A Systematic Review of Mental Health Monitoring and Intervention Using Unsupervised Deep Learning on EEG Data
Abstract
1. Introduction
1.1. Background: EEG and Mental Health
1.2. Limitations of Supervised Learning in EEG Mental Health Analysis
- Subjectivity and Heterogeneity: Psychiatric diagnoses often rely on subjective clinical assessments and symptom reporting, leading to variability and overlap between conditions. Diagnostic categories themselves represent heterogeneous groups, and labels may not fully capture the underlying neurobiological diversity (Vafaei & Hosseini, 2025).
- Label Scarcity and Cost: Acquiring large datasets with precise clinical labels or continuous state annotations is resource-intensive, requiring significant clinical expertise and time.
- High Dimensionality and Noise: EEG data are inherently high-dimensional, noisy, and susceptible to artifacts. While DL models can handle complexity better than traditional ML, they still require substantial data to learn robust representations and avoid overfitting (Al-Saegh et al., 2021).
- Generalizability Issues: Models trained on specific datasets often struggle to generalize to new populations, recording equipment, or experimental conditions due to inter-subject variability and domain-shift issues (Gupta et al., 2021).
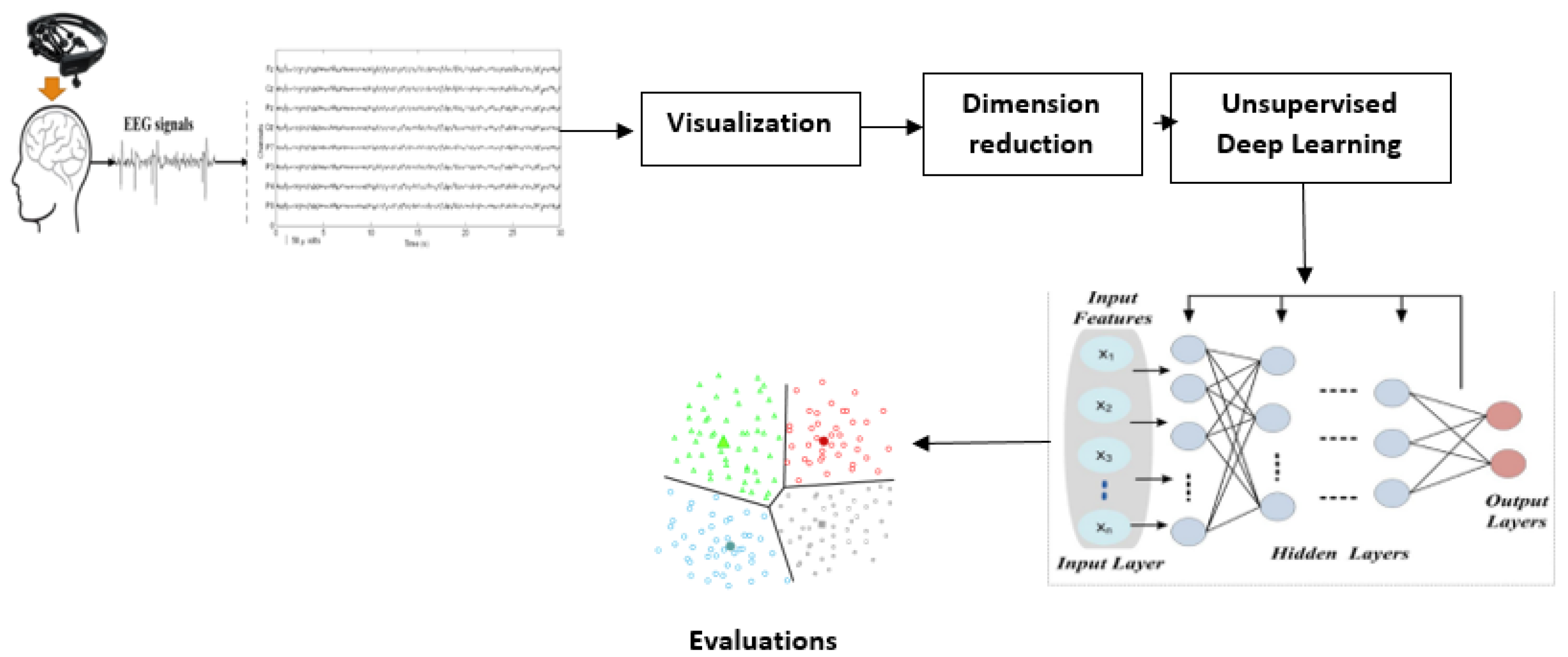
1.3. The Potential of Unsupervised Learning for EEG Mental Health Analysis
- Dimensionality Reduction: Techniques like Principal Component Analysis (PCA) and autoencoders (a type of neural network) can learn compressed representations of high-dimensional EEG data, potentially highlighting the most salient features or variations related to different mental states while reducing noise (Bank et al., 2021; Klein et al., 2025). Autoencoders, in particular, can learn complex non-linear mappings.
- Clustering: Algorithms such as K-Means and Gaussian Mixture Models (GMMs) can group similar EEG patterns or segments together based on intrinsic features (Alhagry et al., 2017; Ezugwu et al., 2022). This could potentially identify distinct neurophysiological subtypes within a diagnostic category, discover different brain states over time, or segment continuous EEG recordings based on underlying activity patterns. Furthermore, prior studies suggest that combining features from different EEG bands, such as the alpha and beta bands, may enhance unsupervised pattern recognition in datasets of emotional or cognitive states (Drzewiecki & Fox, 2024).
- Generative Models: Models like Generative Adversarial Networks (GANs) learn to generate synthetic data that mimics the distribution of the real data (Rathee et al., 2021; K. Wang et al., 2020). In the context of EEG, GANs could be used for data augmentation, anomaly detection (by identifying samples that the generator struggles to create), or learning latent representations that capture the underlying factors of variation in brain activity.
- Discovery of Novel Biomarkers: Previously unknown EEG patterns or features associated with specific mental health conditions or states can be identified, independent of existing diagnostic criteria.
- Identification of Patient Subgroups: Heterogeneity within diagnostic groups can be uncovered based on distinct neurophysiological profiles, potentially leading to more personalized treatment strategies.
- Modeling of Baseline Activity and Detection of Deviations: Normative patterns of brain activity can be learned, and subtle deviations indicative of emerging mental health issues or treatment response can be detected.
- Overcoming of Label Scarcity: Large amounts of unlabeled EEG data can be used, which are often more readily available than labeled datasets.
1.4. Scope and Structure of This Review
2. Materials and Methods
2.1. Search Strategy
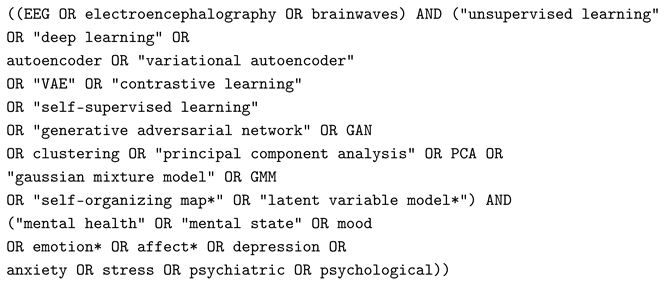
2.2. Inclusion and Exclusion Criteria
- Peer-reviewed journal articles;
- Published in English;
- Published between 2018 and 2025;
- Utilized unsupervised learning or unsupervised deep learning techniques;
- Analyzed electroencephalography (EEG) data;
- Related to mental health, mental state, mood, emotion, or psychological trait monitoring/analysis in humans.
- Non-peer-reviewed materials (e.g., conference abstracts, editorials, and preprints);
- Non-English publications;
- Review articles, meta-analyses, or theoretical papers without original empirical data analysis;
- Animal studies;
- Studies using only supervised learning methods;
- Studies not applying computational analysis to EEG data or using EEG for applications unrelated to mental health (e.g., seizure detection without mental health context).
2.3. Screening and Selection Process (PRISMA)
- Identification: Search results from all databases were aggregated. Duplicates were removed using reference management software.
- Screening: Titles and abstracts of the unique articles were screened against the inclusion and exclusion criteria. This step was performed systematically.
- Eligibility: Full texts of articles deemed potentially relevant during the screening phase were retrieved and assessed in detail for final eligibility.
- Inclusion: Studies that met all inclusion criteria after full-text review were selected for data extraction and synthesis.
2.4. Data Extraction Plan
3. Results
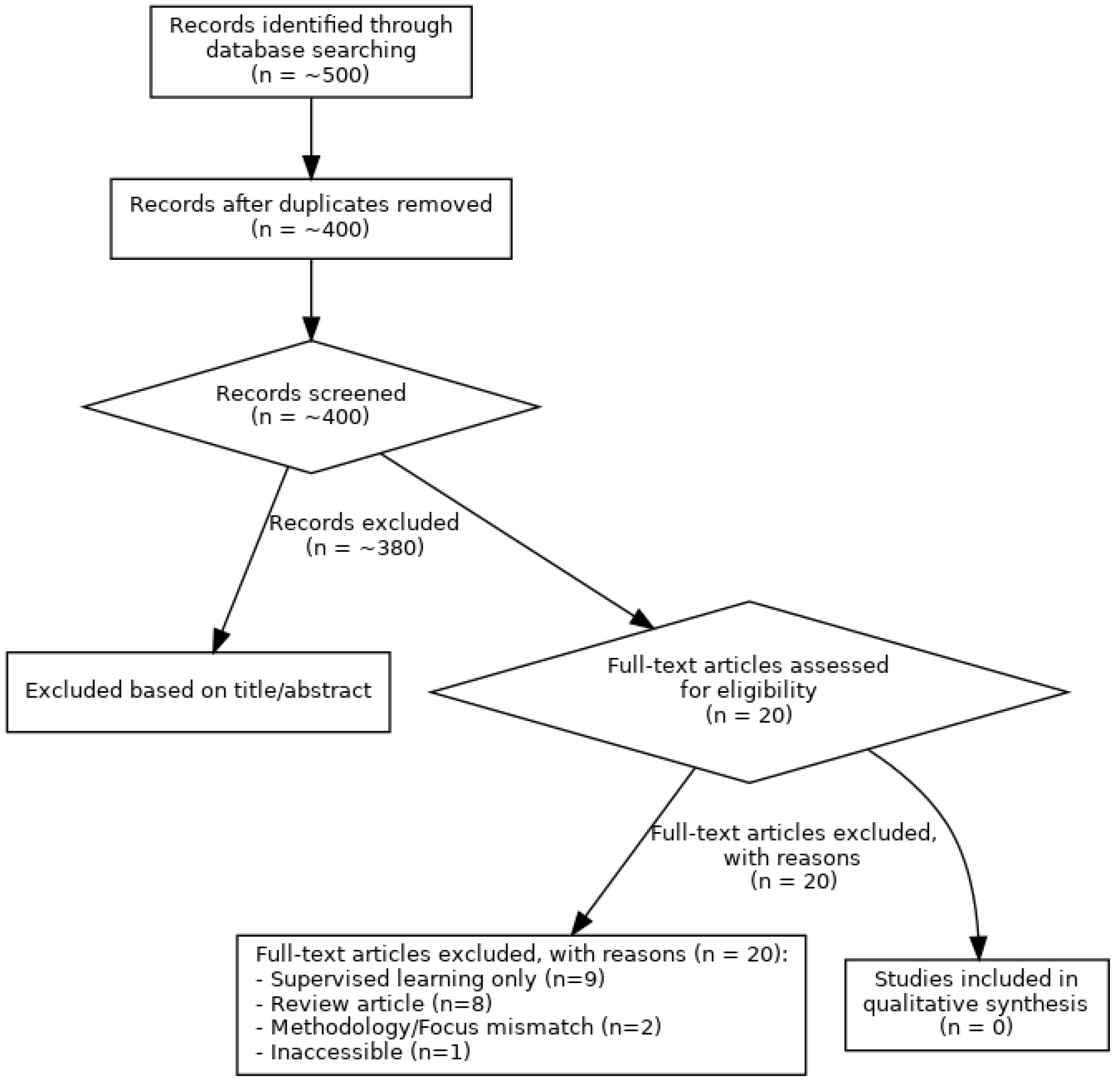
3.1. PRISMA Flow Diagram
3.2. Characteristics of Excluded Studies
- Use of Supervised Learning Only: The majority of excluded studies (12 articles) were removed because they exclusively employed supervised learning methods, failing to meet the core inclusion criterion of utilizing unsupervised techniques.
- Review Articles: Several studies (six articles) were identified as review articles (systematic, narrative, or guideline reviews) rather than original empirical research, leading to their exclusion.
- Incorrect Application Focus: One study focused solely on EEG artifact detection, without a direct link to mental health state analysis.
- Inaccessible Full Text: One article could not be accessed due to a broken link.
- Preprints: Two articles were identified as preprints and, thus, excluded.
3.3. Summary: No Eligible Studies Found
4. Discussion
4.1. Interpretation of Findings: Why the Gap?
- Dominance of Supervised Paradigms: The field of machine learning applied to EEG, particularly in clinical contexts, remains heavily focused on supervised approaches. As highlighted by reviews, much research prioritizes the development and application of supervised models (e.g., SVMs and CNNs) for tasks such as classification and decoding based on predefined labels (Al-Saegh et al., 2021). This focus is understandable, given the clear clinical need for diagnostic classification, prediction of treatment outcomes, or identification of specific brain states corresponding to known conditions. Supervised methods offer a direct path to evaluating performance against established ground truths, aligning well with traditional clinical research goals and validation frameworks (Krol et al., 2023). This inherent alignment with classification-driven objectives may lead researchers to prioritize supervised techniques over the more exploratory nature of unsupervised learning.
- Technical Challenges: Applying unsupervised methods to noisy, high-dimensional EEG data is complex. Discovering meaningful, replicable patterns without labels requires sophisticated algorithms and robust validation strategies, which may be less developed or standardized than supervised counterparts.
- Validation Hurdles: Establishing the clinical relevance of patterns found via unsupervised learning (e.g., novel clusters) is difficult, requiring extensive correlation with external measures or longitudinal tracking.
- Publication Bias and Reporting: Negative or exploratory results from unsupervised analyses might be less likely to be published. Furthermore, unsupervised techniques might be used adjunctively within larger studies focused on supervised outcomes and, thus, not highlighted or indexed appropriately.
- Terminology and Indexing: Our search terms, though broad, might have missed studies using niche terminology for specific unsupervised algorithms.
4.2. Role and Limitations of Supervised Methods in EEG Research
4.3. Potential of Unsupervised Learning in EEG Mental Health: Neuroscience Foundations
4.3.1. Predictive Coding and Free Energy Principle: Theoretical Foundations
- Extracting latent patterns from complex data without predefined labels, similar to how the brain identifies regularities in sensory input;
- Building hierarchical representations that capture increasingly abstract features, paralleling the brain’s cortical hierarchy;
- Minimizing various forms of reconstruction error or statistical divergence, analogous to the brain’s effort to minimize prediction error or free energy.
4.3.2. Neuroscience-Informed Unsupervised Learning for Mental Health
- Identifying natural groupings in neural activity that may correspond to biologically meaningful subtypes within and across diagnostic boundaries;
- Detecting subtle deviations from typical brain dynamics that might represent early markers of mental health conditions;
- Characterizing the dimensional nature of psychopathology by mapping continuous variations in neural patterns.
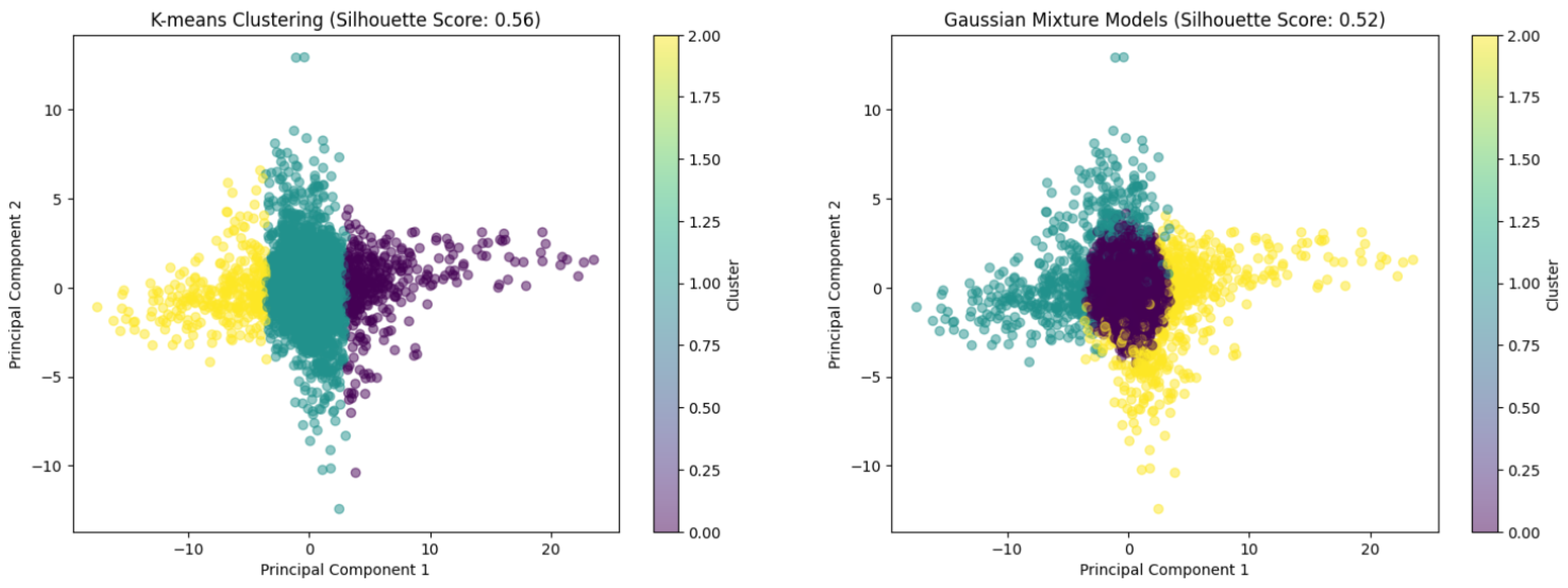
- Discovering Novel Subtypes: Unsupervised clustering techniques hold significant promise for identifying data-driven subtypes within traditional diagnostic categories based on neurophysiological signatures. For example, Y. Zhang et al. (2020) successfully applied unsupervised and supervised machine learning to resting-state EEG functional connectivity patterns (power envelope connectivity) to identify two distinct and clinically relevant subtypes within both PTSD and Major Depressive Disorder (MDD) across multiple datasets (Yan et al., 2023). Crucially, these neurobiologically defined subtypes showed differential responses to treatment (psychotherapy for PTSD and medication for MDD), suggesting their potential utility in personalizing interventions, a goal often difficult to achieve with symptom-based diagnoses alone. Visualizing the results of clustering algorithms applied to dimensionally reduced EEG data can provide insights into the separability of potential groups (Figure 3).
- Dimensionality Reduction and Feature Extraction: The high dimensionality of EEG data presents a challenge. Unsupervised dimensionality reduction methods, such as Principal Component Analysis (PCA) and Independent Component Analysis (ICA) or more complex non-linear techniques like autoencoders, can effectively reduce data complexity, extract salient features representing underlying neural dynamics, denoise signals, and facilitate visualization (Roy et al., 2019). These extracted features can then potentially be used in subsequent supervised or unsupervised analyses.
- Generative Models and Anomaly Detection: Generative models, like generative adversarial networks (GANs) or Variational Autoencoders (VAEs), can learn the distribution of normative brain activity from large EEG datasets. Once trained, these models could be used for anomaly detection, potentially identifying subtle deviations from typical patterns that might indicate early stages of mental health decline or treatment response (K. Wang et al., 2020). They can also be used to generate realistic synthetic EEG data to augment limited datasets for the training of more robust supervised models.
- Exploring Latent Structures: Techniques borrowed from other domains and commonly used in natural language processing, such as topic modeling (e.g., latent Dirichlet allocation), might be adapted to EEG data to uncover latent ‘neural topics’ or recurring patterns of brain activity associated withdifferent mental states or cognitive processes, without prior hypotheses.
4.4. Implications for Clinical Research and Practice
- Objective Biomarker Discovery: Unsupervised learning can analyze complex EEG data without prior assumptions, potentially uncovering novel, objective neurophysiological biomarkers associated with mental health conditions, treatment response, or risk prediction (Yun, 2024). Such biomarkers could augment or even eventually replace subjective symptom scales, leading to more reliable diagnostics and monitoring.
- Patient Stratification and Personalized Medicine: Unsupervised clustering can reveal underlying biological heterogeneity masked by broad diagnostic labels. This approach has shown promise in related neuroimaging domains, such as fMRI-based studies that identified distinct neurobiological subtypes within major depressive disorder (Langer et al., 2022). Similar applications to EEG data could enable more personalized treatment selection, predicting which patients are likely to respond to specific therapies (e.g., medication vs. psychotherapy vs. neuromodulation) and improving overall treatment efficacy (Simmatis et al., 2023).
- Understanding Pathophysiology: Data-driven patterns discovered through unsupervised analysis (e.g., altered connectivity networks and specific oscillatory patterns) can generate new hypotheses about the neurobiological mechanisms underlying mental illness, guiding further basic and clinical research. For instance, recent work in genomics has demonstrated how unsupervised clustering of gene expression data can reveal novel disease subtypes (Cai et al., 2022), suggesting that parallel approaches could be valuable in EEG analysis.
- Early Detection and Monitoring: Unsupervised anomaly detection models trained on normative EEG data could potentially identify subtle, early signs of mental health decline or relapse before symptoms become clinically apparent, enabling proactive interventions (Al-Saegh et al., 2021). Similarly, they could provide objective measures for tracking treatment progress over time. This approach has shown promise in other medical domains, such as in detecting anomalies in cardiac signals (Demirezen et al., 2024).
4.5. Recommendations for Future Research
- Multimodal Integration: While this review focused on EEG data, future research should explore multimodal approaches that combine EEG with complementary neuroimaging techniques such as functional near-infrared spectroscopy (fNIRS), functional magnetic resonance imaging (fMRI), or physiological measures. Multimodal data can provide more comprehensive insights into brain function and potentially enhance the performance of unsupervised learning algorithms by leveraging complementary information (Uyanik et al., 2025).
- Expanded Definition of Unsupervised Learning: Future studies should consider a broader spectrum of unsupervised and self-supervised learning approaches. Self-supervised learning, which creates supervisory signals from the data itself without external labels, represents a promising middle ground between fully supervised and unsupervised approaches (X. Zhang et al., 2022). Similarly, semi-supervised methods that leverage small amounts of labeled data alongside larger unlabeled datasets could help bridge the gap between these paradigms (Krol et al., 2023; Sosulski et al., 2021).
- Hybrid Approaches: Research should explore hybrid models that combine unsupervised components with supervised fine-tuning. For example, using unsupervised methods for feature learning or dimensionality reduction followed by supervised classification has shown promise in other domains and could be particularly valuable for EEG analysis (Kinahan et al., 2024).
- Model Interpretability: As unsupervised methods often discover complex patterns, research should prioritize interpretable models or post hoc explanation techniques that can translate mathematical findings into clinically meaningful insights. This is essential for clinical adoption and scientific advancement (Géron, 2022).
- Standardized Evaluation Frameworks: Developing robust validation frameworks for unsupervised EEG analysis is crucial. This includes creating benchmark datasets with diverse populations, standardized preprocessing pipelines, and metrics for evaluating the clinical relevance of discovered patterns (Khan et al., 2022).
- Algorithm Development and Validation: There is a critical need to develop and rigorously validate unsupervised algorithms specifically tailored for the unique characteristics of EEG data, such as high dimensionality, a low signal-to-noise ratio, non-stationarity, and inter-individual variability (Al-Saegh et al., 2021). Methods should be robust to artifacts and capable of handling longitudinal data. Validation should move beyond technical metrics and focus on establishing the clinical relevance and replicability of discovered patterns. For instance, while various unsupervised techniques like PCA, K-means, and GMM have been conceptualized for EEG analysis, their empirical validation specifically for mental health monitoring in large, diverse cohorts remains limited.
- Exploration of Specific EEG Features: While frequency-band analysis is common, certain features, like gamma-band activity, remain relatively underutilized in unsupervised EEG research for mental health, suggesting a critical gap for future exploration (Newson & Thiagarajan, 2019). Investigating how different unsupervised models capture information from various frequency bands and connectivity measures is essential.
- Standardized Benchmarks and Protocols: The lack of standardized benchmarks hinders the comparison and advancement of unsupervised methods. The field needs to establish common datasets, preprocessing pipelines, evaluation metrics (both technical and clinical), and reporting standards specifically for unsupervised EEG analysis in mental health (Demirezen et al., 2024; Kinahan et al., 2024; Langer et al., 2022). This would facilitate objective comparison of algorithms and improve research reproducibility.
- Large-Scale, Well-Characterized Datasets: Progress in both supervised and unsupervised learning is often limited by data availability. Collaborative initiatives are needed to create and share large-scale, diverse, and richly annotated EEG datasets for mental health research (Khan et al., 2022; X. Zhang et al., 2022). Addressing limitations in dataset labeling and ensuring diverse representation are crucial for developing generalizable models.
- Hybrid and Semi-Supervised Approaches: Given the challenges of purely unsupervised learning and the limitations of purely supervised methods (especially regarding labeled data scarcity), investigating hybrid or semi-supervised approaches is crucial (Cai et al., 2022; Sosulski et al., 2021). These methods aim to leverage the strengths of both paradigms, using limited labeled data to guide the learning process on larger unlabeled datasets, potentially improving model performance, generalizability, and interpretability.
- Cross-Domain Adaptation: EEG signals vary significantly across subjects, sessions, and hardware. Unsupervised domain adaptation techniques are needed to develop models that can generalize effectively across these variations, enabling broader clinical applicability (Sartipi & Cetin, 2024; C. Xu et al., 2025; T. Xu et al., 2022).
- Explainable AI (XAI) for Unsupervised Models: A major barrier to the clinical translation of complex machine learning models, including unsupervised ones, is their often ‘black-box’ nature. Developing and applying XAI methods tailored for unsupervised EEG analysis is essential to understanding which patterns the models are learning and why they group certain data points together or identify specific anomalies (Apicella et al., 2022; Farahani et al., 2022; Ieracitano et al., 2023; Katmah et al., 2021). This interpretability is crucial for building clinical trust, validating findings, and generating actionable insights.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNN | Convolutional Neural Network |
| EEG | Electroencephalography |
| fMRI | Functional Magnetic Resonance Imaging |
| GAN | Generative Adversarial Network |
| GMM | Gaussian Mixture Model |
| ICA | Independent Component Analysis |
| ML | Machine Learning |
| PCA | Principal Component Analysis |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RNN | Recurrent Neural Network |
| SVM | Support Vector Machine |
| UL | Unsupervised Learning |
| VAE | Variational Autoencoder |
References
- Alhagry, S., Fahmy, A. A., & El-Khoribi, R. A. (2017). Emotion recognition based on EEG using LSTM recurrent neural network. International Journal of Advanced Computer Science and Applications, 8(10), 081046. [Google Scholar] [CrossRef]
- Al-Saegh, A., Dawwd, S. A., & Abdul-Jabbar, J. M. (2021). CutCat: An augmentation method for EEG classification. Neural Networks, 141, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Apicella, A., Isgrò, F., Pollastro, A., & Prevete, R. (2022). Toward the application of XAI methods in EEG-based systems. arXiv, arXiv:2210.06554. [Google Scholar] [CrossRef]
- Bank, D., Koenigstein, N., & Giryes, R. (2021). Autoencoders. arXiv, arXiv:2003.05991. [Google Scholar] [CrossRef]
- Baydili, İ., Tasci, B., & Tasci, G. (2025). Artificial Intelligence in psychiatry: A review of biological and behavioral data analyses. Diagnostics, 15(4), 434. [Google Scholar] [CrossRef]
- Cai, H., Yuan, Z., Gao, Y., Sun, S., Li, N., Tian, F., Xiao, H., Li, J., Yang, Z., Li, X., Zhao, Q., Liu, Z., Yao, Z., Yang, M., Peng, H., Zhu, J., Zhang, X., Gao, G., Zheng, F., … Hu, B. (2022). A multi-modal open dataset for mental-disorder analysis. Scientific Data, 9, 178. [Google Scholar] [CrossRef]
- Caucheteux, C., Gramfort, A., & King, J. R. (2023). Evidence of a predictive coding hierarchy in the human brain listening to speech. Nature Human Behaviour, 7, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Craik, A., He, Y., & Contreras-Vidal, J. L. (2019). Deep learning for electroencephalogram (EEG) classification tasks: A review. Journal of Neural Engineering, 16, 031001. [Google Scholar] [CrossRef]
- Demirezen, G., Taşkaya Temizel, T., & Brouwer, A.-M. (2024). Reproducible machine learning research in mental workload classification using EEG. Frontiers in Neuroergonomics, 5, 1346794. [Google Scholar] [CrossRef]
- Drzewiecki, C. M., & Fox, A. S. (2024). Understanding the heterogeneity of anxiety using a translational neuroscience approach. Cognitive, Affective, & Behavioral Neuroscience, 24(2), 228–245. [Google Scholar] [CrossRef]
- Ezugwu, A. E., Ikotun, A. M., Oyelade, O. O., Abualigah, L., Agushaka, J. O., Eke, C. I., & Akinyelu, A. A. (2022). A comprehensive survey of clustering algorithms: State-of-the-art machine learning applications, taxonomy, challenges, and future research prospects. Engineering Applications of Artificial Intelligence, 110, 104743. [Google Scholar] [CrossRef]
- Farahani, F. V., Fiok, K., Lahijanian, B., Karwowski, W., & Douglas, P. K. (2022). Explainable AI: A review of applications to neuroimaging data. Frontiers in Neuroscience, 16, 906290. [Google Scholar] [CrossRef]
- Ficco, L., Mancuso, L., Manuello, J., Teneggi, A., Liloia, D., Duca, S., Costa, T., & Cauda, F. (2021). Disentangling predictive processing in the brain: A meta-analytic study in favour of a predictive network. Scientific Reports, 11, 16258. [Google Scholar] [CrossRef]
- Friston, K. J., Da Costa, L., Sajid, N., Heins, C., Parr, T., & Ramstead, M. J. D. (2023). The free energy principle made simpler but not too simple. Physics Reports, 1024, 1–29. [Google Scholar] [CrossRef]
- Gao, X., Lin, Y., & Liu, F. (2021). Monitoring of mental health conditions using EEG: A systematic review. Frontiers in Neuroscience, 15, 684765. [Google Scholar] [CrossRef]
- GBD 2019 Mental Disorders Collaborators. (2022). Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. The Lancet Psychiatry, 9, 137–150. [Google Scholar] [CrossRef]
- Géron, A. (2022). Hands-on machine learning with scikit-learn, keras, and TensorFlow (3rd ed.). O’Reilly Media. [Google Scholar]
- Gupta, D., Bhatia, M. P. S., & Kumar, A. (2021). Real-time mental health analytics using IoMT and social media datasets: Research and challenges. In Proceedings of the international conference on innovative computing & communication (ICICC). SSRN. [Google Scholar] [CrossRef]
- Hodson, R., Sajid, N., & Friston, K. J. (2024). The empirical status of predictive coding and active inference. Neuroscience & Biobehavioral Reviews, 156, 105487. [Google Scholar] [CrossRef]
- Huidobro, N., Meza-Andrade, R., Méndez-Balbuena, I., Trenado, C., Tello Bello, M., & Tepichin Rodríguez, E. (2025). Electroencephalographic biomarkers for neuropsychiatric diseases: The state of the art. Bioengineering, 12(3), 295. [Google Scholar] [CrossRef]
- Ieracitano, C., Mahmud, M., Doborjeh, M., & Lay-Ekuakille, A. (2023). Editorial topical collection: “Explainable and augmented machine learning for biosignals and biomedical images”. Sensors, 23(24), 9722. [Google Scholar] [CrossRef]
- Isomura, T., Shimazaki, H., & Friston, K. J. (2023). Experimental validation of the free-energy principle via in vitro neural networks. Nature Communications, 14, 4422. [Google Scholar] [CrossRef]
- Katmah, R., Al-Shargie, F., Tariq, U., Babiloni, F., Al-Mughairbi, F., & Al-Nashash, H. (2021). A review on mental stress assessment methods using EEG signals. Sensors, 21(15), 5043. [Google Scholar] [CrossRef]
- Khan, H. A., Ul Ain, R., Kamboh, A. M., Butt, H. T., Shafait, S., Alamgir, W., Stricker, D., & Shafait, F. (2022). The NMT scalp EEG dataset: An open-source annotated dataset of healthy and pathological EEG recordings for predictive modeling. Frontiers in Neuroscience, 15, 755817. [Google Scholar] [CrossRef]
- Kinahan, S., Saidi, P., Daliri, A., Liss, J., & Berisha, V. (2024). Achieving reproducibility in EEG-based machine learning. In Proceedings of the 2024 ACM conference on fairness, accountability, and transparency (pp. 1464–1474). ACM. [Google Scholar] [CrossRef]
- Klein, T., Minakowski, P., & Sager, S. (2025). Flexible patched brain transformer model for EEG decoding. Scientific Reports, 15, 10935. [Google Scholar] [CrossRef]
- Krol, L. R., Pawlitzki, J., Lotte, F., Gramann, K., & Zander, T. O. (2023). Cognitive neuroscience meets BCI: Assessing the brain-behavior relationship using event-related potentials and deep learning. Journal of Neural Engineering, 20, 026004. [Google Scholar] [CrossRef]
- Langer, N., Plomecka, M. B., Tröndle, M., Negi, A., Popov, T., Milham, M., & Haufe, S. (2022). A benchmark for prediction of psychiatric multimorbidity from resting EEG data in a large pediatric sample. NeuroImage, 258, 119348. [Google Scholar] [CrossRef]
- Liu, Z., & Zhao, J. (2025). Leveraging deep learning for robust EEG analysis in mental health monitoring. Frontiers in Neuroinformatics, 18, 1494970. [Google Scholar] [CrossRef]
- Marais, A. L., Brock, J., & Nordt, M. (2025). Predictive coding and attention in developmental cognitive neuroscience: A systematic review. Developmental Cognitive Neuroscience, 65, 101341. [Google Scholar] [CrossRef]
- Newson, J. J., & Thiagarajan, T. C. (2019). EEG frequency bands in psychiatric disorders: A review of resting-state studies. Frontiers in Human Neuroscience, 12, 521. [Google Scholar] [CrossRef]
- Rathee, D., Raza, H., Roy, S., & Prasad, G. (2021). A magnetoencephalography dataset for motor and cognitive imagery-based brain-computer interface. Scientific Data, 8, 120. [Google Scholar] [CrossRef]
- Roy, Y., Banville, H., Albuquerque, I., Gramfort, A., Falk, T. H., & Faubert, J. (2019). Deep learning-based electroencephalography analysis: A systematic review. Journal of Neural Engineering, 16, 051001. [Google Scholar] [CrossRef]
- Sartipi, S., & Cetin, M. (2024, April 14–19). Multi-source domain adaptation with transformer-based feature generation for subject-independent EEG-based emotion recognition. 2024 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP) (pp. 2086–2090), Seoul, Republic of Korea. [Google Scholar] [CrossRef]
- Schilling, A., Krauss, P., Gerum, R., Metzner, C., Tziridis, K., & Schulze, H. (2023). Predictive coding and stochastic resonance as fundamental principles of auditory phantom perception. Brain, 146, 4809–4825. [Google Scholar] [CrossRef]
- Simmatis, L., Russo, E. E., Geraci, J., Harmsen, I. E., & Samuel, N. (2023). Technical and clinical considerations for electroencephalography-based biomarkers for major depressive disorder. npj Mental Health Research, 2(1), 18. [Google Scholar] [CrossRef]
- Sosulski, J., Hortal, E., Iáñez, E., Úbeda, A., & Azorín, J. M. (2021). Online semi-supervised learning from heterogeneous decoders for motor imagery BCI systems. Neurocomputing, 453, 673–683. [Google Scholar] [CrossRef]
- Uyanik, H., Sengur, A., Salvi, M., Tan, R.-S., Tan, J. H., & Acharya, U. R. (2025). Automated detection of neurological and mental health disorders USING EEG Signals and artificial intelligence: A systematic review. WIREs Data Mining and Knowledge Discovery, 15, e70002. [Google Scholar] [CrossRef]
- Vafaei, E., & Hosseini, M. (2025). Transformers in EEG analysis: A review of architectures and applications in motor imagery, seizure, and emotion classification. Sensors, 25(5), 1293. [Google Scholar] [CrossRef]
- Wang, J., & Biljecki, F. (2022). Unsupervised machine learning in urban studies: A systematic review of applications. Cities, 129, 103925. [Google Scholar] [CrossRef]
- Wang, K., Gou, C., Duan, Y., Lin, Y., Zheng, X., & Wang, F.-Y. (2020). Generative adversarial networks: Introduction and outlook. IEEE/CAA Journal of Automatica Sinica, 7, 588–598. [Google Scholar] [CrossRef]
- Xu, C., Song, Y., Zheng, Q., Wang, Q., & Heng, P.-A. (2025). Unsupervised multi-source domain adaptation via contrastive learning for EEG classification. Expert Systems with Applications, 261, 125452. [Google Scholar] [CrossRef]
- Xu, T., Chen, W., Wang, P., Wang, F., Li, H., & Jin, R. (2022). CDTrans: Cross-domain transformer for unsupervised domain adaptation. arXiv, arXiv:2109.06165. [Google Scholar] [CrossRef]
- Yan, W., He, B., & Zhao, J. (2023). SSVEP unsupervised adaptive feature recognition method based on self-similarity of same-frequency signals. Frontiers in Neuroscience, 17, 1161511. [Google Scholar] [CrossRef]
- Yun, S. (2024). Advances, challenges, and prospects of electroencephalography-based biomarkers for psychiatric disorders: A narrative review. Journal of Yeungnam Medical Science, 41(4), 261–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X., Yao, L., Huang, C., Sheng, Q. Z., & Wang, X. (2022). Semi-supervised learning for EEG-based brain-computer interfaces: A review. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 30, 2766–2777. [Google Scholar] [CrossRef]
- Zhang, Y., Wu, W., Toll, R. T., Naparstek, S., Maron-Katz, A., Watts, M., Gordon, J., Jeong, J., Astolfi, L., Shpigel, E., & Longwell, P. (2020). Identification of psychiatric disorder subtypes from functional connectivity patterns in resting-state EEG. Nature Biomedical Engineering, 4, 1226–1238. [Google Scholar] [CrossRef]
| EEG Band | Frequency Range (Hz) | Associated Mental States/Cognitive Processes | Example Source(s) |
|---|---|---|---|
| Delta | <4 | Deep sleep, certain brain injuries, and some continuous attention tasks | (Newson & Thiagarajan, 2019) |
| Theta | 4–8 | Drowsiness, light sleep, memory processing, creativity, and meditative states | (Newson & Thiagarajan, 2019) |
| Alpha | 8–12 | Relaxed wakefulness (eyes closed), attention modulation, and inhibition control | (Newson & Thiagarajan, 2019) |
| Beta | 13–30 | Active thinking, alertness, concentration, anxiety, and motor control | (Newson & Thiagarajan, 2019) |
| Gamma | >30 | Higher cognitive functions, sensory processing, attention, and memory formation | (Newson & Thiagarajan, 2019) |
| Study ID Citation/URL | Reason for Exclusion |
|---|---|
| S1#1 Frontiers Unsupervised EEG Artifact Detection (https://doi.org/10.3389/fdgth.2020.608920) | Incorrect application focus |
| S1#2 Sp64n3r Feature engineering of EEG applied to mental disorders… (https://doi.org/10.1007/s10489-023-04702-5) | Supervised learning only |
| S1#3 Clin Pract Epidemiol Ment Health Machine Learning Techniques to Predict Mental Health Diagnoses… (https://doi.org/10.2174/0117450179315688240607052117) | Supervised learning only |
| S1#4 Frontiers Machine learning in biosignals processing for mental health… (https://doi.org/10.3389/fpsyg.2022.1066317) | Review article |
| S1#5 arXiv Stress Monitoring Using Low-Cost EEG Devices… (https://arxiv.org/abs/2403.05577) | Preprint |
| S2#1 PMC Electroencephalography-Based Depression Detection… (https://doi.org/10.3390/s23115079) | Supervised learning only |
| S2#2 PubMed Depression Detection and Diagnosis Based on EEG Analysis… (PMID: 39857094) | Supervised learning only |
| S2#3 PMC Leveraging deep learning for robust EEG analysis… (https://doi.org/10.3389/fninf.2024.1494970) | Supervised learning only |
| S2#4 PubMed EEG-based Signatures of Schizophrenia, Depression… (PMID: 39248267) | Supervised learning only |
| S2#5 PubMed EEG-derived brainwave patterns for depression diagnosis… (PMID: 39879638) | Supervised learning only |
| S2#6 PubMed Machine-Learning-Based Depression Detection Model from EEG… (PMID: 39595870) | Supervised learning only |
| S2#7 PubMed Machine Learning-Based EEG Phenotypes of Schizophrenia… (PMID: 34721112) | Supervised learning only |
| S2#8 PubMed Comparing resting state and task-based EEG using ML… (PMID: 37156879) | Supervised learning only |
| S3#1 ScienceDirect Psychiatric disorders from EEG signals through deep learning… (https://doi.org/10.1016/j.biopsycho.2021.108117) | Supervised learning only |
| S3#2 Wiley Automated Detection of Neurological and Mental Health Disorders… (https://doi.org/10.1002/widm.70002) | Review article |
| S3#3 ScienceDirect Deep learning-based feature extraction for EEG signals… (https://doi.org/10.1016/j.neucom.2023.126805) | Supervised learning only |
| S3#4 Frontiers Reproducible machine learning research in mental workload… (https://doi.org/10.3389/fnrgo.2024.1346794) | Review article |
| S3#5 PMC Enhancing EEG-Based Emotion Detection with Hybrid Models… (https://doi.org/10.3390/s24072217) | Supervised learning only |
| S3#6 Nature Predicting treatment response using EEG in major depressive disorder… (https://doi.org/10.1038/s41398-022-02064-z) | Supervised learning only |
| S3#7 Research Square Mental Health Monitoring And Intervention Using Unsupervised… (https://doi.org/10.21203/rs.3.rs-5014270/v1) | Preprint |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadulla, A.R.; Sajja, G.S.; Addula, S.R.; Maturi, M.H.; Nadella, G.S.; De La Cruz, E.; Meduri, K.; Gonaygunta, H. A Systematic Review of Mental Health Monitoring and Intervention Using Unsupervised Deep Learning on EEG Data. Psychol. Int. 2025, 7, 61. https://doi.org/10.3390/psycholint7030061
Yadulla AR, Sajja GS, Addula SR, Maturi MH, Nadella GS, De La Cruz E, Meduri K, Gonaygunta H. A Systematic Review of Mental Health Monitoring and Intervention Using Unsupervised Deep Learning on EEG Data. Psychology International. 2025; 7(3):61. https://doi.org/10.3390/psycholint7030061
Chicago/Turabian StyleYadulla, Akhila Reddy, Guna Sekhar Sajja, Santosh Reddy Addula, Mohan Harish Maturi, Geeta Sandeep Nadella, Elyson De La Cruz, Karthik Meduri, and Hari Gonaygunta. 2025. "A Systematic Review of Mental Health Monitoring and Intervention Using Unsupervised Deep Learning on EEG Data" Psychology International 7, no. 3: 61. https://doi.org/10.3390/psycholint7030061
APA StyleYadulla, A. R., Sajja, G. S., Addula, S. R., Maturi, M. H., Nadella, G. S., De La Cruz, E., Meduri, K., & Gonaygunta, H. (2025). A Systematic Review of Mental Health Monitoring and Intervention Using Unsupervised Deep Learning on EEG Data. Psychology International, 7(3), 61. https://doi.org/10.3390/psycholint7030061










