roles and interactions based on domain content and homology [139]. Finally, experimental validation of gene models by CRISPR/cas9 knockouts and RT-PCR will be required to confirm the functions, and structure will be needed.
- (c)
- Uncharacterized Immune System Components: Certain immune mechanisms, such as cytokine interactions and T-cell responses, are not fully understood in hamsters, making it difficult to extrapolate findings directly to humans. Comparative analysis of immune responses in simplified experimental models (human and murine) and corresponding disease states may elucidate cytokine/chemokine secretion patterns. Analysis of diverse trials across various cancers and infectious disease models, stratified by disease severity, will elucidate the patterns of immune mediator secretion, including source, target cells, concentrations, and biological effects.
- (d)
- Unknown ability to correlate with human outcomes: Usually, nine out of ten drugs that appear promising in animal studies fail in human clinical trials. By characterizing the immune response and performing genomic annotation, we can better understand the disparities between human and murine models, thereby improving the translation of hamster study results to human outcomes.
- (e)
- Lack of Standardization: The disease outcomes can be influenced by variables such as inoculation dose and volume, requiring careful experimental design for reproducibility and comparison across studies [142]. Once fundamental biological data from hamsters, including immune mechanisms, available immunological CD markers, cytokine/chemokine secretion, genomic annotation, and metabolic processes, are established, subsequent cancer research will benefit from enhanced standardization and reproducibility.
7. Conclusions
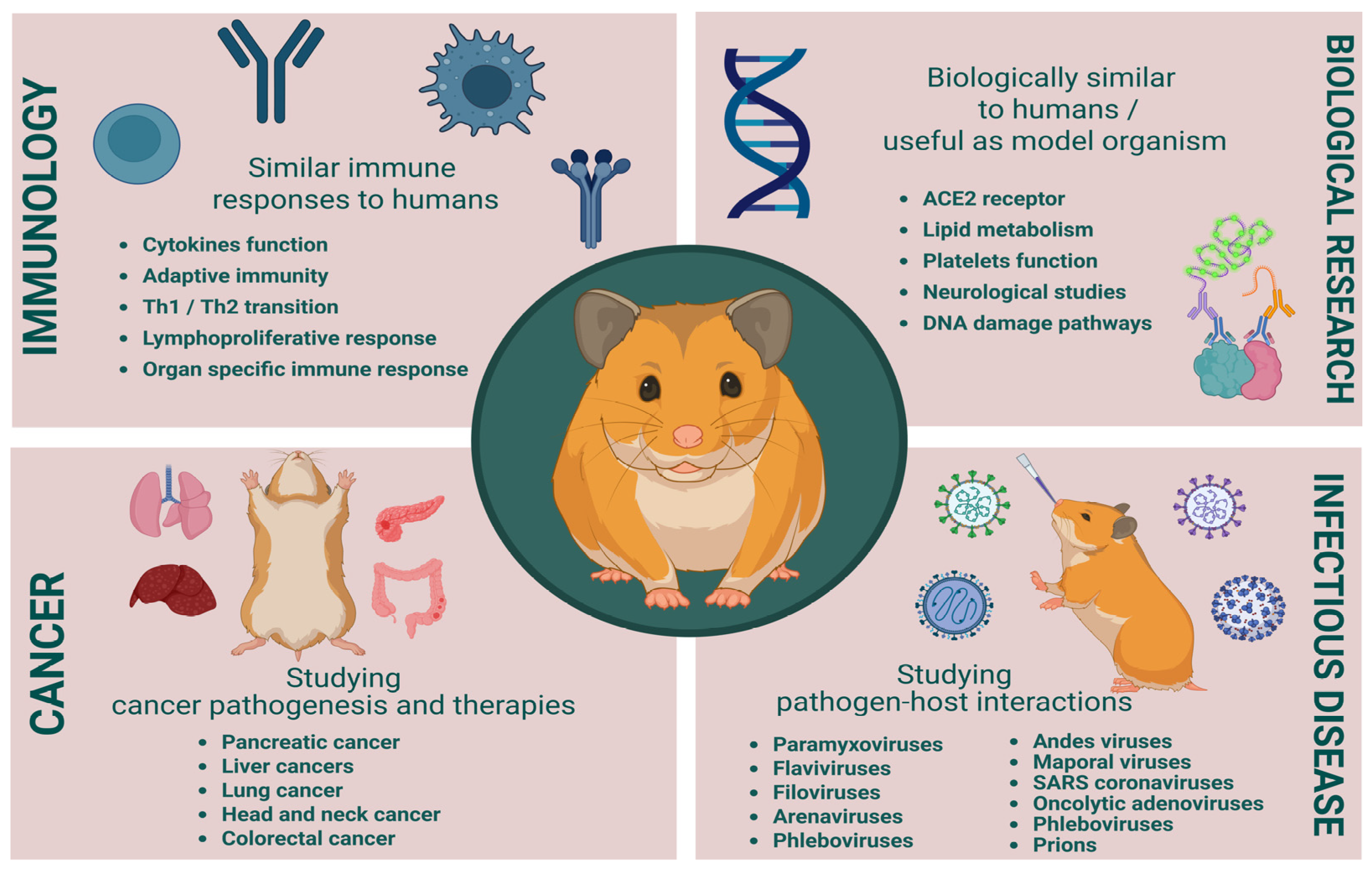
Syrian hamsters bridge critical gaps in cancer research by combining immunocompetence, human-like carcinogenesis, and viral permissiveness. Golden hamsters have significantly contributed to advancements in cancer and infectious disease research, (Figure 3). Their unique biological traits make them a valuable model for studying tumor biology, viral pathogenesis, and bacterial infections. However, the incomplete immunological and molecular knowledge of hamsters presents challenges in fully utilizing their potential as a model. Several studies are using the hamster antibodies based on cross-creative antibodies without verification of these antibodies’ binding specificities or comparable functionalities, which makes the data generated misleading and deceptive. For greater utilization of hamsters in biomedical research, these limitations must be addressed by further genetic and immunological studies, leading to better therapeutic strategies and disease management.
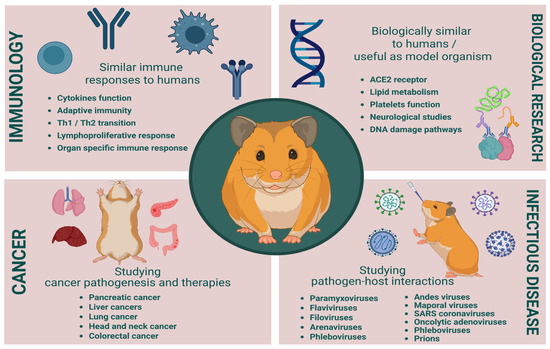
Figure 3.
Benefits and challenges in cancer and infectious disease research. Created in BioRender. Singer, M. (2025), https://BioRender.com/5gq1a5u.
Author Contributions
M.S., D.K.I., M.A. and N.A.-J. contribute to Conceptualization, investigation, writing of this manuscript, editing and review of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dominguez-Oliva, A.; Hernandez-Avalos, I.; Martinez-Burnes, J.; Olmos-Hernandez, A.; Verduzco-Mendoza, A.; Mota-Rojas, D. The Importance of Animal Models in Biomedical Research: Current Insights and Applications. Animals 2023, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Soufizadeh, P.; Mansouri, V.; Ahmadbeigi, N. A review of animal models utilized in preclinical studies of approved gene therapy products: Trends and insights. Lab. Anim. Res. 2024, 40, 17. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.J.; Grieder, F.B. The continued importance of animals in biomedical research. Lab Anim. 2024, 53, 295–297. [Google Scholar] [CrossRef]
- Zhao, X.; Bhattacharyya, A. Human Models Are Needed for Studying Human Neurodevelopmental Disorders. Am. J. Hum. Genet. 2018, 103, 829–857. [Google Scholar] [CrossRef] [PubMed]
- Bjornson-Hooper, Z.B.; Fragiadakis, G.K.; Spitzer, M.H.; Chen, H.; Madhireddy, D.; Hu, K.; Lundsten, K.; McIlwain, D.R.; Nolan, G.P. A Comprehensive Atlas of Immunological Differences Between Humans, Mice, and Non-Human Primates. Front. Immunol. 2022, 13, 867015. [Google Scholar] [CrossRef]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Part 2: Potential Alternatives to the Use of Animals in Preclinical Trials. JACC Basic Transl. Sci. 2020, 5, 387–397. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, S.; Li, X.J. Genetically modified large animal models for investigating neurodegenerative diseases. Cell Biosci. 2021, 11, 218. [Google Scholar] [CrossRef]
- MacDougall, G.; Brown, L.Y.; Kantor, B.; Chiba-Falek, O. The Path to Progress Preclinical Studies of Age-Related Neurodegenerative Diseases: A Perspective on Rodent and hiPSC-Derived Models. Mol. Ther. 2021, 29, 949–972. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: A review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef]
- Bracken, M.B. Why animal studies are often poor predictors of human reactions to exposure. J. R. Soc. Med. 2009, 102, 120–122. [Google Scholar] [CrossRef]
- Monteggia, L.M.; Heimer, H.; Nestler, E.J. Meeting Report: Can We Make Animal Models of Human Mental Illness? Biol. Psychiatry 2018, 84, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Hylander, B.L.; Repasky, E.A.; Sexton, S. Using Mice to Model Human Disease: Understanding the Roles of Baseline Housing-Induced and Experimentally Imposed Stresses in Animal Welfare and Experimental Reproducibility. Animals 2022, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Nishikawa, M.; Kutsuzawa, N.; Tokito, F.; Kobayashi, T.; Kurniawan, D.A.; Shioda, H.; Cao, W.; Shinha, K.; Nakamura, H.; et al. Advancements in Microphysiological systems: Exploring organoids and organ-on-a-chip technologies in drug development focus on pharmacokinetics related organs. Drug Metab. Pharmacokinet. 2025, 60, 101046. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Li, C.; Cao, J.; Cui, Z.; Du, J.; Fu, Z.; Yang, H.; Chen, P. Organ-on-a-chip meets artificial intelligence in drug evaluation. Theranostics 2023, 13, 4526–4558. [Google Scholar] [CrossRef]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Wang, Z.; Cormier, R.T. Golden Syrian Hamster Models for Cancer Research. Cells 2022, 11, 2395. [Google Scholar] [CrossRef]
- Miao, J.; Chard, L.S.; Wang, Z.; Wang, Y. Syrian Hamster as an Animal Model for the Study on Infectious Diseases. Front. Immunol. 2019, 10, 2329. [Google Scholar] [CrossRef]
- Warner, B.M.; Safronetz, D.; Kobinger, G.P. Syrian Hamsters as a Small Animal Model for Emerging Infectious Diseases: Advances in Immunologic Methods. Adv. Exp. Med. Biol. 2017, 972, 87–101. [Google Scholar] [CrossRef]
- Saini, S.; Rai, A.K. Hamster, a close model for visceral leishmaniasis: Opportunities and challenges. Parasite Immunol. 2020, 42, e12768. [Google Scholar] [CrossRef] [PubMed]
- Miedel, E.L.; Hankenson, F.C. Chapter 5—Biology and Diseases of Hamsters. In Laboratory Animal Medicine, 3rd ed.; Fox, J.G., Anderson, L.C., Otto, G.M., Pritchett-Corning, K.R., Whary, M.T., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 209–245. [Google Scholar]
- Kliman, R.M.; Lynch, G.R. Evidence for independence of circadian characters and extent of photoresponsiveness in the Djungarian hamster, Phodopus sungorus. J. Biol. Rhythm 1991, 6, 159–166. [Google Scholar] [CrossRef]
- Hankenson, F.C.; Van Hoosier, G.L. Chapter 5—Biology and Diseases of Hamsters. In Laboratory Animal Medicine, 2nd ed.; Fox, J.G., Anderson, L.C., Loew, F.M., Quimby, F.W., Eds.; Academic Press: Burlington, MA, USA, 2002; pp. 167–202. [Google Scholar]
- Martin, B.; Ji, S.; Maudsley, S.; Mattson, M.P. “Control” laboratory rodents are metabolically morbid: Why it matters. Proc. Natl. Acad. Sci. USA 2010, 107, 6127–6133. [Google Scholar] [CrossRef]
- Dedoni, S.; Scherma, M.; Camoglio, C.; Siddi, C.; Dazzi, L.; Puliga, R.; Frau, J.; Cocco, E.; Fadda, P. An overall view of the most common experimental models for multiple sclerosis. Neurobiol. Dis. 2023, 184, 106230. [Google Scholar] [CrossRef]
- Masopust, D.; Sivula, C.P.; Jameson, S.C. Of Mice, Dirty Mice, and Men: Using Mice To Understand Human Immunology. J. Immunol. 2017, 199, 383–388. [Google Scholar] [CrossRef]
- Lai, F.; Chen, Q. Humanized Mouse Models for the Study of Infection and Pathogenesis of Human Viruses. Viruses 2018, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Coers, J.; Starnbach, M.N.; Howard, J.C. Modeling infectious disease in mice: Co-adaptation and the role of host-specific IFNgamma responses. PLoS Pathog. 2009, 5, e1000333. [Google Scholar] [CrossRef]
- Ottaiano, A.; Ianniello, M.; Santorsola, M.; Ruggiero, R.; Sirica, R.; Sabbatino, F.; Perri, F.; Cascella, M.; Di Marzo, M.; Berretta, M.; et al. From Chaos to Opportunity: Decoding Cancer Heterogeneity for Enhanced Treatment Strategies. Biology 2023, 12, 1183. [Google Scholar] [CrossRef] [PubMed]
- Walrath, J.C.; Hawes, J.J.; Van Dyke, T.; Reilly, K.M. Genetically engineered mouse models in cancer research. Adv. Cancer Res. 2010, 106, 113–164. [Google Scholar] [CrossRef]
- Sarkar, S.; Heise, M.T. Mouse Models as Resources for Studying Infectious Diseases. Clin. Ther. 2019, 41, 1912–1922. [Google Scholar] [CrossRef]
- Avdoshina, D.V.; Kondrashova, A.S.; Belikova, M.G.; Bayurova, E.O. Murine Models of Chronic Viral Infections and Associated Cancers. Mol. Biol. 2022, 56, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Kiani, A.K.; Pheby, D.; Henehan, G.; Brown, R.; Sieving, P.; Sykora, P.; Marks, R.; Falsini, B.; Capodicasa, N.; Miertus, S.; et al. Ethical considerations regarding animal experimentation. J. Prev. Med. Hyg. 2022, 63, E255–E266. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A. The flaws and human harms of animal experimentation. Camb. Q. Healthc. Ethics 2015, 24, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Cui, W.; Zhang, B.; Fonseca, P.; Zhao, Q.; Zhang, P.; Xu, B.; Zhang, Q.; Li, Z.; Seashore-Ludlow, B.; et al. Patient-derived organoids in precision cancer medicine. Med 2024, 5, 1351–1377. [Google Scholar] [CrossRef]
- Azar, J.; Bahmad, H.F.; Daher, D.; Moubarak, M.M.; Hadadeh, O.; Monzer, A.; Al Bitar, S.; Jamal, M.; Al-Sayegh, M.; Abou-Kheir, W. The Use of Stem Cell-Derived Organoids in Disease Modeling: An Update. Int. J. Mol. Sci. 2021, 22, 7667. [Google Scholar] [CrossRef]
- Wang, H.; Brown, P.C.; Chow, E.C.Y.; Ewart, L.; Ferguson, S.S.; Fitzpatrick, S.; Freedman, B.S.; Guo, G.L.; Hedrich, W.; Heyward, S.; et al. 3D cell culture models: Drug pharmacokinetics, safety assessment, and regulatory consideration. Clin. Transl. Sci. 2021, 14, 1659–1680. [Google Scholar] [CrossRef]
- Kondo, J.; Inoue, M. Application of Cancer Organoid Model for Drug Screening and Personalized Therapy. Cells 2019, 8, 470. [Google Scholar] [CrossRef]
- Bose, S.; Clevers, H.; Shen, X. Promises and Challenges of Organoid-Guided Precision Medicine. Med 2021, 2, 1011–1026. [Google Scholar] [CrossRef]
- El Harane, S.; Zidi, B.; El Harane, N.; Krause, K.H.; Matthes, T.; Preynat-Seauve, O. Cancer Spheroids and Organoids as Novel Tools for Research and Therapy: State of the Art and Challenges to Guide Precision Medicine. Cells 2023, 12, 1001. [Google Scholar] [CrossRef]
- Rudroff, T. Artificial Intelligence as a Replacement for Animal Experiments in Neurology: Potential, Progress, and Challenges. Neurol. Int. 2024, 16, 805–820. [Google Scholar] [CrossRef]
- Ezanno, P.; Picault, S.; Beaunee, G.; Bailly, X.; Munoz, F.; Duboz, R.; Monod, H.; Guegan, J.F. Research perspectives on animal health in the era of artificial intelligence. Vet. Res. 2021, 52, 40. [Google Scholar] [CrossRef] [PubMed]
- Madden, J.C.; Enoch, S.J.; Paini, A.; Cronin, M.T.D. A Review of In Silico Tools as Alternatives to Animal Testing: Principles, Resources and Applications. Altern. Lab. Anim. 2020, 48, 146–172. [Google Scholar] [CrossRef]
- Barre-Sinoussi, F.; Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Future Sci. OA 2015, 1, FSO63. [Google Scholar] [CrossRef]
- VandeBerg, J.L.; Williams-Blangero, S. Advantages and limitations of nonhuman primates as animal models in genetic research on complex diseases. J. Med. Primatol. 1997, 26, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Estes, J.D.; Wong, S.W.; Brenchley, J.M. Nonhuman primate models of human viral infections. Nat. Rev. Immunol. 2018, 18, 390–404. [Google Scholar] [CrossRef]
- Proetzel, G.; Wiles, M.V.; Roopenian, D.C. Genetically engineered humanized mouse models for preclinical antibody studies. BioDrugs 2014, 28, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Legrand, N.; Ploss, A.; Balling, R.; Becker, P.D.; Borsotti, C.; Brezillon, N.; Debarry, J.; de Jong, Y.; Deng, H.; Di Santo, J.P.; et al. Humanized mice for modeling human infectious disease: Challenges, progress, and outlook. Cell Host Microbe 2009, 6, 5–9. [Google Scholar] [CrossRef]
- Ma, M.; Ge, J.Y.; Nie, Y.Z.; Li, Y.M.; Zheng, Y.W. Developing Humanized Animal Models with Transplantable Human iPSC-Derived Cells. Front. Biosci. 2024, 29, 34. [Google Scholar] [CrossRef]
- Allen, T.M.; Brehm, M.A.; Bridges, S.; Ferguson, S.; Kumar, P.; Mirochnitchenko, O.; Palucka, K.; Pelanda, R.; Sanders-Beer, B.; Shultz, L.D.; et al. Humanized immune system mouse models: Progress, challenges and opportunities. Nat. Immunol. 2019, 20, 770–774. [Google Scholar] [CrossRef]
- Zhou, Q.; Facciponte, J.; Jin, M.; Shen, Q.; Lin, Q. Humanized NOD-SCID IL2rg−/− mice as a preclinical model for cancer research and its potential use for individualized cancer therapies. Cancer Lett. 2014, 344, 13–19. [Google Scholar] [CrossRef]
- Tillman, H.; Janke, L.J.; Funk, A.; Vogel, P.; Rehg, J.E. Morphologic and Immunohistochemical Characterization of Spontaneous Lymphoma/Leukemia in NSG Mice. Vet. Pathol. 2020, 57, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Vaeteewoottacharn, K.; Kariya, R. Application of Highly Immunocompromised Mice for the Establishment of Patient-Derived Xenograft (PDX) Models. Cells 2019, 8, 889. [Google Scholar] [CrossRef]
- de Arruda, M.S.; Montenegro, M.R. The hamster cheek pouch: An immunologically privileged site suitable to the study of granulomatous infections. Rev. Inst. Med. Trop. Sao Paulo 1995, 37, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.A. Chapter 27—Anatomy, Physiology, and Behavior. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Suckow, M.A., Stevens, K.A., Wilson, R.P., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 753–763. [Google Scholar]
- Williams, D.E.; Evans, D.M.; Blamey, R.W. The primary implantation of human tumours to the hamster cheek pouch. Br. J. Cancer 1971, 25, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.M.; Li, S.; Gumin, J.; Daou, M.; Ledbetter, D.; Yang, J.; Singh, S.; Parker Kerrigan, B.C.; Hossain, A.; Yuan, Y.; et al. An immune-competent, replication-permissive Syrian Hamster glioma model for evaluating Delta-24-RGD oncolytic adenovirus. Neuro-Oncology 2021, 23, 1911–1921. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Y.; Dunmall, L.S.C.; Lemoine, N.R.; Wang, P.; Wang, Y. Syrian hamster as an ideal animal model for evaluation of cancer immunotherapy. Front. Immunol. 2023, 14, 1126969. [Google Scholar] [CrossRef]
- Phelps, C.; Huey, D.D.; Niewiesk, S. Production of Humanized Mice through Stem Cell Transfer. Curr. Protoc. 2023, 3, e800. [Google Scholar] [CrossRef]
- Puchalapalli, M.; Zeng, X.; Mu, L.; Anderson, A.; Hix Glickman, L.; Zhang, M.; Sayyad, M.R.; Mosticone Wangensteen, S.; Clevenger, C.V.; Koblinski, J.E. NSG Mice Provide a Better Spontaneous Model of Breast Cancer Metastasis than Athymic (Nude) Mice. PLoS ONE 2016, 11, e0163521. [Google Scholar] [CrossRef]
- Kaur, K.; Jewett, A. Differences in Tumor Growth and Differentiation in NSG and Humanized-BLT Mice; Analysis of Human vs. Humanized-BLT-Derived NK Expansion and Functions. Cancers 2022, 15, 112. [Google Scholar] [CrossRef]
- Hanazawa, A.; Ito, R.; Katano, I.; Kawai, K.; Goto, M.; Suemizu, H.; Kawakami, Y.; Ito, M.; Takahashi, T. Generation of Human Immunosuppressive Myeloid Cell Populations in Human Interleukin-6 Transgenic NOG Mice. Front. Immunol. 2018, 9, 152. [Google Scholar] [CrossRef]
- Barker, C.F.; Billingham, R.E. The lymphatic status of hamster cheek pouch tissue in relation to its properties as a graft and as a graft site. J. Exp. Med. 1971, 133, 620–639. [Google Scholar] [CrossRef]
- Banerjee, J.; Papu John, A.M.; Al-Wadei, M.H.; Schuller, H.M. Prevention of pancreatic cancer in a hamster model by cAMP decrease. Oncotarget 2016, 7, 44430–44441. [Google Scholar] [CrossRef]
- Wiblin, C.N.; MacPherson, I.A. The transformation of BHK 21 hamster cells by simian virus 40. Int. J. Cancer 1972, 10, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.M.; Keir, H.M. Excretion of polyamines from baby hamster kidney cells (BHK-21/C13: Effect of infection with Herpes Simplex Virus Type 1. J. Gen. Virol. 1981, 56, 251–258. [Google Scholar] [CrossRef]
- Taylor, H.C.; Fallon, M.D.; Velasco, M.E. Oncogenic osteomalacia and inappropriate antidiuretic hormone secretion due to oat-cell carcinoma. Ann. Intern. Med. 1984, 101, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Sandham, J.S. Health Promotion Authority for Wales. Br. Dent. J. 1989, 167, 370–371. [Google Scholar] [CrossRef]
- Fossati, C. [Collateral effects of an anti-arrhythmic agent: Amiodarone]. Clin. Ter. 1985, 114, 509–514. [Google Scholar] [PubMed]
- Young, B.A.; Spencer, J.F.; Ying, B.; Tollefson, A.E.; Toth, K.; Wold, W.S. The role of cyclophosphamide in enhancing antitumor efficacy of an adenovirus oncolytic vector in subcutaneous Syrian hamster tumors. Cancer Gene Ther. 2013, 20, 521–530. [Google Scholar] [CrossRef]
- Urmanova, M.A.; Tsareva, A.A. [The cytogenetic study of established Syrian hamster cell lines. II. A comparative analysis of the karyotypes of cell lines HaK, CER and BHK-21 (C-13)]. Tsitologiia 1996, 38, 639–645. [Google Scholar]
- Suklabaidya, S.; Das, B.; Ali, S.A.; Jain, S.; Swaminathan, S.; Mohanty, A.K.; Panda, S.K.; Dash, P.; Chakraborty, S.; Batra, S.K.; et al. Characterization and use of HapT1-derived homologous tumors as a preclinical model to evaluate therapeutic efficacy of drugs against pancreatic tumor desmoplasia. Oncotarget 2016, 7, 41825–41842. [Google Scholar] [CrossRef]
- Abraham, A.T.; Shah, S.R.; Davidson, B.R. The HaP-T1 Syrian golden hamster pancreatic cancer model: Cell implantation is better than tissue implantation. Pancreas 2004, 29, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Vanathi, M.; Azimeera, S.; Gupta, N.; Tandon, R. Study on change in corneal biomechanics and effect of percent tissue altered in myopic laser-assisted in situ keratomileusis. Indian J. Ophthalmol. 2020, 68, 2964–2974. [Google Scholar] [CrossRef]
- Maistro, E.L.; Carvalho, J.C.; Mantovani, M.S. Evaluation of the genotoxic potential of the Casearia sylvestris extract on HTC and V79 cells by the comet assay. Toxicol. In Vitro 2004, 18, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Kitsak, V.; Mikhailova, G.R.; Tsareva, A.A.; Gushchina, E.A.; Novokhatskii, A.S. [Morphological, cytogenetic and proliferative characteristics of Syrian hamster HTC-2 and HTC-1 cells and of the HTCT tumor cell line transformed by herpes simplex type 2 virus]. Vopr. Virusol. 1982, 27, 415–418. [Google Scholar] [PubMed]
- Silva Gunawardene, Y.I.; Bendena, W.G.; Tobe, S.S.; Chan, S.M. Comparative immunohistochemistry and cellular distribution of farnesoic acid O-methyltransferase in the shrimp and the crayfish. Peptides 2003, 24, 1591–1597. [Google Scholar] [CrossRef]
- Oswiecimska, J.; Brus, R.; Szkilnik, R.; Nowak, P.; Kostrzewa, R.M. 7-OH-DPAT, unlike quinpirole, does not prime a yawning response in rats. Pharmacol. Biochem. Behav. 2000, 67, 11–15. [Google Scholar] [CrossRef]
- Englebienne, P. The serum steroid transport proteins: Biochemistry and clinical significance. Mol. Asp. Med. 1984, 7, 313–396. [Google Scholar] [CrossRef]
- Byers, T.L.; Bitonti, A.J.; McCann, P.P. bis(benzyl)polyamine analogues are substrates for a mammalian cell-transport system which is distinct from the polyamine-transport system. Biochem. J. 1990, 269, 35–40. [Google Scholar] [CrossRef]
- Jeon, M.J.; Ko, H.; Shin, S.J.; Kim, M. Cytotoxicity and genotoxicity of various types of endodontic sealers in Chinese hamster ovary (CHO-K1) cells. Dent. Mater. J. 2023, 42, 774–779. [Google Scholar] [CrossRef]
- Moore, G.J.; Bebchuk, J.M.; Hasanat, K.; Chen, G.; Seraji-Bozorgzad, N.; Wilds, I.B.; Faulk, M.W.; Koch, S.; Glitz, D.A.; Jolkovsky, L.; et al. Lithium increases N-acetyl-aspartate in the human brain: In vivo evidence in support of bcl-2’s neurotrophic effects? Biol. Psychiatry 2000, 48, 1–8. [Google Scholar] [CrossRef]
- Mire-Sluis, A.R.; Hoffbrand, A.V.; Wickremasinghe, R.G. Evidence that guanine-nucleotide binding regulatory proteins couple cell-surface receptors to the breakdown of inositol-containing lipids during T-lymphocyte mitogenesis. Biochem. Biophys. Res. Commun. 1987, 148, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- De, S. Bronchus-associated Lymphoid Tissue Lymphoma. J. Bronchol. Interv. Pulmonol. 2011, 18, 295–296. [Google Scholar] [CrossRef]
- Braxton, A.M.; Creisher, P.S.; Ruiz-Bedoya, C.A.; Mulka, K.R.; Dhakal, S.; Ordonez, A.A.; Beck, S.E.; Jain, S.K.; Villano, J.S. Hamsters as a Model of Severe Acute Respiratory Syndrome Coronavirus-2. Comp. Med. 2021, 71, 398–410. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Desrosiers, A.; Terzaghi, M.; Little, J.B. Morphometric and histological analysis of the lungs of Syrian golden hamsters. J. Anat. 1978, 125, 527–553. [Google Scholar]
- Imai, M.; Iwatsuki-Horimoto, K.; Hatta, M.; Loeber, S.; Halfmann, P.J.; Nakajima, N.; Watanabe, T.; Ujie, M.; Takahashi, K.; Ito, M.; et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl. Acad. Sci. USA 2020, 117, 16587–16595. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.R.; Little, J.B. Respiratory System Differences Relevant to Lung Carcinogenesis between Syrian Hamsters and Other Species. In Progress in Tumor Research, Proceedings of the Symposium on the Syrian Hamster in Toxicology and Carcinogenesis Research, Boston, MA, USA, 30 November–2 December 1977; Homburger, F., Ed.; Karger International: Basel, Switzerland, 1979; Volume 24, pp. 302–314. [Google Scholar]
- Kennedy, A.R.; McGandy, R.B.; Little, J.B. Morphologic and histochemical characteristics of cell lines derived from hamster peripheral lung tumors. Eur. J. Cancer 1977, 13, 1341–1350. [Google Scholar] [CrossRef]
- Hammond, W.G.; Teplitz, R.L.; Benfield, J.R. Lung cancer model for study of the metastatic process. Ann. Thorac. Surg. 1991, 52, 732–736; discussion 737. [Google Scholar] [CrossRef] [PubMed]
- Tsuchitani, M.; Sato, J.; Kokoshima, H. A comparison of the anatomical structure of the pancreas in experimental animals. J. Toxicol. Pathol. 2016, 29, 147–154. [Google Scholar] [CrossRef]
- Wang, Y.; Kayoumu, A.; Lu, G.; Xu, P.; Qiu, X.; Chen, L.; Qi, R.; Huang, S.; Li, W.; Wang, Y.; et al. Experimental Models in Syrian Golden Hamster Replicate Human Acute Pancreatitis. Sci. Rep. 2016, 6, 28014. [Google Scholar] [CrossRef]
- Takahashi, M.; Pour, P.; Althoff, J.; Donnelly, T. The pancreas of the Syrian hamster (Mesocricetus auratus). I Anatomical study. Lab. Anim. Sci. 1977, 27, 336–342. [Google Scholar]
- Jewell, H.A.; Charipper, H.A. The morphology of the pancreas of the golden hamster, Cricetus auratus, with special reference to the histology and cytology of the islets of Langerhans. Anat. Rec. 1951, 111, 401–415. [Google Scholar] [CrossRef]
- Takahashi, M.; Runge, R.; Donnelly, T.; Pour, P. The morphologic and biologic patterns of chemically induced pancreatic adenocarcinoma in Syrian golden hamsters after homologous transplantation. Cancer Lett. 1979, 7, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Egami, H.; Tomioka, T.; Tempero, M.; Kay, D.; Pour, P.M. Development of intrapancreatic transplantable model of pancreatic duct adenocarcinoma in Syrian golden hamsters. Am. J. Pathol. 1991, 138, 557–561. [Google Scholar] [PubMed]
- Schneider, M.B.; Matsuzaki, H.; Haorah, J.; Ulrich, A.; Standop, J.; Ding, X.Z.; Adrian, T.E.; Pour, P.M. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology 2001, 120, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Nistal-Villan, E.; Bunuales, M.; Poutou, J.; Gonzalez-Aparicio, M.; Bravo-Perez, C.; Quetglas, J.I.; Carte, B.; Gonzalez-Aseguinolaza, G.; Prieto, J.; Larrea, E.; et al. Enhanced therapeutic effect using sequential administration of antigenically distinct oncolytic viruses expressing oncostatin M in a Syrian hamster orthotopic pancreatic cancer model. Mol. Cancer 2015, 14, 210. [Google Scholar] [CrossRef]
- Bortolanza, S.; Bunuales, M.; Otano, I.; Gonzalez-Aseguinolaza, G.; Ortiz-de-Solorzano, C.; Perez, D.; Prieto, J.; Hernandez-Alcoceba, R. Treatment of pancreatic cancer with an oncolytic adenovirus expressing interleukin-12 in Syrian hamsters. Mol. Ther. 2009, 17, 614–622. [Google Scholar] [CrossRef]
- Yanagi, K.; Onda, M.; Uchida, E. Effect of angiostatin on liver metastasis of pancreatic cancer in hamsters. Jpn. J. Cancer Res. 2000, 91, 723–730. [Google Scholar] [CrossRef]
- Valentine, H.; Daugherity, E.K.; Singh, B.; Maurer, K.J. The Experimental Use of Syrian Hamsters. In The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Academic Press: Cambridge, MA, USA, 2012; pp. 875–906. [Google Scholar]
- Hickman, D.L.; Johnson, J.; Vemulapalli, T.H.; Crisler, J.R.; Shepherd, R. Commonly Used Animal Models. In Principles of Animal Research for Graduate and Undergraduate Students; Academic Press: Cambridge, MA, USA, 2017; pp. 117–175. [Google Scholar] [CrossRef]
- Souza, A.J.S.; Souza Filho, A.F.; Zimpel, C.K.; Ayupe, M.C.; Araujo, M.V.; Machado, R.R.G.; Salles, E.; Salgado, C.L.; Tavares, M.S.; Silva-Pereira, T.T.; et al. Hepatic endotheliitis in Golden Syrian hamsters (Mesocricetus auratus) experimentally infected with SARS-CoV-2. Rev. Inst. Med. Trop. Sao Paulo 2024, 66, e44. [Google Scholar] [CrossRef]
- Bhamarapravati, N.; Thammavit, W.; Vajrasthira, S. Liver changes in hamsters infected with a liver fluke of man, Opisthorchis viverrini. Am. J. Trop. Med. Hyg. 1978, 27, 787–794. [Google Scholar] [CrossRef]
- Russell, P.S.; Hong, J.; Windsor, J.A.; Itkin, M.; Phillips, A.R.J. Renal Lymphatics: Anatomy, Physiology, and Clinical Implications. Front. Physiol. 2019, 10, 251. [Google Scholar] [CrossRef]
- Kruepunga, N.; Hakvoort, T.B.M.; Hikspoors, J.; Kohler, S.E.; Lamers, W.H. Anatomy of rodent and human livers: What are the differences? Biochim. Biophys. Acta—Mol. Basis Dis. 2019, 1865, 869–878. [Google Scholar] [CrossRef]
- Zhao, Y.; Qu, H.; Wang, Y.; Xiao, W.; Zhang, Y.; Shi, D. Small rodent models of atherosclerosis. Biomed. Pharmacother. 2020, 129, 110426. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Lai, P.; Guo, J.; Wang, Y.; Xian, X. Genetically-engineered hamster models: Applications and perspective in dyslipidemia and atherosclerosis-related cardiovascular disease. Med. Rev. 2021, 1, 92–110. [Google Scholar] [CrossRef] [PubMed]
- Dillard, A.; Matthan, N.R.; Lichtenstein, A.H. Use of hamster as a model to study diet-induced atherosclerosis. Nutr. Metab. 2010, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Horn, P.S.; Heubi, J.E.; Woollett, L.A. The liver plays a key role in whole body sterol accretion of the neonatal Golden Syrian hamster. Biochim. Biophys. Acta 2007, 1771, 550–557. [Google Scholar] [CrossRef]
- Chen, G.; Dai, Z.K.; Liang, R.G.; Xiao, S.J.; He, S.Q.; Zhao, H.L.; Xu, Q. Characterization of diethylnitrosamine-induced liver carcinogenesis in Syrian golden hamsters. Exp. Ther. Med. 2012, 3, 285–292. [Google Scholar] [CrossRef]
- Martinez, I.; Wallace, G.; Zhang, C.; Legge, R.; Benson, A.K.; Carr, T.P.; Moriyama, E.N.; Walter, J. Diet-induced metabolic improvements in a hamster model of hypercholesterolemia are strongly linked to alterations of the gut microbiota. Appl. Environ. Microbiol. 2009, 75, 4175–4184. [Google Scholar] [CrossRef]
- Clarke, S.F.; Murphy, E.F.; Nilaweera, K.; Ross, P.R.; Shanahan, F.; O’Toole, P.W.; Cotter, P.D. The gut microbiota and its relationship to diet and obesity: New insights. Gut Microbes 2012, 3, 186–202. [Google Scholar] [CrossRef]
- Wan, S.; You, P.; Shi, Q.; Hu, H.; Zhang, L.; Chen, L.; Wu, Z.; Lin, S.; Song, X.; Luo, Y.; et al. Gut microbiome changes in mouse, Mongolian gerbil, and hamster models following Clostridioides difficile challenge. Front. Microbiol. 2024, 15, 1368194. [Google Scholar] [CrossRef]
- Boswald, L.F.; Popper, B.; Matzek, D.; Neuhaus, K.; Wenderlein, J. Characterization of the gastrointestinal microbiome of the Syrian hamster (Mesocricetus auratus) and comparison to data from mice. FEBS Open Bio 2024, 14, 1701–1717. [Google Scholar] [CrossRef]
- Xiao, J.; Metzler-Zebeli, B.U.; Zebeli, Q. Gut Function-Enhancing Properties and Metabolic Effects of Dietary Indigestible Sugars in Rodents and Rabbits. Nutrients 2015, 7, 8348–8365. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Zheng, Y.; Xue, H.; Xu, J.; Wu, M.; Chen, L.; Xu, L. Different gut microbial types were found in captive striped hamsters. PeerJ 2023, 11, e16365. [Google Scholar] [CrossRef] [PubMed]
- Takiyama, Y.; Egami, H.; Pour, P.M. Expression of human tumor-associated antigens in pancreatic cancer induced in Syrian hamsters. Am. J. Pathol. 1990, 136, 707–715. [Google Scholar]
- Nambiar, P.R.; Kirchain, S.; Fox, J.G. Gastritis-associated Adenocarcinoma and Intestinal Metaplasia in a Syrian Hamster Naturally Infected with Helicobacter Species. Vet. Pathol. 2005, 42, 386–390. [Google Scholar] [CrossRef]
- Yapijakis, C.; Kalogera, S.; Papakosta, V.; Vassiliou, S. The Hamster Model of Sequential Oral Carcinogenesis: An Update. In Vivo 2019, 33, 1751–1755. [Google Scholar] [CrossRef]
- Doetschman, T.; Georgieva, T. Gene Editing with CRISPR/Cas9 RNA-Directed Nuclease. Circ. Res. 2017, 120, 876–894. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.A.; Spencer, J.F.; La Regina, M.C.; Dhar, D.; Tollefson, A.E.; Toth, K.; Wold, W.S. Syrian hamster as a permissive immunocompetent animal model for the study of oncolytic adenovirus vectors. Cancer Res. 2006, 66, 1270–1276. [Google Scholar] [CrossRef]
- Nelles, M.J.; Duncan, W.R.; Streilein, J.W. Immune response to acute virus infection in the Syrian hamster. II. Studies on the identity of virus-induced cytotoxic effector cells. J. Immunol. 1981, 126, 214–218. [Google Scholar] [CrossRef]
- Avula, L.R.; Hagerty, B.; Alewine, C. Molecular mediators of peritoneal metastasis in pancreatic cancer. Cancer Metastasis Rev. 2020, 39, 1223–1243. [Google Scholar] [CrossRef]
- Zivcec, M.; Safronetz, D.; Haddock, E.; Feldmann, H.; Ebihara, H. Validation of assays to monitor immune responses in the Syrian golden hamster (Mesocricetus auratus). J. Immunol. Methods 2011, 368, 24–35. [Google Scholar] [CrossRef]
- Samant, M.; Sahu, U.; Pandey, S.C.; Khare, P. Role of Cytokines in Experimental and Human Visceral Leishmaniasis. Front. Cell. Infect. Microbiol. 2021, 11, 624009. [Google Scholar] [CrossRef] [PubMed]
- Poutou, J.; Bunuales, M.; Gonzalez-Aparicio, M.; Garcia-Aragoncillo, E.; Quetglas, J.I.; Casado, R.; Bravo-Perez, C.; Alzuguren, P.; Hernandez-Alcoceba, R. Safety and antitumor effect of oncolytic and helper-dependent adenoviruses expressing interleukin-12 variants in a hamster pancreatic cancer model. Gene Ther. 2015, 22, 696–706. [Google Scholar] [CrossRef]
- Loukas, A.; Prociv, P. Immune responses in hookworm infections. Clin. Microbiol. Rev. 2001, 14, 689–703, table of contents. [Google Scholar] [CrossRef]
- Francis, M.E.; Goncin, U.; Kroeker, A.; Swan, C.; Ralph, R.; Lu, Y.; Etzioni, A.L.; Falzarano, D.; Gerdts, V.; Machtaler, S.; et al. SARS-CoV-2 infection in the Syrian hamster model causes inflammation as well as type I interferon dysregulation in both respiratory and non-respiratory tissues including the heart and kidney. PLoS Pathog. 2021, 17, e1009705. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, Y.; Zhu, Z.; Lu, C.; Zhang, C.; Zeng, L.; Xie, F.; Zhang, L.; Zhou, F. Mucosal immune response in biology, disease prevention and treatment. Signal Transduct. Target. Ther. 2025, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Siurala, M.; Vaha-Koskela, M.; Havunen, R.; Tahtinen, S.; Bramante, S.; Parviainen, S.; Mathis, J.M.; Kanerva, A.; Hemminki, A. Syngeneic syrian hamster tumors feature tumor-infiltrating lymphocytes allowing adoptive cell therapy enhanced by oncolytic adenovirus in a replication permissive setting. OncoImmunology 2016, 5, e1136046. [Google Scholar] [CrossRef]
- Mendez, S.; Valenzuela, J.G.; Wu, W.; Hotez, P.J. Host cytokine production, lymphoproliferation, and antibody responses during the course of Ancylostoma ceylanicum infection in the Golden Syrian hamster. Infect. Immun. 2005, 73, 3402–3407. [Google Scholar] [CrossRef]
- Thomas, M.A.; Spencer, J.F.; Toth, K.; Sagartz, J.E.; Phillips, N.J.; Wold, W.S. Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol. Ther. 2008, 16, 1665–1673. [Google Scholar] [CrossRef]
- Prakash, S.; Dhanushkodi, N.R.; Singer, M.; Quadiri, A.; Zayou, L.; Vahed, H.; Coulon, P.-G.; Ibraim, I.C.; Tafoya, C.; Hitchcock, L. A Broad-Spectrum Multi-Antigen mRNA/LNP-Based Pan-Coronavirus Vaccine Induced Potent Cross-Protective Immunity Against Infection and Disease Caused by Highly Pathogenic and Heavily Spike-Mutated SARS-CoV-2 Variants of Concern in the Syrian Hamster Model. bioRxiv 2024. [Google Scholar] [CrossRef]
- Dhanushkodi, N.R.; Prakash, S.; Quadiri, A.; Zayou, L.; Srivastava, R.; Shaik, A.M.; Suzer, B.; Ibraim, I.C.; Landucci, G.; Tifrea, D.F. Antiviral and anti-inflammatory therapeutic effect of RAGE-Ig protein against multiple SARS-CoV-2 variants of concern demonstrated in K18-hACE2 mouse and Syrian golden hamster models. J. Immunol. 2024, 212, 576–585. [Google Scholar] [CrossRef]
- Zabelina, D.S.; Osipov, I.D.; Maslov, D.E.; Kovner, A.V.; Vasikhovskaia, V.A.; Demina, D.S.; Romanov, S.E.; Shishkina, E.V.; Davydova, J.; Netesov, S.V.; et al. Ad6-Based GM-CSF Expressing Vector Displays Oncolytic and Immunostimulatory Effects in an Immunocompetent Syrian Hamster Model of Cholangiocarcinoma. Viruses 2025, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Jiao, Y.; Gao, J.; Wang, T.; Xia, Y.; Li, K.; Yang, Y.; Zhang, J.; Bao, H.; Wang, L.; et al. Enhancement of immune responses to classical swine fever virus E2 in mice by fusion or mixture with the porcine IL-28B. Appl. Microbiol. Biotechnol. 2025, 109, 44. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Hamdy, R.; Ghonaim, J.H.; Husseiny, M.I. Metabolic Imbalance in Immune Cells in Relation to Metabolic Disorders, Cancer, and Infections. In Metabolic Dynamics in Host-Microbe Interaction; Springer Nature: Singapore, 2025; pp. 187–218. [Google Scholar]
- Singer, M.; Hamdy, R.; Elsayed, T.M.; Husseiny, M.I. The Mechanisms and Therapeutic Implications of Metabolic Communication in the Tumor-Immune Microenvironment. In Metabolic Dynamics in Host-Microbe Interaction; Springer Nature: Singapore, 2025; pp. 291–315. [Google Scholar]
- Wang, C.; Cheng, Z.; Miao, J.; Xue, X.; Dong, Y.; Zhao, L.; Guo, H.; Wang, J.; Wang, Z.; Lu, S.; et al. Genomic-transcriptomic analysis identifies the Syrian hamster as a superior animal model for human diseases. BMC Genom. 2025, 26, 286. [Google Scholar] [CrossRef] [PubMed]
- Handley, A.; Ryan, K.A.; Davies, E.R.; Bewley, K.R.; Carnell, O.T.; Challis, A.; Coombes, N.S.; Fotheringham, S.A.; Gooch, K.E.; Charlton, M.; et al. SARS-CoV-2 Disease Severity in the Golden Syrian Hamster Model of Infection Is Related to the Volume of Intranasal Inoculum. Viruses 2023, 15, 748. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).