Evaluating the Effects of Novel Enrichment Strategies on Dog Behaviour Using Collar-Based Accelerometers
Abstract
1. Introduction
2. Materials and Methods
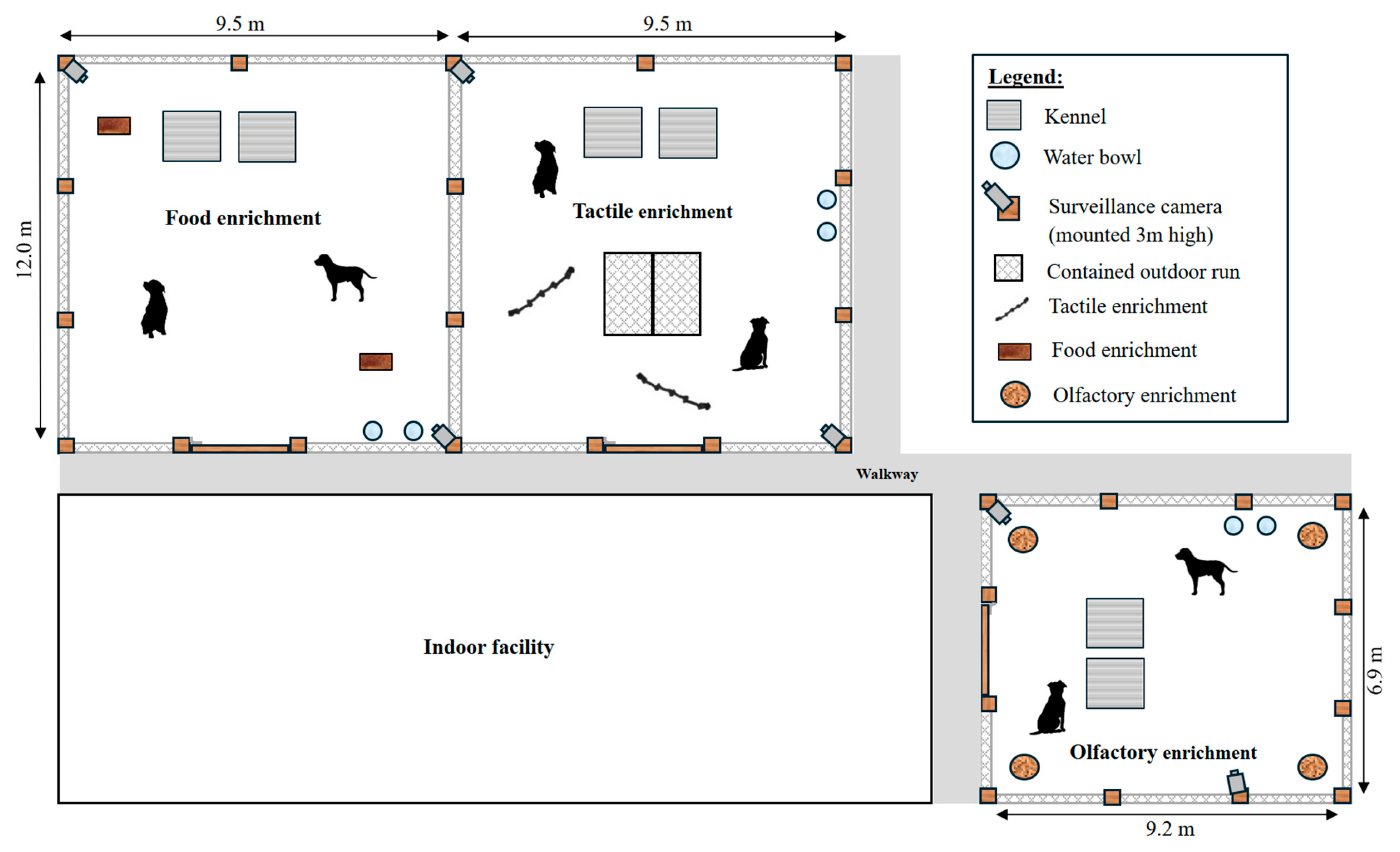
2.1. Animal Husbandry
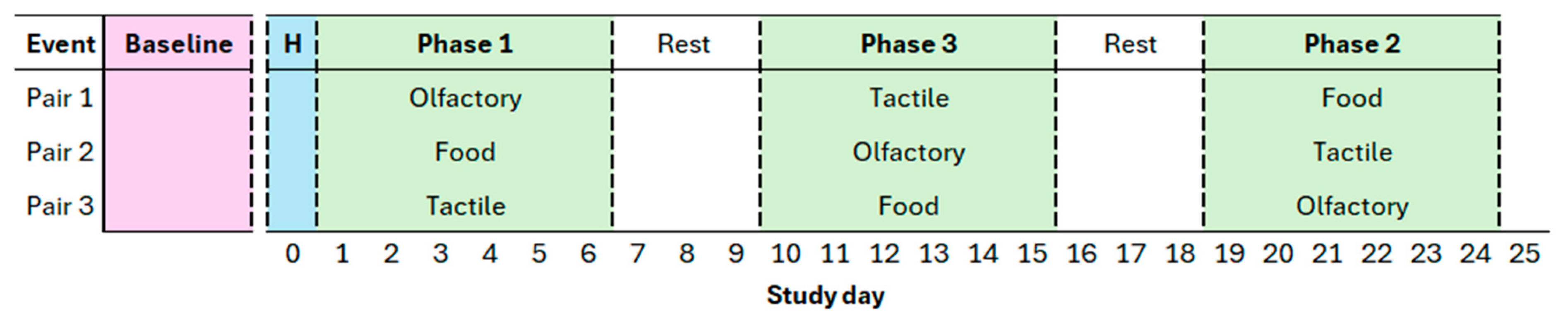
2.2. Study Design
2.3. Enrichment Treatments
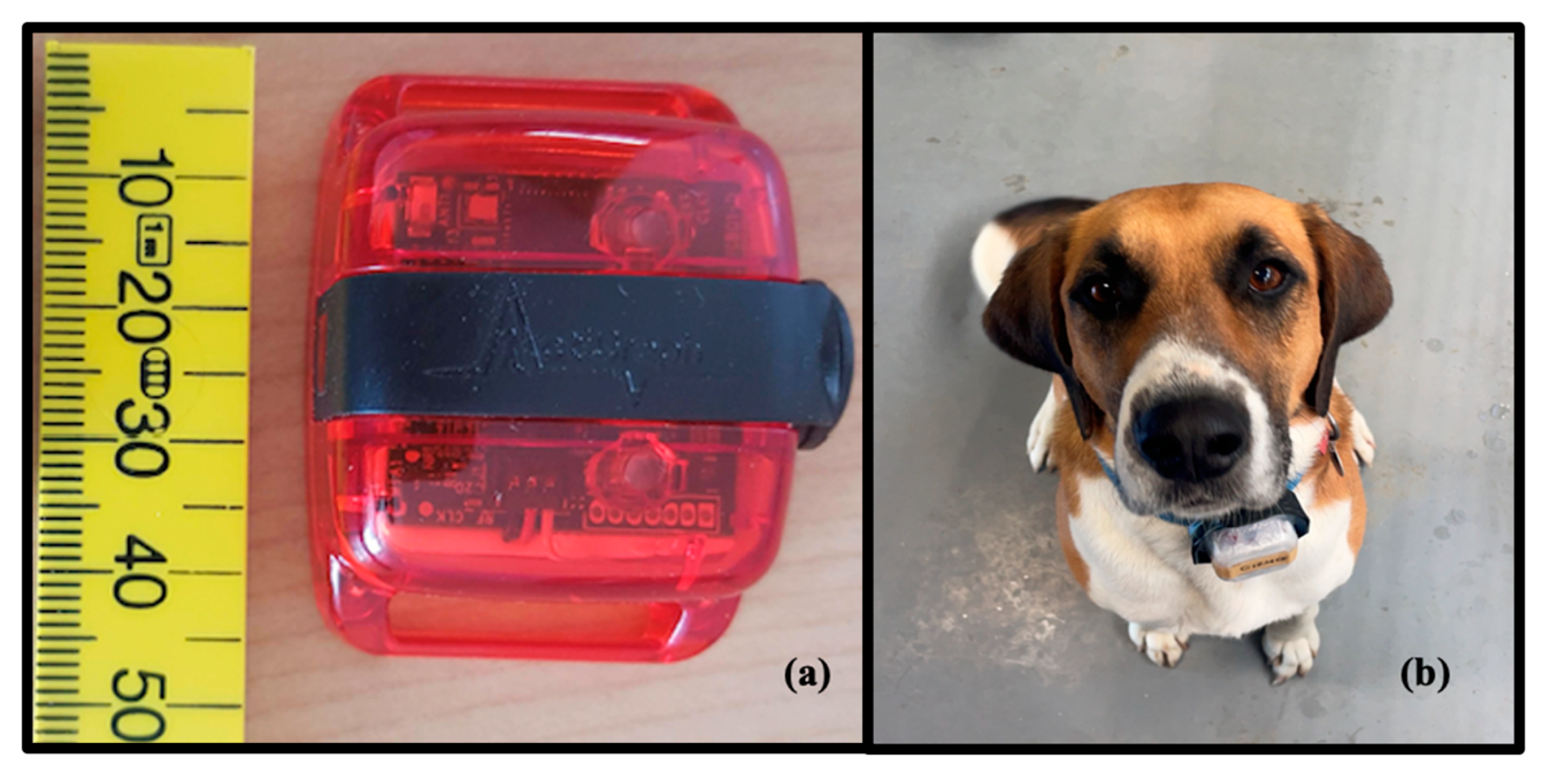
2.4. Acceleration Data
2.5. Video Footage
2.6. Data Processing and Analysis
2.6.1. Data Cleaning and Modelling
2.6.2. Statistical Analyses
3. Results
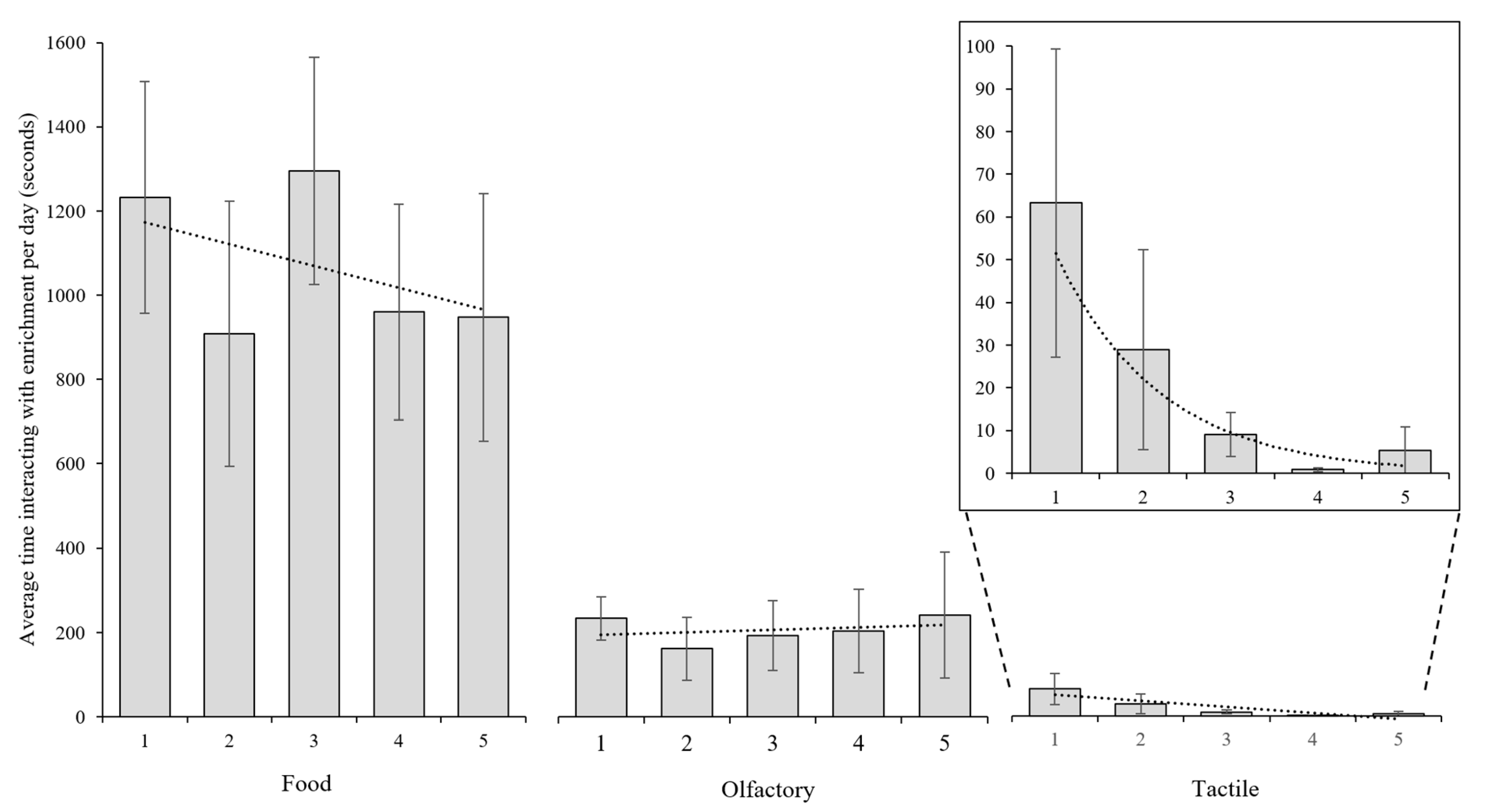
3.1. Interaction with Enrichment Objects
3.2. Overall Body Dynamic Acceleration
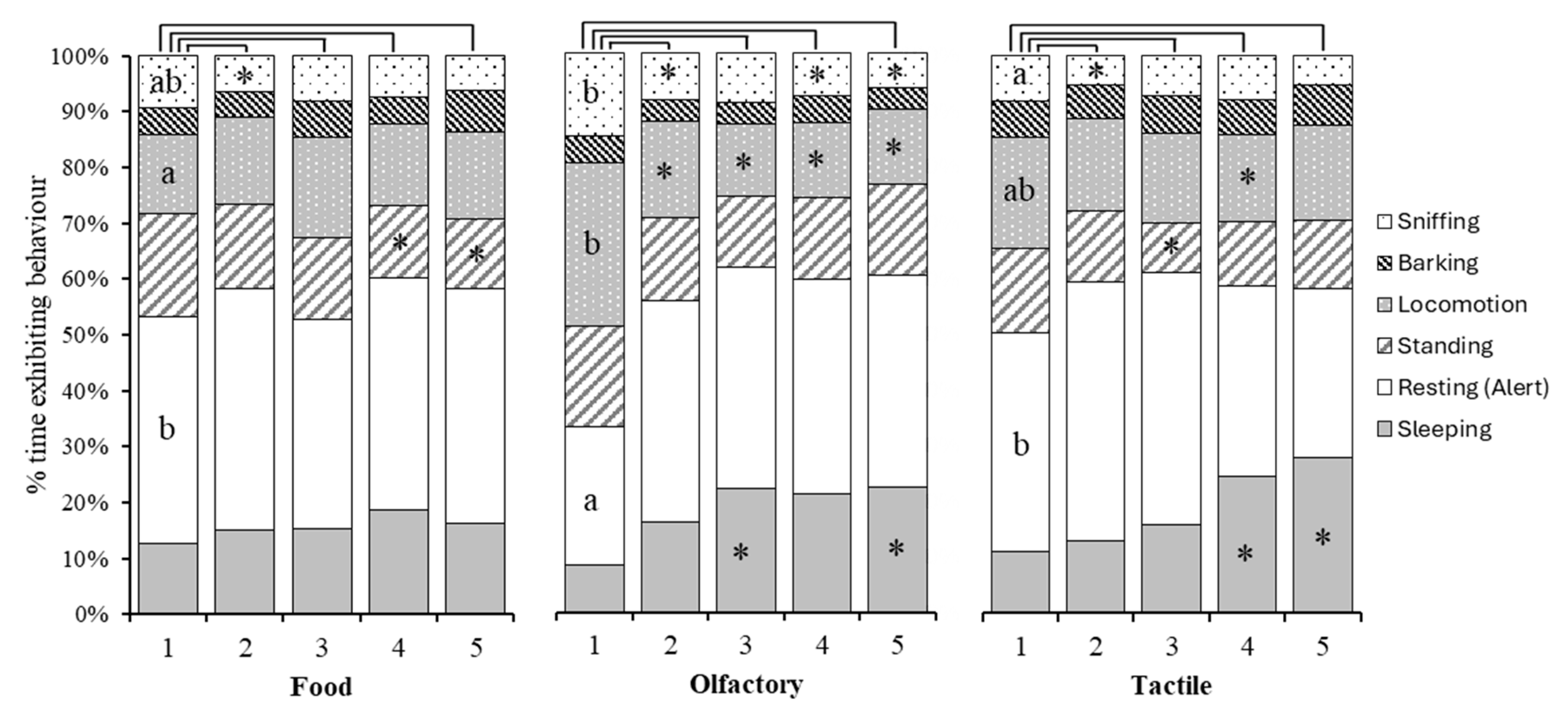
3.3. Behaviours
Time Spent Active
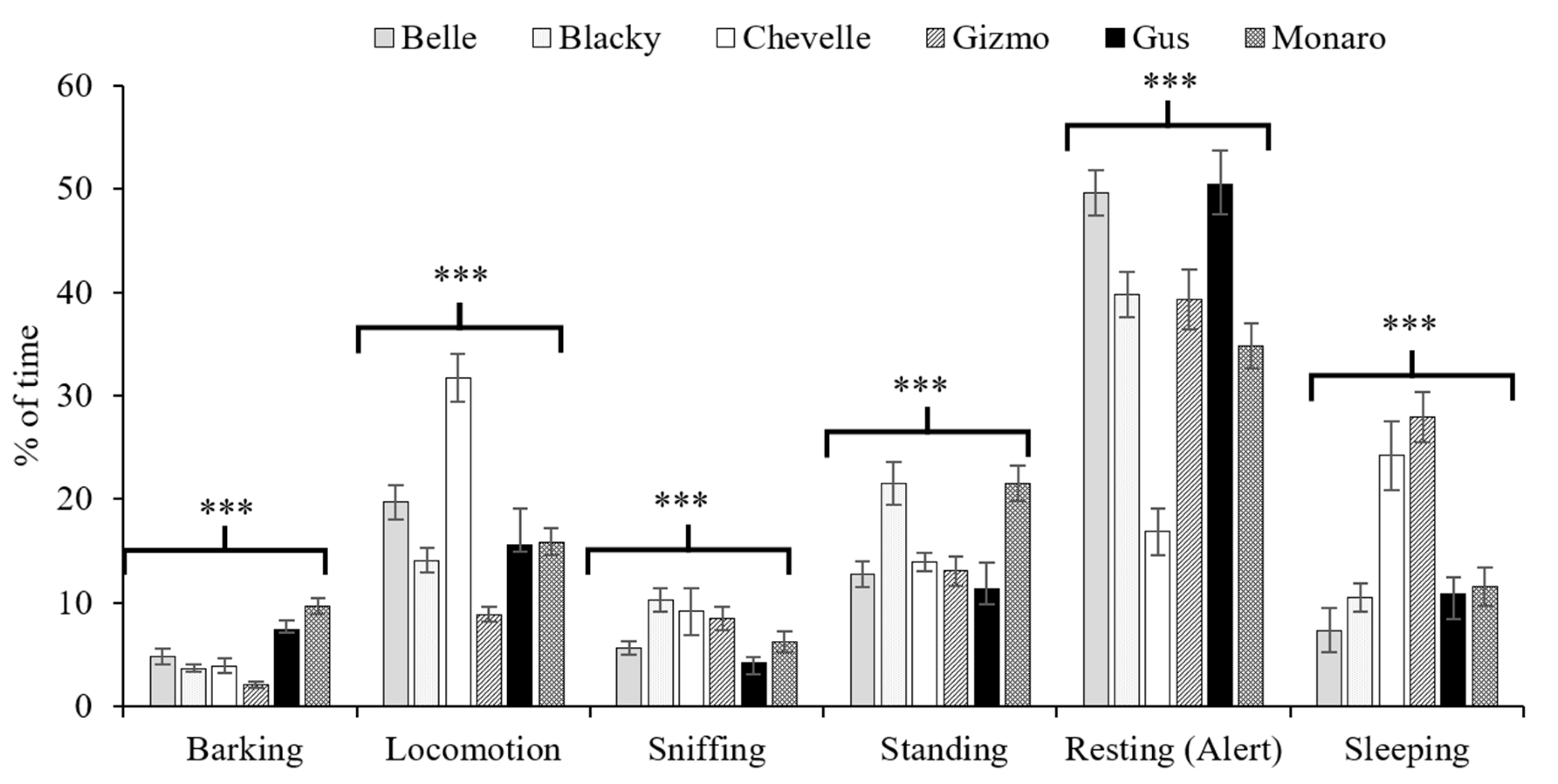
3.4. Specific Behaviours
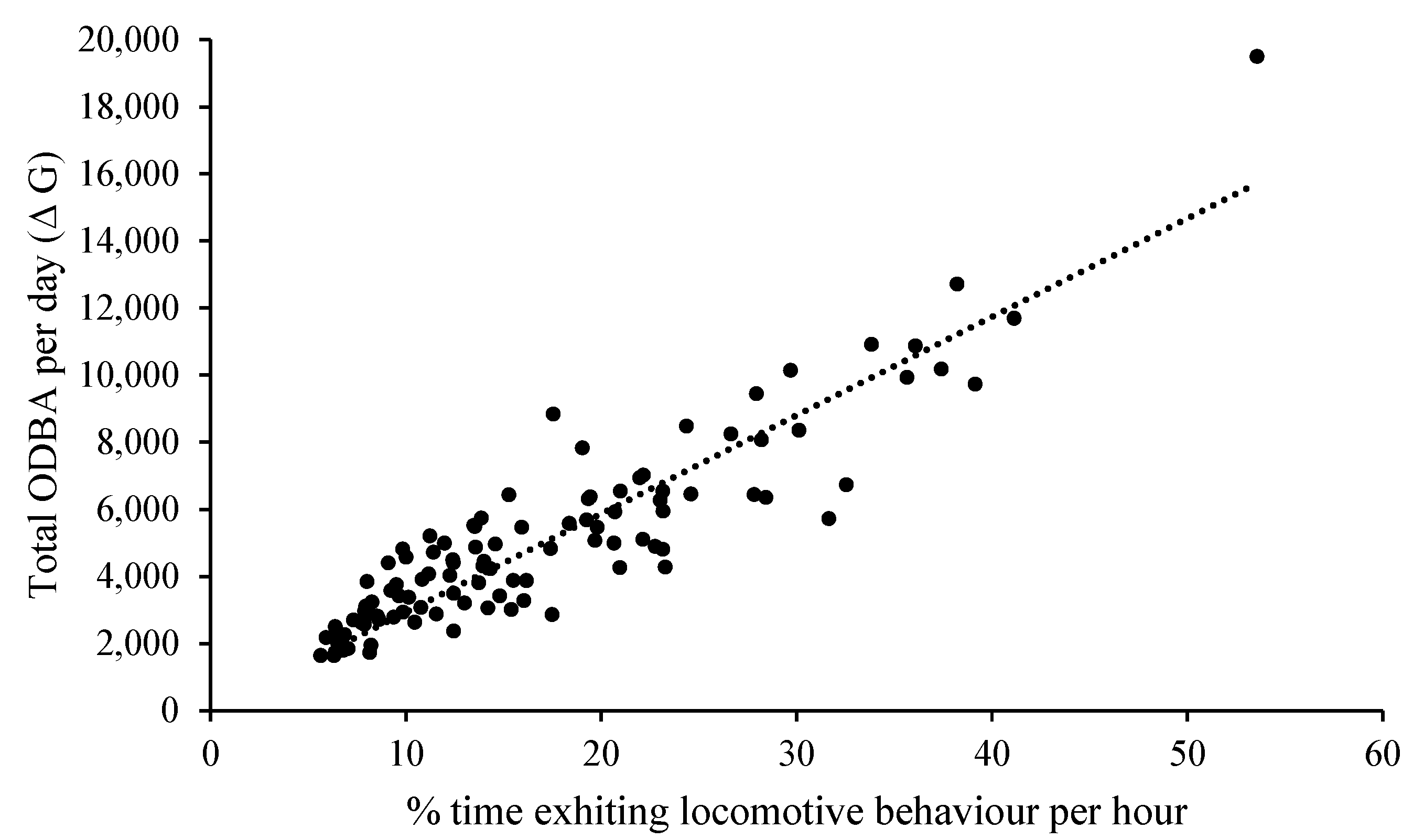
3.5. Correlation of ODBA and Behaviour
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desforges, E. Challenges and solutions surrounding environmental enrichment for dogs and cats in a scientific environment. Animals 2021, 11, 2980. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.L.; Whiteside, H.; Prankel, S. Effects of environmental enrichment on dog behaviour: Pilot study. Animals 2022, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Schipper, L.L.; Vinke, C.M.; Schilder, M.B.; Spruijt, B.M. The effect of feeding enrichment toys on the behaviour of kennelled dogs (Canis familiaris). Appl. Anim. Behav. Sci. 2008, 114, 182–195. [Google Scholar] [CrossRef]
- Herron, M.E.; Kirby-Madden, T.M.; Lord, L.K. Effects of environmental enrichment on the behavior of shelter dogs. J. Am. Vet. Med. Assoc. 2014, 244, 687–692. [Google Scholar] [CrossRef]
- Kang, O.-D. Effects of environment enrichment on behavioral problems in dogs with separation anxiety. J. Environ. Sci. Int. 2022, 31, 131–139. [Google Scholar] [CrossRef]
- Hubrecht, R.C. A comparison of social and environmental enrichment methods for laboratory housed dogs. Appl. Anim. Behav. Sci. 1993, 37, 345–361. [Google Scholar] [CrossRef]
- Wells, D.L. A review of environmental enrichment for kennelled dogs, Canis familiaris. Appl. Anim. Behav. Sci. 2004, 85, 307–317. [Google Scholar] [CrossRef]
- Newberry, R.C. Environmental enrichment: Increasing the biological relevance of captive environments. Appl. Anim. Behav. Sci. 1995, 44, 229–243. [Google Scholar] [CrossRef]
- Young, R.J. The importance of food presentation for animal welfare and conservation. Proc. Nutr. Soc. 1997, 56, 1095–1104. [Google Scholar] [CrossRef]
- Gaines, S.A.; Rooney, N.J.; Bradshaw, J.W. The effect of feeding enrichment upon reported working ability and behavior of kenneled working dogs. J. Forensic Sci. 2008, 53, 1400–1404. [Google Scholar] [CrossRef]
- Wells, D.L. Aromatherapy for travel-induced excitement in dogs. J. Am. Vet. Med. Assoc. 2006, 229, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.L. Sensory stimulation as environmental enrichment for captive animals: A review. Appl. Anim. Behav. Sci. 2009, 118, 1–11. [Google Scholar] [CrossRef]
- Tod, E.; Brander, D.; Waran, N. Efficacy of dog appeasing pheromone in reducing stress and fear related behaviour in shelter dogs. Appl. Anim. Behav. Sci. 2005, 93, 295–308. [Google Scholar] [CrossRef]
- Dare, P.; Strasser, R. Ruff Morning? The Use of Environmental Enrichment during an Acute Stressor in Kenneled Shelter Dogs. Animals 2023, 13, 1506. [Google Scholar] [CrossRef]
- Bruno, K.; DeSocio, E.; White, J.; Wilson, B.K. Effect of environmental enrichment devices on behavior of individually housed beef heifers. Transl. Anim. Sci. 2020, 4, txaa220. [Google Scholar] [CrossRef]
- Liu, Y. Use of Triaxial Accelerometers and Machine Learning Algorithms for Behavioural Identification to Assess the Effectiveness of a Joint Supplement in Old Domestic Cats (Felis catus): A Thesis Presented in Partial Fulfilment of the Requirements for the Degree of MSc (Animal Science). Master’s Thesis, Massey University, Palmerston North, New Zealand, 2024. [Google Scholar]
- Pullin, A.N.; Temple, S.M.; Bennett, D.C.; Rufener, C.B.; Blatchford, R.A.; Makagon, M.M. Pullet rearing affects collisions and perch use in enriched colony cage layer housing. Animals 2020, 10, 1269. [Google Scholar] [CrossRef]
- Sudo, M.; Nagamatsu, T.; Ando, S. Does environmental enrichment increase locomotor activity in rats? Evidence from an implanted sensor device. Bull. Phys. Fit. Res. Inst. 2018, 116, 29–32. [Google Scholar]
- Veldkamp, F.; Garcia-Faria, T.I.; Witjes, V.L.; Rebel, J.M.; de Jong, I.C. Validation of non-invasive sensor technologies to measure interaction with enrichment material in weaned fattening pigs. Appl. Anim. Behav. Sci. 2023, 263, 105923. [Google Scholar] [CrossRef]
- Whitham, J.; Miller, L. Using technology to monitor and improve zoo animal welfare. Anim. Welf. 2016, 25, 395–409. [Google Scholar] [CrossRef]
- Jones, S.; Dowling-Guyer, S.; Patronek, G.J.; Marder, A.R.; Segurson D’Arpino, S.; McCobb, E. Use of accelerometers to measure stress levels in shelter dogs. J. Appl. Anim. Welf. Sci. 2014, 17, 18–28. [Google Scholar] [CrossRef]
- Kumpulainen, P.; Cardó, A.V.; Somppi, S.; Törnqvist, H.; Väätäjä, H.; Majaranta, P.; Gizatdinova, Y.; Antink, C.H.; Surakka, V.; Kujala, M.V. Dog behaviour classification with movement sensors placed on the harness and the collar. Appl. Anim. Behav. Sci. 2021, 241, 105393. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Kim, H.-C. Activity detection for the wellbeing of dogs using wearable sensors based on deep learning. IEEE Access 2022, 10, 53153–53163. [Google Scholar] [CrossRef]
- Den Uijl, I.; Gómez Álvarez, C.B.; Bartram, D.; Dror, Y.; Holland, R.; Cook, A. External validation of a collar-mounted triaxial accelerometer for second-by-second monitoring of eight behavioural states in dogs. PLoS ONE 2017, 12, e0188481. [Google Scholar] [CrossRef] [PubMed]
- AAFCO. Official Publication/Association of American Feed Control Officials; Association of American Feed Control Officials: Champaign, IL, USA, 2021. [Google Scholar]
- Redmond, C.; Smit, M.; Draganova, I.; Corner-Thomas, R.; Thomas, D.; Andrews, C. The Use of Triaxial Accelerometers and Machine Learning Algorithms for Behavioural Identification in Domestic Dogs (Canis familiaris): A Validation Study. Sensors 2024, 24, 5955. [Google Scholar] [CrossRef]
- Hansen, B.D.; Lascelles, B.D.X.; Keene, B.W.; Adams, A.K.; Thomson, A.E. Evaluation of an accelerometer for at-home monitoring of spontaneous activity in dogs. Am. J. Vet. Res. 2007, 68, 468–475. [Google Scholar] [CrossRef]
- Smit, M.; Ikurior, S.J.; Corner-Thomas, R.A.; Andrews, C.J.; Draganova, I.; Thomas, D.G. The Use of Triaxial Accelerometers and Machine Learning Algorithms for Behavioural Identification in Domestic Cats (Felis catus): A Validation Study. Sensors 2023, 23, 7165. [Google Scholar] [CrossRef]
- Brown, D.D.; Kays, R.; Wikelski, M.; Wilson, R.; Klimley, A.P. Observing the unwatchable through acceleration logging of animal behavior. Anim. Biotelem. 2013, 1, 20. [Google Scholar] [CrossRef]
- Tatler, J.; Cassey, P.; Prowse, T.A. High accuracy at low frequency: Detailed behavioural classification from accelerometer data. J. Exp. Biol. 2018, 221, jeb184085. [Google Scholar] [CrossRef]
- Nielsen, B.L.; Jezierski, T.; Bolhuis, J.E.; Amo, L.; Rosell, F.; Oostindjer, M.; Christensen, J.W.; McKeegan, D.; Wells, D.L.; Hepper, P. Olfaction: An overlooked sensory modality in applied ethology and animal welfare. Front. Vet. Sci. 2015, 2, 69. [Google Scholar] [CrossRef]
- Polgár, Z.; Blackwell, E.J.; Rooney, N.J. Assessing the welfare of kennelled dogs—A review of animal-based measures. Appl. Anim. Behav. Sci. 2019, 213, 1–13. [Google Scholar] [CrossRef]
- Sampaio, R.A.G.; Martins, Y.N.d.F.; Barbosa, F.M.S.; Franco, C.I.Q.; Kobayashi, M.D.; Talieri, I.C. Behavioral assessment of shelter dogs submitted to different methods of environmental enrichment. Ciência Rural 2019, 49, e20180181. [Google Scholar] [CrossRef]
- Siwak, C.T.; Tapp, P.D.; Zicker, S.C.; Murphey, H.L.; Muggenburg, B.A.; Head, E.; Cotman, C.W.; Milgram, N.W. Locomotor activity rhythms in dogs vary with age and cognitive status. Behav. Neurosci. 2003, 117, 813. [Google Scholar] [CrossRef] [PubMed]
- Hiby, E.F.; Rooney, N.J.; Bradshaw, J.W. Behavioural and physiological responses of dogs entering re-homing kennels. Physiol. Behav. 2006, 89, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Rooney, N.; Gaines, S.; Hiby, E. A practitioner’s guide to working dog welfare. J. Vet. Behav. 2009, 4, 127–134. [Google Scholar] [CrossRef]
- Rooney, N.J.; Parr-Cortes, Z. Olfaction and Dog Welfare. In Olfactory Research in Dogs; Springer: Princton, NJ, USA, 2023; pp. 205–234. [Google Scholar]
- Domjan, M. The Essentials of Conditioning and Learning; American Psychological Association: Worcester, MA, USA, 2018. [Google Scholar]
- Kuczaj, S.; Lacinak, T.; Fad, O.; Trone, M.; Solangi, M.; Ramos, J. Keeping environmental enrichment enriching. Int. J. Comp. Psychol. 2002, 15, 127–137. [Google Scholar] [CrossRef]
- Tarou, L.R.; Bashaw, M.J. Maximizing the effectiveness of environmental enrichment: Suggestions from the experimental analysis of behavior. Appl. Anim. Behav. Sci. 2007, 102, 189–204. [Google Scholar] [CrossRef]
- Andrews, C.J.; Potter, M.A.; Thomas, D.G. Quantification of activity in domestic cats (Felis catus) by accelerometry. Appl. Anim. Behav. Sci. 2015, 173, 17–21. [Google Scholar] [CrossRef]
- Mason, G.; Clubb, R.; Latham, N.; Vickery, S. Why and how should we use environmental enrichment to tackle stereotypic behaviour? Appl. Anim. Behav. Sci. 2007, 102, 163–188. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, M.B.; van Hooff, J.A.; de Vries, H.W. Manifestations of chronic and acute stress in dogs. Appl. Anim. Behav. Sci. 1997, 52, 307–319. [Google Scholar] [CrossRef]
- Beerda, B.; Schilder, M.B.; Van Hooff, J.A.; De Vries, H.W.; Mol, J.A. Chronic stress in dogs subjected to social and spatial restriction. I. Behavioral responses. Physiol. Behav. 1999, 66, 233–242. [Google Scholar] [CrossRef]
- Roth, L.S.; Faresjö, Å.; Theodorsson, E.; Jensen, P. Hair cortisol varies with season and lifestyle and relates to human interactions in German shepherd dogs. Sci. Rep. 2016, 6, 19631. [Google Scholar] [CrossRef]
- Wirobski, G.; Range, F.; Schaebs, F.; Palme, R.; Deschner, T.; Marshall-Pescini, S. Endocrine changes related to dog domestication: Comparing urinary cortisol and oxytocin in hand-raised, pack-living dogs and wolves. Horm. Behav. 2021, 128, 104901. [Google Scholar] [CrossRef]

| Name | Sex | Age (Years) | Breed * | Weight (kg) | Pair |
|---|---|---|---|---|---|
| Belle | Female | 7.9 | Huntaway | 23.4 | 3 |
| Blacky | Male | 4.3 | Huntaway/Heading | 23.6 | 3 |
| Chevelle | Female | 7.9 | Huntaway | 21.5 | 2 |
| Gizmo | Male | 6.1 | Harrier hound | 30.8 | 1 |
| Gus | Male | 4.4 | Huntaway/Smithfield terrier | 22.4 | 2 |
| Monaro | Male | 7.9 | Huntaway | 32.1 | 1 |
| Dog | Baseline | Food | Olfactory | Tactile | p Value |
|---|---|---|---|---|---|
| Belle | 8713 ± 1103 b | 3935 ± 721 a | 3996 ± 365 a | 5900 ± 1077 ab | 0.005 |
| Blacky | 6068 ± 303 c | 3246 ± 226 a | 4483 ± 196 b | 5342 ± 426 bc | <0.001 |
| Chevelle | 13,209 ± 3150 b | 8471 ± 1392 ab | 4467 ± 1797 a | 8164 ± 1267 ab | 0.045 |
| Gizmo | 3291 ± 266 b | 2552 ± 187 ab | 2222 ± 194 a | 2062 ± 213 a | 0.010 |
| Gus | 5898 ± 1510 b | 5474 ± 676 b | 2041 ± 511 a | 5993 ± 563 b | 0.004 |
| Monaro | 7489 ± 502 b | 4504 ± 640 a | 4594 ± 497 a | 3964 ± 280 a | 0.003 |
| Average | 7445 ± 1371 c | 4697 ± 860 ab | 3634 ± 483 a | 5210 ± 842 b | <0.001 |
| Baseline | Food | Olfactory | Tactile | |
|---|---|---|---|---|
| Active (%) | ||||
| Barking | 4.7 ± 0.8 b | 5.6 ± 0.8 bc | 4.2 ± 0.4 a | 6.5 ± 0.8 c |
| Locomotion | 22.8 ± 2.7 b | 15.6 ± 1.6 a | 17.3 ± 2.3 a | 17.1 ± 1.9 a |
| Sniffing | 4.8 ± 0.6 a | 7.6 ± 0.8 bc | 9.1 ± 1.4 c | 6.8 ± 0.9 b |
| Standing | 23.5 ± 2.4 c | 14.7 ± 1.3 b | 15.3 ± 1.3 b | 12.1 ± 1.2 a |
| Total | 55.8 ± 3.1 a | 43.8 ± 2.5 b | 45.8 ± 3.2 b | 42.5 ± 4.5 b |
| Inactive (%) | ||||
| Resting | 35.6 ± 2.8 | 41.0 ± 3.0 | 36.1 ± 3.0 | 35.6 ± 2.8 |
| Sleeping | 8.0 ± 1.9 a | 15.5 ± 1.8 b | 18.0 ± 2.6 b | 17.8 ± 2.4 b |
| Total | 43.7 ± 3.1 a | 56.5 ± 2.5 b | 54.0 ± 3.2 b | 57.4 ± 5.5 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Redmond, C.; Draganova, I.; Corner-Thomas, R.; Thomas, D.; Andrews, C. Evaluating the Effects of Novel Enrichment Strategies on Dog Behaviour Using Collar-Based Accelerometers. Pets 2025, 2, 23. https://doi.org/10.3390/pets2020023
Redmond C, Draganova I, Corner-Thomas R, Thomas D, Andrews C. Evaluating the Effects of Novel Enrichment Strategies on Dog Behaviour Using Collar-Based Accelerometers. Pets. 2025; 2(2):23. https://doi.org/10.3390/pets2020023
Chicago/Turabian StyleRedmond, Cushla, Ina Draganova, Rene Corner-Thomas, David Thomas, and Chris Andrews. 2025. "Evaluating the Effects of Novel Enrichment Strategies on Dog Behaviour Using Collar-Based Accelerometers" Pets 2, no. 2: 23. https://doi.org/10.3390/pets2020023
APA StyleRedmond, C., Draganova, I., Corner-Thomas, R., Thomas, D., & Andrews, C. (2025). Evaluating the Effects of Novel Enrichment Strategies on Dog Behaviour Using Collar-Based Accelerometers. Pets, 2(2), 23. https://doi.org/10.3390/pets2020023






