Abstract
Associations between age-related diseases and behavioral alterations have been highlighted in previous studies. This study investigates the prevalence of diseases and behavioral changes in non-diseased and diseased senior cats before and after diagnosis, concentrating on four prevalent diseases: 1. osteoarthritis, 2. chronic kidney disease, 3. hyperthyroidism, and 4. cognitive dysfunction syndrome. An online survey was performed by 594 German cat-owners with a cat older than nine years; prevalent diseases, related medications, and scaled behavioral questions before and after diagnosis were queried. Chi-Quadrat-Test and Spearman’s rank correlation were used to detect correlations between behavioral changes and diseases. Multiple linear regression was used to determine dependencies between behavioral changes and each disease pre- and post-diagnosis. Half of the cats had at least one disease diagnosed (54.6%). The most prevalent diseases were osteoarthritis (18.9%), chronic kidney disease (12.3%), and hyperthyroidism (8.9%). Cognitive dysfunction syndrome was diagnosed in 2.9% of the cats. With increasing age, the likelihood of developing at least one disease rose (rs = 0.204, p < 0.001). Disease-associated behavioral changes were found in the four mentioned diseases, with some behavioral changes occurring before diagnosis. These findings underscore the relevance of early detection of underlying diseases to decelerate ongoing behavioral changes in treatable diseases.
1. Introduction
Cats have been Germany’s favorite pet for many years, with every fourth household owning a cat in 2023 [1]. Advances in breeding, nutrition, health care and management have resulted in a prolonged life expectancy [2]. A study in the UK illustrated a median longevity of 14 years for crossbred cats, in comparison to a median longevity of 12.5 years in purebred cats [3]. Due to the increasing number of senior cats, the knowledge about specific age-related physical and cognitive alterations is of particular importance to diagnose covert diseases. Alterations in behavior are frequently seen due to underlying health issues such as systemic diseases, intracranial pathology or neuronal degeneration [4,5,6]. In a study with 206 cats in the UK, 47% of middle-aged cats are reported to show behavioral changes [7]. The influence of age was illustrated by Gunn-Moore et al. [8], who suggested that 28% of cats between 11 and 14 years showed behavioral changes, unlike 50% of cats aged 15 years and older. Most common changes were altered social interactions, elimination problems, aimless activity and increased vocalization [9]. Sordo et al. [6] identified increased affection towards their owners and increased vocalization, as well as house-soiling as one of the most named behavioral problems stated in two owner questionnaires in 1995 and between 2010 and 2015.
Being susceptible to diseases, old cats have health problems that often affect mobility (e.g., osteoarthritis), and endocrine disorders (e.g., hyperthyroidism), organ failure (e.g., chronic kidney disease), hypertension, neoplasia, sensory deficits and cognitive dysfunction syndrome are often seen in elderly cats [4,5,6]. Osteoarthritis (OA) is a chronic progressive joint disease, which results in the destruction of joint cartilage and reorganization of bone tissue [10]. Radiographic findings suggest the prevalence of OA in cats older than six years to be 61%, and in cats older than 12 years up to 90% [11,12,13]. Being one of the main reasons for chronic pain in old cats, behavioral changes like decreased activities (e.g., jumping, grooming, using the litter box) are common in cats suffering from OA [13]. As diagnosing OA exclusively on clinical findings can be challenging, a precise anamnesis considering difficulties in performing daily activities or simply decreased activities is necessary [6,12].
Chronic kidney disease (CKD) is caused by structural or functional damage of one or both kidneys that lasts longer than three months. An underlying cause can frequently not be detected. Often only tubulointerstitial fibrosis is present at the time of diagnosis [14]. Limited data are available about the prevalence of CKD in geriatric cats, and range from 3% to 80% in senior cats [14,15,16]. Most cats are geriatric (>12 years old) at the time of diagnosis [15]. The main clinical signs observed by owners are lethargy, anorexia, polydipsia and weight loss [14]. Sordo et al. [6] observed behavioral changes such as increased daytime and nighttime vocalizations, prolonged sleep time and alterations in social interactions with other animals or people.
The most common endocrine disorder in old cats is hyperthyroidism which is caused in 98% by benign adenomatous hyperplasia of one or both thyroid glands [17,18]. This condition causes an excessive circulation of the thyroid hormones (thyroxine T4 and triiodothyronine T3) [19] leading to an increased metabolic rate and energy metabolism. Hyperthyroidism is diagnosed at a median age of 12–13 years [20]. The prevalence of hyperthyroidism in a suburban cat population in Germany was 11.4% in cats eight years of age and older [21], although there was wide geographic variation [22]. Weight loss despite normal or increased appetite, restlessness, polyuria, polydipsia and gastrointestinal signs are clinical findings observed in hyperthyroid cats [20,23]. Behavioral changes have also been found by Sordo et al. [6], with increased vocalization being one of the most evident behavioral symptoms. In addition, decreased tolerance to stress, as well as aggressiveness resulting from hyperactivity, are common findings [24]. Comorbidities complicate the diagnosis as there are overlapping clinical signs for CKD and hyperthyroidism. Milner et al. [25] found that 14% of hyperthyroid cats had a pre-existing CKD.
Cognitive dysfunction syndrome (CDS) is a recognized disorder in cats [8,26]. Amongst others, neuropathological findings like the extracellular accumulation of β-amyloid and tau-hyperphosphorylation are found in many cats with symptoms suitable for CDS [27]. However, CDS is still a diagnosis of exclusion after ruling out all potential medical causes. In 28% of cats between 11 and 14 years, and 50% of those over 15 years, at least one symptom consistent with CDS is present [8]. The acronym VISH-DAAL describes common behavioral alterations seen in cats with CDS: excessive vocalization, altered interaction with owners, altered sleep–wake cycle, house-soiling, disorientation, alterations in activity, anxiety and/or learning/memory deficit [9]. In a survey, the signs of CDS were described by 75% of pet owners when specifically asked, and only 12% described these symptoms on their own [28].
This study examines the prevalence of behavioral changes in German old cats by comparing cats older than nine years without any diagnosed disease(s) and those with one or more diagnosed disease(s) before and after diagnosis. An owner-based survey was used to determine diagnosed disease(s), and behavioral changes pre- and post-diagnosis for the four specific diseases, namely: OA, CKD, Hyperthyroidism and CDS. Potential risk factors for each disease were analyzed. The association between specific diseases and the occurrence of a behavioral problem, especially before and after diagnosis, were examined. It is hypothesized that age-related behavioral changes occur not only after diagnosis, but also before. Lastly, symptoms consistent with CDS were analyzed in the group of non-diseased cats to indicate a potential oversight of this syndrome.
When referring to behavioral problems, any behavior is meant that is not tolerated by the owner, whether it is abnormal or not [29,30].
2. Materials and Methods
Data were collected through an online survey addressed to owners of cats older than 9 years. Owners who lived in a multi-cat-household were supposed to answer questions for the oldest cat living in the household. The questionnaire contained questions regarding demographic details about the cat, medical history, prevalence of diagnosed diseases and medication, prevalence of veterinary visits and scaled behavioral questions. Finally, demographic data of owners were queried. The behavioral question set was divided between those whose cat had no disease (1) and those whose cat had at least one disease (2). While owners of cats without disease were only asked about current behavior, the behavioral questionnaire for owners with a “sick” cat was divided into a retrospective part asking about behavior before (2.1) and after diagnosis (2.2) of (the) disease(s). Behavioral questions emphasized food and water intake, house-soiling, activity patterns, social interaction (cat-human, cat-animal), cognitive fitness, sleep–wake cycle and vocalization. In addition, possible conditioning by owners was asked about. While answering the questionnaire, giving no answer or skipping an answer was possible. A pilot study with five people was accomplished to verify coherence. The average processing time was 15 min. The survey was available online via LimeSurvey® (LimeSurvey GmbH, 112 Hamburg, Germany) and promoted via veterinary clinics, Facebook, Instagram, university email distributor and (online) cat magazines (Geliebte Katze, Good4Pets, Haustiger) from March until July 2023. (See Supplementary Material).
2.1. Survey Response
In total, 594 cat owners answered the survey, which included incomplete questionnaires. Participants with “healthy” cats were asked 62 questions, while those with diseased cats were asked 102 questions. Answering every question was not obligatory, therefore, data were analyzed for each individual question. In total, 79.1% of participants answered every question (n = 471/594), while 20.9% did not complete the whole survey (n = 123/594). (See Supplementary Material).
2.2. Data Analysis
The survey was analyzed using IBM SPSS® Version 29 (SPSS Inc., Chicago, IL, USA). All questions were descriptively analyzed. Depending on the variables, either χ2 test or Spearman’s rank correlation was used to detect dependencies between behavioral changes, diseases, medication and potential correlating factors. Fisher’s exact test was used when the number of cell frequencies was less than five. Multiple linear regression was used to determine dependencies between behavioral changes and each disease. A reduction of behavioral variables in cats without a disease and with a disease (before and after diagnosis) was performed by operating principal component analysis (PCA) with varimax rotation. p < 0.05 was considered significant in all tests.
3. Results
3.1. Demographics
Most respondents were women (92.3%, n = 431/467), while 7.3% (n = 34/467) were male and 0.4% (n = 2/467) diverse. On average, respondents were 51–60 years old, 21.7% (n = 100/460) owned a cat for the first time, 78.3% (n = 360/460) had a cat before. The median age of the cats was 13–15 years, sex was distributed comparably between male 44.3% (n = 262/592) and female 55.7% (n = 330/592). Nearly all cats were neutered (99.0%, n = 568/575). Domestic shorthair was the most prominent race with 67.5% (n = 397/588). 39.1% (n = 230/588) lived in a single-cat household, 34.5% (n = 203/588) lived in a household with two cats, whereas 26.4% (n = 155/588) of the households kept more than two cats. Additionally, 30.8% (n = 179/582) also kept one or more other pets. Most cats lived in the household for more than 10 years (77.4%, n = 460/591). (See Supplementary Material).
3.2. Prevalence of Diseases
Half of the participants reported taking their cats once to twice annually (52.8%, n = 301/570) for veterinary visits, while 20.9% (n = 119/570) had more than three yearly visits, and 26.3% (n = 150/570) had less than one or no visits per year. The last veterinarian visit was less than three months ago for 42.6% of the cats (n = 240/564).
Owners were asked whether their cats suffered from “arthritis” or “joint pain”, and both answers were used as synonyms for osteoarthritis [OA]. The answering options for cognitive dysfunction syndrome [CDS] were “dementia” or “cognitive dysfunction”.
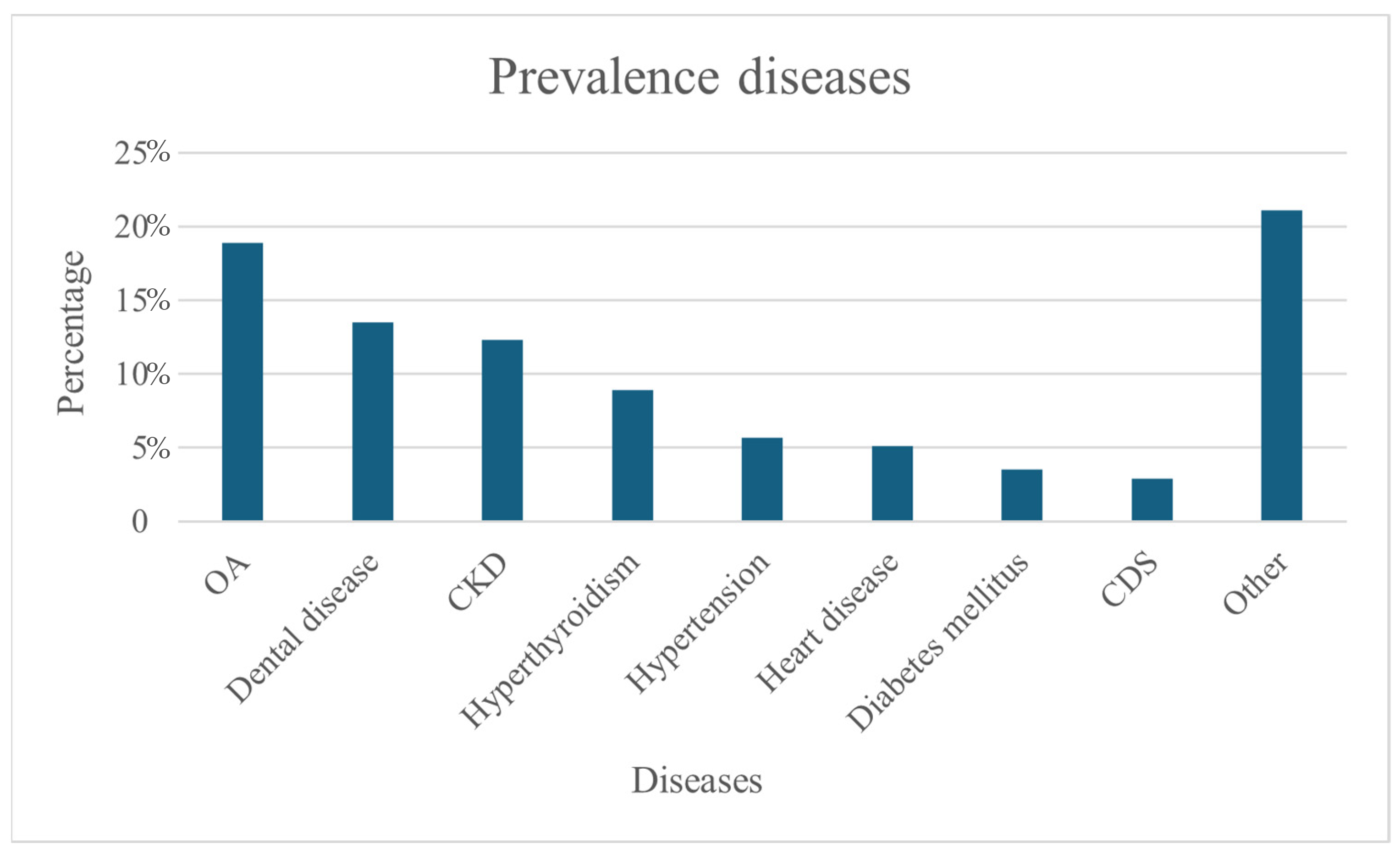
More than half of the cats had at least one disease diagnosed (54.6%, n = 308/564). The most prevalent diseases were OA with 18.9% (n = 112/564), dental disease with 13.5% (n = 80/564), CKD with 12.3% (n = 75/564) and hyperthyroidism with 8.9% (n = 53/564). CDS was diagnosed in only 2.9% (n = 17/564) (Figure 1). On average, respondents reported that their cat had 1.79 diseases.
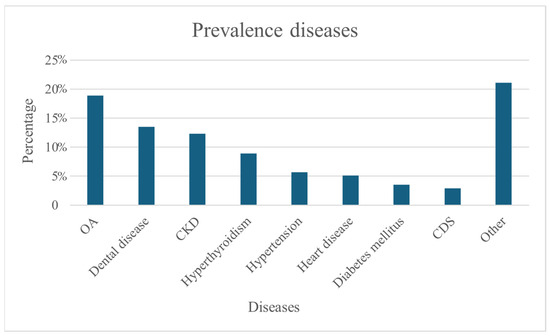
Figure 1.
Percentage prevalence diseases (N = 564).
3.3. Risk Factors for Developing a Disease
The likelihood of developing at least one disease increased with age (rs = 0.204, p < 0.001). Osteoarthritis (rs = −0.154, p = 0.007), joint pain (rs = −0.16, p = 0.005), dementia (rs = −0.186, p = 0.001), kidney disease (rs = −0.192, p < 0.001), hypertension (rs = −0.167, p = 0.003) and hyperthyroidism (rs = −0.164, p = 0.004) were associated with an increased age. Weight had an influence on the occurrence of diabetes mellitus (rs = 0.201, p < 0.001), but no association was found between weight and the other disease mentioned above. In addition, sex, breed or keeping type had no significant influence on the occurrence of disease. (See Supplementary Material).
3.4. Behavior
An alteration in behavior was seen to occur with increasing age for almost all behavioral categories. The felines were divided into three distinct groups, (1) cats without any known disease, (2.1) cats with diseases pre-diagnosis, and (2.2) cats with diseases post-diagnosis. To reduce behavioral categories, a principal component analysis was performed for each group. In Group 1, a single component was identified, which was classified as ‘vocalization’ (p < 0.001). Group 2.1 evolved five components, which were summarized into the following: 1. vocalization, 2. mobility, 3. cognitive decline, 4. sleep patterns, and 5. owner-directed social interaction (p < 0.001). Finally, in Group 2.2 nine components were detected: 1. vocalization, 2. social interaction with other animals, 3. polyuria/polydipsia, 4. owner-directed social interaction, 5. sleep patterns, 6. exploratory behavior, 7. mobility, 8. anxiety, and 9. food intake (p < 0.001) (Table 1).

Table 1.
Results of PCA showing behavioral components in cats when non diseased (1), diseased before diagnosis (2.1) and diseased after diagnosis (2.2).
3.5. Behavior Changes During Aging
3.5.1. Food Intake
As cats without a diagnosed disease age, their food intake decreased (rs = −0.146, p = 0.021). After a diagnosis of disease, food intake also decreased (rs = −0.174, p = 0.006).
3.5.2. Polyuria/Polydipsia
Participants reported that cats with one or more diseases consumed more water during the process of aging. Pre- and post-diagnosis, cats exhibited increased water consumption relative to their previous level (Group 2.1: rs = 0.131, p = 0.030; Group 2.2 rs = 0.181, p = 0.004). Almost half of the cats with diseases drank more before and after diagnosis (Group 2.1: 42.2%, n = 117/277; Group 2.2: 39.7%, n = 98/247), whereas only 20.6% of cats without disease displayed an increased thirst (n = 50/242). Polyuria was reported in 11.5% of cats without disease (n = 23/199). In those with a disease before diagnosis, every fourth cat urinated more (25.7%, n = 64/249). After diagnosis, nearly every third cat showed polyuria (31.3%, n = 70/223). Furthermore, an increase in polyuria was observed by the participants within cats of rising age, independently of a diagnosed disease (Group 1: rs = 0.149, p = 0.037; Group 2.1: rs = 0.235, p < 0.001; Group 2.2: rs = 0.248, p < 0.001).
3.5.3. House-Soiling
The occurrence of urination outside the litter box was reported from every tenth cat without disease and to a similar extent in cats with one or more diseases before and after diagnosis (Group 1: 10.5%, n = 23/219; Group 2.1: 11.5%, n = 30/261; Group 2.2: 10.2%, n = 23/226). A significant increase was mentioned in cats with disease before and after diagnosis (Group 2.1: rs = 0.210, p < 0.001; Group 2.2: rs = 0.186, p = 0.005). With regard to defecation patterns, 7.2% of cats without disease defecated outside the litter box (n = 16/220), while 6.4% did so before diagnosis (n = 17/265) and 10.2% after (n = 23/226). When diseased, a significant increase in defecation outside the litter box was observed by the participants (Group 2.1: rs = 0.208, p < 0.001; Group 2.2: rs = 0.143, p = 0.031).
3.5.4. Activity Patterns
Playful behavior decreased in all three groups during aging (Group 1: rs = −0.176, p = 0.006; Group 2.1: rs = −0.296, p < 0.001; Group 2.2.: rs = −0.311, p < 0.001). A comparative analysis of cats pre- and post-diagnosis revealed a decline in playful behavior, with 54.9% (n = 147/268) of cats demonstrating reduced playfulness before diagnosis, and 56.3% (n = 125/222) after diagnosis. Moreover, a decline in grooming behavior while aging was reported within all the studied groups (Group 1: rs = −0.133, p = 0.039; Group 2.1: rs = −0.260, p < 0.001; Group 2.2: rs = −0.197, p = 0.003). Before diagnosis, 9.7% (n = 25/258) of cats were noted to groom less, and this increased to 15.8% after diagnosis (n = 35/222).
3.5.5. Cat–Human Interaction
A reduced attachment tendency was perceived in cats without disease (15.7%, n = 38/242) compared to cats with disease before diagnosis (18.6%, n = 51/274) and after diagnosis (19.6%, n = 43/231). As cats aged, the desire to be petted was significantly more common in cats without disease (rs = 0.131, p = 0.043). Less desire to be petted was noted in 16.9% (n = 41/242) of cats without disease, compared to 21.2% (n = 58/274) of the cats with disease before diagnosis and 24.3% (n = 56/231) after diagnosis. In contrast, interaction time raised in cats without disease (47.3%, n = 113/239) to cats with disease before and after diagnosis (Group 2.1: 52.6%, n = 143/272; Group 2.2: 62.1%, n = 141/227).
3.5.6. Cat-Animal Interaction
Among the cats living in a multi-pet household, less compatibility was reported in the diseased cats before and after diagnosis (Group 2.1: 23.0%, n = 42/183; Group 2.2: 26.0%, n = 40/154). There was also a significant decrease in compatibility with other animals within the course of aging in the group of cats with at least one disease after diagnosis (rs = −0.163, p = 0.043).
3.5.7. Cognitive Fitness
Disorientation increased significantly within aging in all groups (Group 1: rs = 0.352, p < 0.001; Group 2.1: rs = 0.203, p < 0.001; Group 2.2: rs = 0.322, p < 0.001). Only 3.5% (n = 8/232) of the healthy cats displayed disorientation, whereas 6.3% (n = 17/268) of the diseased cats showed disorientation before diagnosis and 13.4% (n = 31/230) after diagnosis. A similar increase was seen in restlessness in cats without disease, with 5.6% (n = 13/233) exhibiting restless behavior, compared to 8.5% (n = 23/268) before and 13.5% (n = 32/230) after diagnosis. During aging, a significant increase in restlessness was reported by the participants in non-diseased cats (rs = 0.134, p = 0.043) and in diseased cats after diagnosis (rs = 0.197, p = 0.003). A slight decrease in joyful recognition is described when comparing cats without disease (91.7%, n = 210/229) and cats with disease before (89.9%, n = 241/268) and after diagnosis (87.6%, n = 199/227). More cats with disease performed irritable behavior after diagnosis (13.3%, n = 30/226), than before diagnosis (10.4%, n = 28/267). 11.3% (n = 26/230) of the cats without disease displayed irritability.
3.5.8. Sleep–Wake Cycle
Cats in all groups generally slept more with age (Group 1: rs = 0.301, p < 0.001; Group 2.1: rs = 0.261, p < 0.001; Group 2.2: rs = 0.295, p < 0.001). More than half of the cats without disease slept more in general (58.0%, n = 131/230), with disease this increased to 65.2% (n = 174/266) before diagnosis and 61.3% (n = 139/228) after diagnosis. Similarly, daytime sleepiness increased with age in all groups (Group 1: rs = 0.305, p < 0.001; rs = 0.200, p < 0.001; rs = 0.262, p < 0.001). In 56.1% (n = 129/233) of the non-diseased cats more sleep during the day was reported, 63.9% (n = 170/266) before diagnosis and 60.0% (n = 137/228) after diagnosis. Nocturnal activity was observed in 8.0% (n = 18/223) of cats without disease and 9.1% (n = 24/261) of cats before and 11.6% (n = 26/224) after diagnosis.
3.5.9. Vocalization
A significant increase in general vocalization was described in all groups (Group 1: rs = 0.170, p = 0.009; Group 2.1: rs = 0.258, p < 0.001; Group 2.2: rs = 0.257, p < 0.001). More generalized vocalization was noticed in one third of the cats without disease (32.9%, n = 78/237), comparable prevalences were noticeable in the diseased cats before and after diagnosis (Group 2.1: 34.1%, n = 91/267; Group 2.2: 34.4%, n = 78/227). However, only one out of ten cats (10.9%, n = 25/230) without diseases and almost one out of five cats with disease before diagnosis (18.8%, n = 50/267) vocalized more nocturnally, after diagnosis vocalization is described in 18.4% (n = 43/222). (See Supplementary Material). A significant increase in nocturnal vocalization with age was reported in all groups (Group 1: rs = 0.200, p = 0.00; Group 2.1: rs = 0.309, p = 0.001; Group 2.2: rs = 0.270, p < 0.001). Daytime vocalization increased with age only when a disease was present before and after diagnosis (Group 2.1: rs = 0.237, p < 0.001; Group 2.2: rs = 0.270, p < 0.001). In 23.0% (n = 53/231) of cats without disease more daytime vocalization was shown. In diseased cats more vocalization during the day is reported, 25.0% (n = 66/263) before diagnosis and 29.2% (n = 66/226) after diagnosis. Finally, undirected vocalization was significantly more frequent in diseased cats (Group 2.1: rs = 0.275, p < 0.001; Group 2.2: rs = 0.233, p < 0.001). Vocalization was noticed by the participants in 24.1% (n = 56/232) of cats without disease and in 25.8% (n = 68/264) of the cats with disease before and 28.9% (n = 66/229) after diagnosis (Table 2).

Table 2.
Correlations between aging and cats without disease (1), with disease before diagnosis (2.1) and with disease after diagnosis (2.2) and individual behavioral traits.
3.6. Behavior Disease Associations
3.6.1. Osteoarthritis (OA)
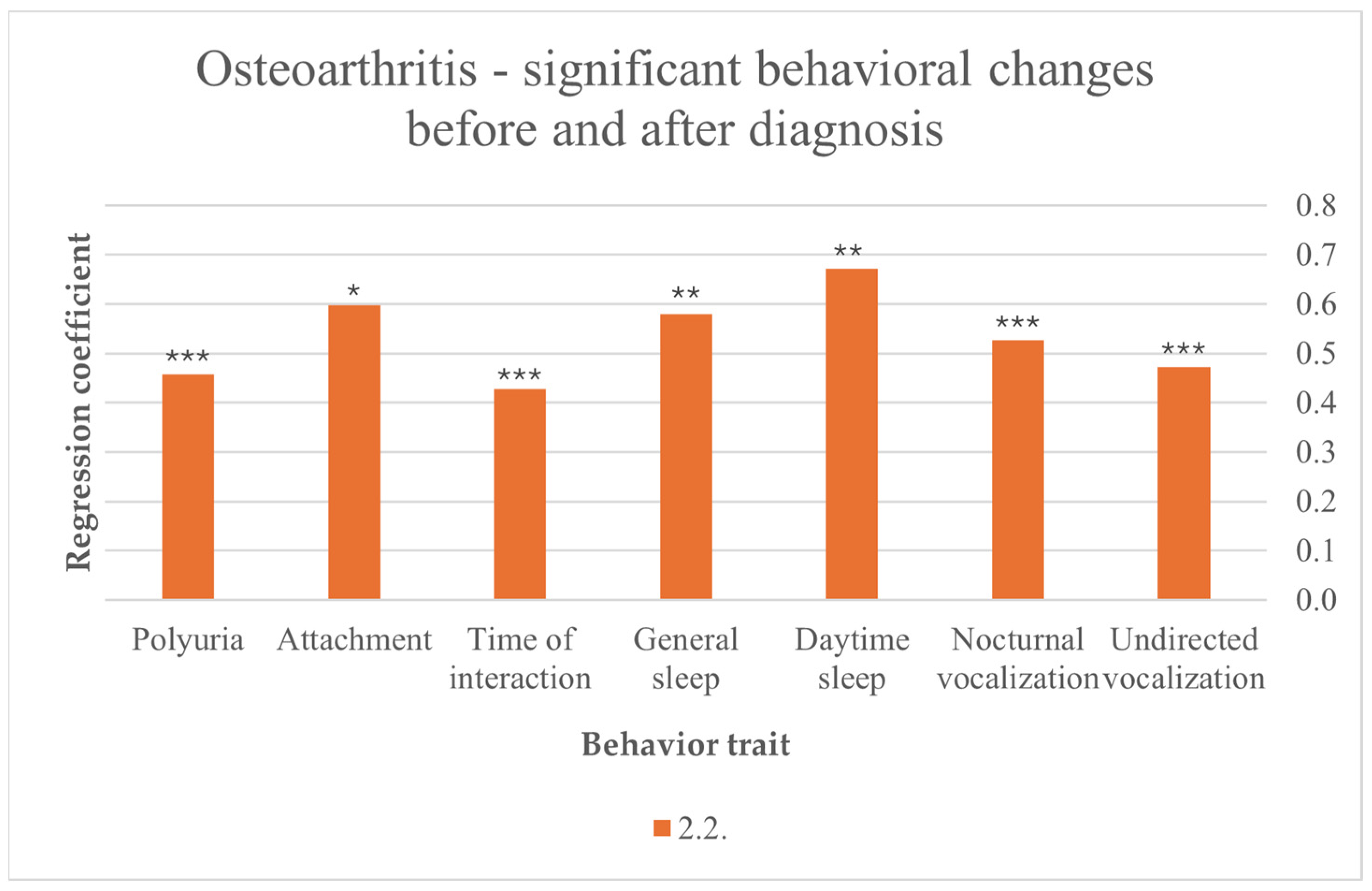
After cats were diagnosed with OA, their disease contributed to more sleep in general (F (10,216) = 2.457, p = 0.008) and more day-time sleep (F (10,217) = 2.530, p = 0.007). Another noticed behavior is vocalization after OA diagnosis, with statistically more general vocalization (F (10,216) = 3.050, p = 0.001), nocturnal vocalization (F (10,211) = 6.011, p < 0.001) and undirected vocalization (F (10,218) = 3.402, p < 0.001). Increasing attachment was associated with OA after diagnosis (F (7,219) = 2.342, p = 0.015). In addition, cat–owner interaction time increased after diagnosis (F (9,213) = 3.609, p < 0.001). Furthermore, polyuria was associated with OA after diagnosis (F (10,212) = 8.521, p < 0.001) (Figure 2).
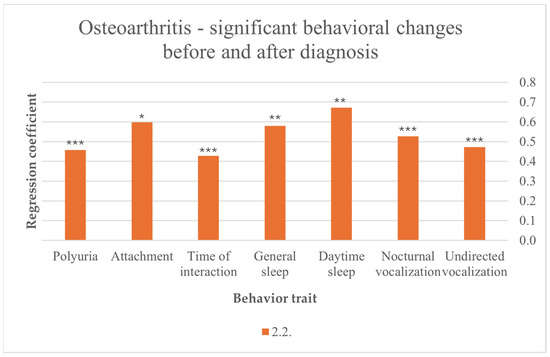
Figure 2.
Significant behavioral changes before and after diagnosis in cats suffering from OA; results from multiple linear regression; p < 0.001 ***, p < 0.01 **, p < 0.05 *.
3.6.2. Chronic Kidney Disease (CKD)
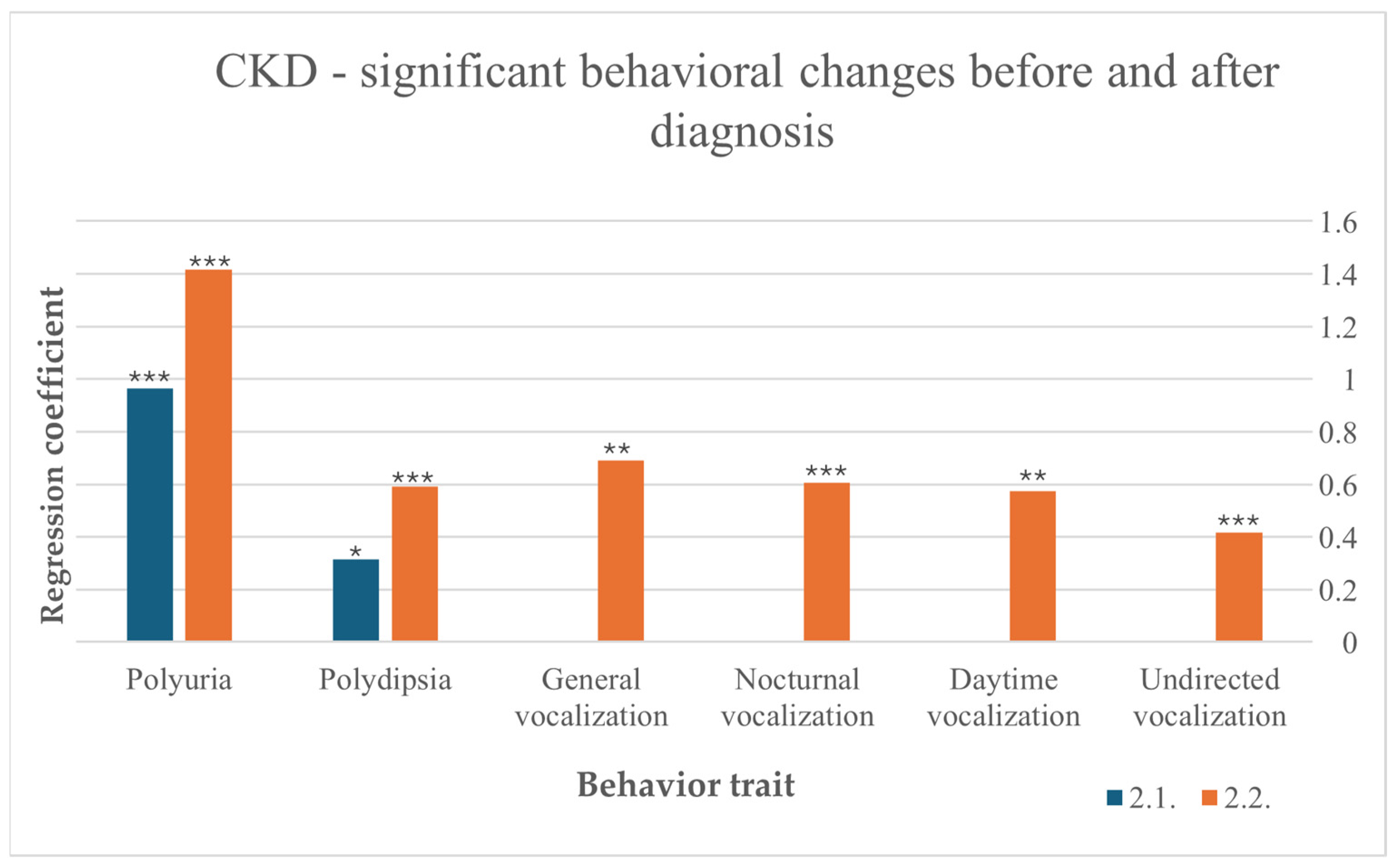
Among cats with CKD, the disease led to an increase in water intake before (F (10,266) = 1.996, p = 0.034) and after diagnosis (F (10,236) = 4.880, p < 0.001). After diagnosis, increased water intake indicates a preexisting CKD more noticeable than before diagnosis. Polyuria was also induced by CKD, with a stronger effect after diagnosis (F (10,212) = 8.521, p < 0.001) than before (F (10,238) = 5.279, p < 0.001). After diagnosis, vocalization was more frequent in general (F (10,216) = 3.050, p = 0.00), during the day (F (10,215) = 2.685, p = 0.004), at night (F (10,211) = 6.011, p < 0.001) and undirected (F (10,218) = 3.402, p < 0.001) (Figure 3).
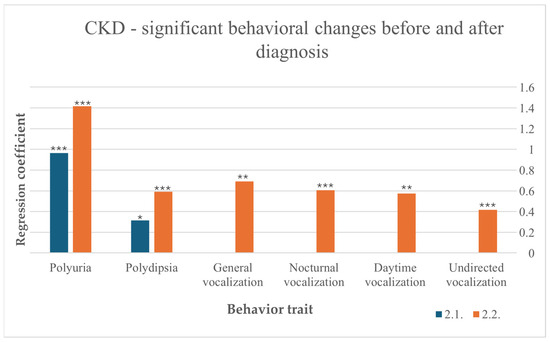
Figure 3.
Significant behavioral changes before and after diagnosis in cats suffering from CKD, results from multiple linear regression; p < 0.001 ***, p < 0.01 **, p < 0.05 *.
3.6.3. Hyperthyroidism
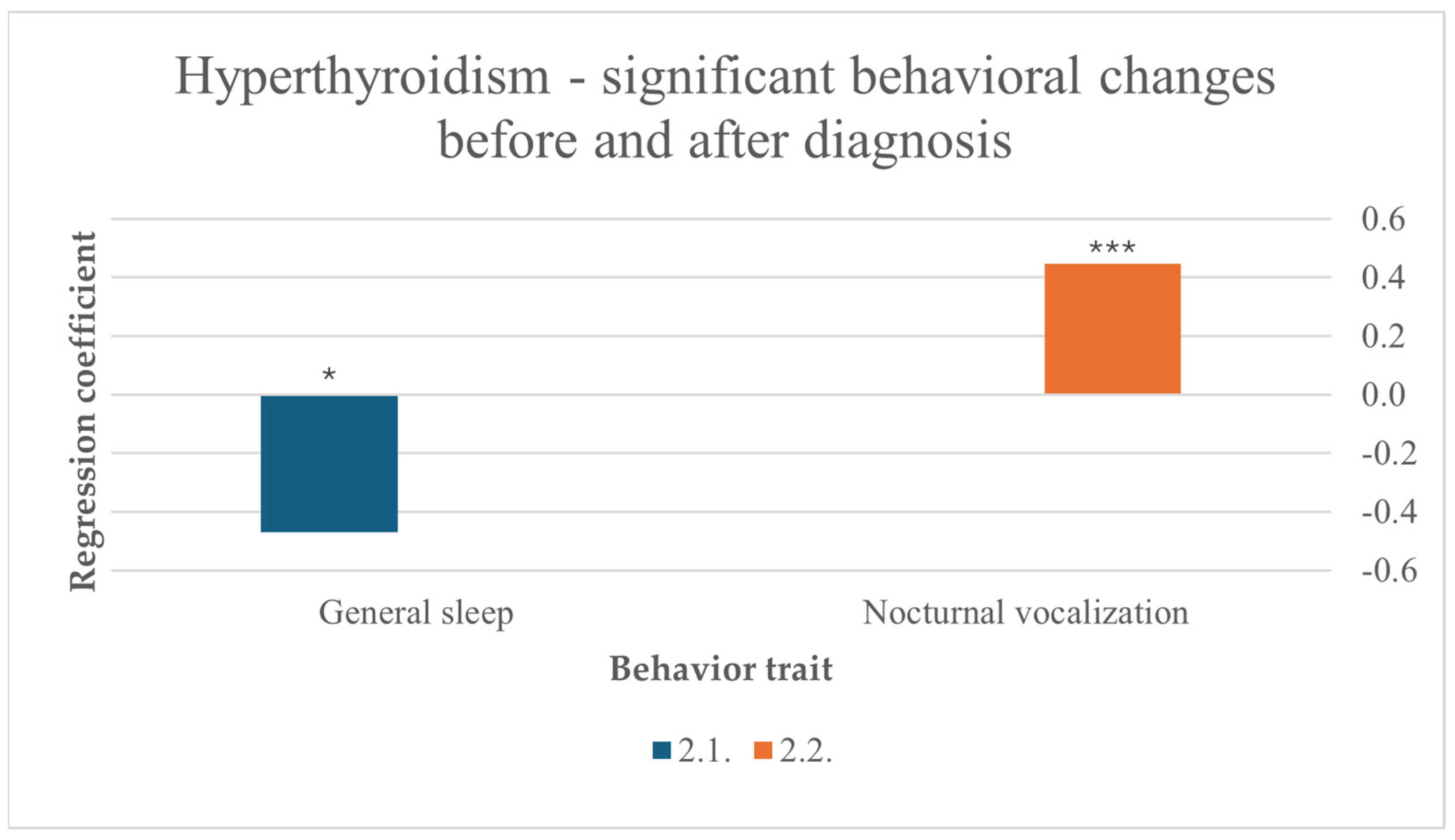
Before diagnosis, more sleep was a predictor for hyperthyroidism (F (10,256) = 2.078, p = 0.027). Hyperthyroidism also significantly influenced nocturnal vocalization in cats after diagnosis (F (10,211) = 6.011, p < 0.001) (Figure 4).
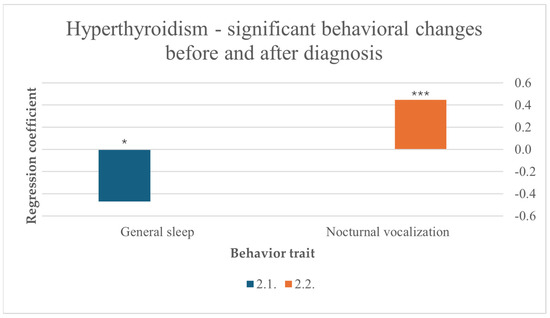
Figure 4.
Significant behavioral changes before and after diagnosis in cats suffering from hyperthyroidism, results from multiple linear regression; nocturnal vocalization: p < 0.001 ***, general sleep: p < 0.05 *.
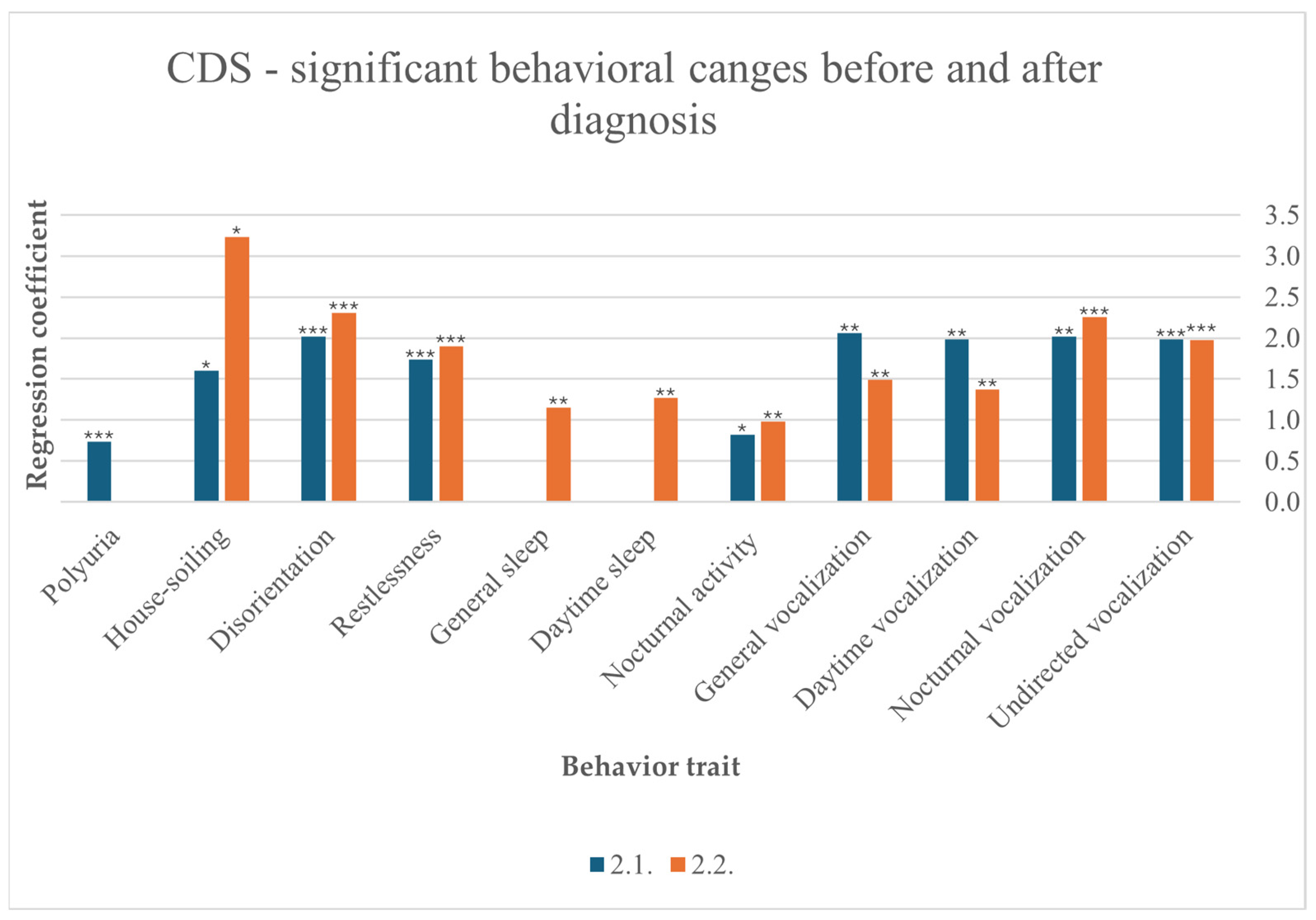
3.6.4. Cognitive Dysfunction Syndrome (CDS)
Disorientation was statistically associated with CDS before (F (10,257) = 5.855, p < 0.001) and after (F (10,220) = 3.976, p < 0.001) diagnosis. Restlessness also occurred significantly before (F (10,257) = 4.702, p < 0.001) and after (F (10,219) = 3.749, p < 0.001) diagnosis. Increased nocturnal vocalization was one of the strongest behavioral changes observed in cats diagnosed with CDS before (F (10,256) = 4.650, p < 0.001) and after diagnosis (F (10,211) = 6.011, p < 0.001). Furthermore, vocalization in general increased before (F(10,256) = 2.727, p = 0.003) and after diagnosis (F(10,216) = 3.050, p = 0.001), at daytime before (F(10,252) = 2.693, p = 0.004) and after diagnosis (F(10,215) = 2.685, p = 0.004), and undirected (F(10,253) = 3.408, p < 0.001) and after diagnosis (F(10,218) = 3.402, p < 0.001). Participants reported that their cats slept more after diagnosis in general (F (10,216) = 2.457, p = 0.008) and specifically during the day (F (10,217) = 2.5030, p = 0.007). CDS significantly contributed to increased nocturnal activity before (F (9248) = 1.956, p = 0.045) and after diagnosis (F (10,213) = 2.727, p = 0.004). Polyuria was significantly diagnosed before CDS (F (10,238) = 5.279, p < 0.001). Finally, urinating outside the litter box is another behavioral change that was observed by participants before (F (10,250) = 2.091, p = 0.032) and after diagnosis (F (10,212) = 2.418, p = 0.010) (Figure 5).
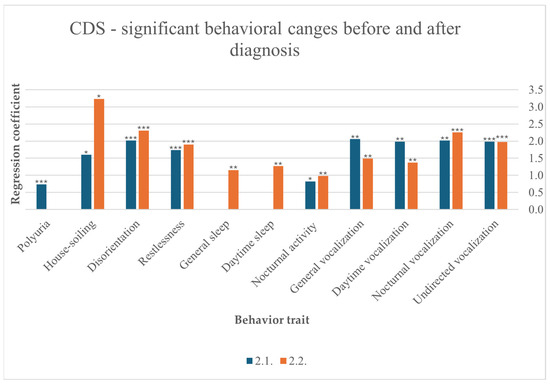
Figure 5.
Significant behavioral changes before and after diagnosis in cats suffering from CDS, results from multiple linear regression; p < 0.001 ***, p < 0.01 **, p < 0.05 *.
3.7. Symptoms Associated with CDS in “Healthy” Cats
Few participants reported a diagnosis of dementia or CDS in the questionnaire, although CDS is believed to be very common and increases with age. The following results focus on the symptoms of CDS observed in cats without disease, as they may indicate a possible undiagnosed disease.
The acronym VISH-DAAL [9] was used to decide which behavioral changes to focus on. For excessive vocalization, general vocalization (rs = 0.170, p = 0.009) and nocturnal vocalization (rs = 0.200, p = 0.002) were selected, both increased with age in healthy cats. The desire to be petted (rs = 0.131, p = 0.043) increased with age, reflecting changes in cat–human interaction. Changes in the sleep–wake cycle were registered with advanced general sleep (rs = 0.301, p < 0.001) and daytime sleep (rs = 0.305, p < 0.001). No association was found between CDS and house-soiling, either urination or defecation, in aging. Disorientation as a behavioral trait was found to increase in old cats (rs = 0.353, p < 0.001). Changes in activity levels were analyzed, with activity (rs = −0.178, p = 0.005), jumping (rs = −0.240, p < 0.001), grooming (rs = −0.133, p = 0.039), and playfulness (rs = −0.176, p = 0.006) all tending to decrease with age. For anxiety, the focus was on restlessness (rs = 0.134, p = 0.043), which increased with age. No questions were asked about learning. Memory was assessed with a question about joyful recognition, but there was no correlation with age.
4. Discussion
More than half of the cat owners participating in the survey reported that their cats were diagnosed with at least one disease, and most of these cats had at least one additional disease. This finding underscores the importance of comorbidity as a major factor in interpreting the results of diseased cats, particularly in geriatric cats, where such comorbidities are a common finding. The presence of more than one disease in a geriatric cat is thought to be influenced by various factors, including oxidative stress, infectious diseases and a weakened immune system [31]. Our study revealed correlations between hypertension and CKD, as well as between hyperthyroidism and heart disease. CKD and hyperthyroidism are the main causes of secondary hypertension, while cardiac disease does not cause secondary hypertension. The prevalences of hypertension are likely higher than previously diagnosed [31,32]. As systemic hypertension (systolic arterial pressure > 140 mmHG) is mainly secondary to the above-described diseases and can, if untreated, lead to peripheral vascular breakdown in target organs such as the heart, eyes, brain and kidneys, it is essential to put an emphasis on controlling blood pressure during routine health screenings [33,34]. Estimates in previous studies suggest that every tenth hyperthyroid cat is hypertensive at the time of diagnosis [32]. Furthermore, every fifth cat is expected to develop hypertension during treatment, which may unmask an underlying renal problem [23]. The prevalence of CKD-induced hypertension varies greatly, ranging from 19% to 65% in prior studies [32]. Interestingly, a noteworthy comorbidity that was not found in our study is the presence of preexisting CKD in hyperthyroid cats, which has been observed in 14% [25] and 23% [20] of affected cats.
Considering risk factors for developing one of the above-mentioned diseases (OA, CKD, hyperthyroidism and CDS), our study illustrated that only age had a direct influence, with rising numbers of each disease with age. Sex, breed, weight and form of keeping had no influence. The biological changes associated with aging, particularly the decreased capacity for internal self-regulation in the face of physiological and environmental stressors, have been identified as a potential underlying mechanism for the development of diseases in previous studies [5]. Consequently, age is considered the predominant risk factor.
Our study revealed that several behavioral traits have been shown to increase in the “healthy” cat during the process of aging, including polyuria, a desire to be petted, prolonged sleep (especially during the day), vocalization (especially nocturnal), disorientation and restlessness. On the other hand, activity patterns and food intake have been observed to decrease. These findings are in accordance with the physical age-related behavioral changes found in previous studies [4,5] that have been found in aging cats. In contrast to the group of cats without disease, those with a disease demonstrated an association between toileting outside the litter box (both urination and defecation) when aging in our study, which makes house-soiling an important indicator for possible diseases (e.g., idiopathic cystitis, pain, and CKD). Still, previous studies have shown that other factors might contribute to house-soiling, such as anxiety, living in a multi-cat household and social and environmental stress [35]. Furthermore, our study revealed that vocalization increased in all cat groups, but it was particularly pronounced in those with disease during daytime hours and in the absence of any discernible reason. This finding suggests that vocalization may serve as a potential precursor for disease in elderly cats, particularly in cases where it is excessively increased and undirected.
The prevalence of OA as the most diagnosed disease in our study is consistent with the findings of other studies, which indicate that radiological findings of OA are found in almost 90% of elderly cats [11]. However, in our study, not even every fifth cat was diagnosed with OA, which suggests a higher number of undiagnosed cases [11,36]. Cat owners in our study primarily observed behavioral changes after diagnosis, which might be due to the fact that behavioral changes and clinical findings in OA are often subtle and unspecific [12,13]. Polyuria is significantly increased in cats with OA after diagnosis in our results, suggesting a potential comorbidity with another disease that causes polyuria either due to primary polydipsia or more likely due to primary polyuria, most commonly due to CKD and diabetes mellitus in older cats [37]. The influence of psychogenic polydipsia resulting from chronic pain on polyuria is another noteworthy consideration, although psychogenic polydipsia is a rare finding in cats [37]. Furthermore, cats diagnosed with OA may spend more time indoors and may be observed more thoroughly by their owners, potentially leading to an overinterpretation of this finding. Participants in our study reported an increased level of attachment, which is possibly initiated by the cat realizing its decreasing level of mobilization and therefore the increasing dependency on its owners’ care. This attachment might also be intensified by the owner as special care and management is offered to cats with chronic pain. A prolonged sleep, especially during the day, was detected and is suitable for an increased level of resting and a decreased level of daily activities seen in cats with chronic pain. Finally, heightened vocalization, especially nocturnal and seemingly without an identifiable reason, was noted in those cats with OA. A prior study suggests that sudden vocalizations in cats with chronic pain are often a response to unexpected pain [13] and may also reflect an attention seeking behavior towards the owners. However, the presence of comorbidities, such as hypertension, cannot be ruled out.
A recent study revealed that the prevalence of CKD in aged cats is higher than previously suspected with 42.1% of cats aged 10–15 years and 80.9% of cats older than 15 years [16]. In our own study, we found that every eighth cat suffered from renal disease. It is possible that these numbers are even higher, as diagnoses are frequently made based solely on the presence of azotemia [16]. Our study demonstrated that an increased water intake is directly associated with the diagnosis of CKD, both before and after diagnosis, with a growth in water intake seen after diagnosis. Polydipsia is one of the first clinical signs observed by over 75% of owners in a previous study [14] and results from the compensatory response to polyuria [37], which is another predicator for CKD in our study. Our results demonstrate that similarly to polydipsia, polyuria worsens after diagnosis. Prior results show that both symptoms are usually already detected in the year before diagnosis [14] and are the only symptoms found in our study before diagnosis. After diagnosis, participants reported that increased vocalization in general, at nighttime, in the daytime, and without apparent reason, which might be caused by hypertension, which correlates with CKD seen in 19–65% of cats with CKD in foregoing studies [38,39].
In the present study, an increasing number of cats has been diagnosed with hyperthyroidism since the syndrome was first recognized, which leads to numerous investigations to identify possible causes, including immunological, nutritional, environmental and genetic factors. However, the underlying mechanisms are not yet completely understood. The prevalence of hyperthyroidism varies geographically. In a prior study, every tenth cat older than ten years was diagnosed with hyperthyroidism, which matches the number of cats found with hyperthyroidism in our study [40,41]. Before diagnosis, participants mentioned decreased sleep, which could be a cause of hyperactivity leading to restlessness and nervousness seen in prior studies in hyperthyroid cats [24]. After diagnosis, nocturnal vocalization was prevalent in the present study, maybe due to restlessness that is often described in hyperthyroid cats. It is also important to consider the role of hypertension leading to nocturnal vocalization, as a study has shown that approximately 20% of cats develop hypertension after treatment and reach an euthyroid stage [23].
In the present study, a small percentage of cats (2.9%) was diagnosed with CDS. Even though a previous study suggests that at least one symptom consistent with CDS is suspected to affect every fourth cat between 11–14 years and every second cat over 15 years [8], it is still a diagnosis of exclusion [26]. In addition, another study revealed that many cat owners will only mention symptoms consistent with CDS if asked specifically [28]. Despite the small number of cats diagnosed with CDS, the behavioral symptoms found in our own study fit mostly with the VISH-DAAL acronym. In our study a particularly strong rise of vocalization to night- and daytime, in general and without apparent reason, was perceived before and after diagnosis. Daytime vocalization seemed to decrease a little after diagnosis, in contrast to nocturnal vocalization, which increases after diagnosis. Nevertheless, the number of cats with a link to CDS is small, which lowers the informative value. A change in sleep–wake cycle is also described by the participants, with cats sleeping more in general and especially at daytime after diagnosis. It must be taken into consideration that behavior like sleeping may have been observed more closely post-diagnosis especially in a behavior-pronounced disease such as CDS, therefore overinterpretation by owners cannot be excluded. In addition, an increase in nocturnal activity is reported before and after diagnosis. Urination outside the litter box is stated before and after CDS, even though polyuria was noted by the cat owners before diagnosis. Polyureic cats might also have other underlying diseases, which can result in house-soiling. Disorientation is also reported before and after diagnosis, with a notable increase after diagnosis. Anxiety is associated with restlessness, which is evident before and after diagnosis. It is crucial to note that the survey did not include questions regarding learning and memory. In general, a pronounced increase in behavioral symptoms is detected in cats with CDS, suggesting that this condition has been diagnosed only when particularly pronounced or that its symptoms in this condition might be inherently intense.
As CDS remains an underdiagnosed disease and is believed to have a much greater prevalence with increasing age, a correlation between age and the above-described changes (VISH-DAAL) was examined in cats diagnosed with no disease. These results may reflect a considerable number of undiagnosed cases, as the prevalence of diagnosis and prevalence described in literature strongly disagree.
The study implies several limitations. The main limitation of the study is the method used to generate the data. The owner-addressed survey displays only respondent’s perception, which are subjective and do not always reflect reality. Recall bias might be present as behavioral changes before diagnosis were queried retrospectively. Diseases stated have not been confirmed by veterinarians. When diving into groups pre- and postdiagnosis, diagnosis was used to indicate the veterinarian recognition of a specific disease, not the beginning of it. Furthermore, the behavior observed might have been misinterpreted. Additionally, not all respondents answered all questions, therefore some respondents’ data about their cat’s behavior could not be analyzed. Additionally, the survey’s dissemination through veterinary clinics might overrepresent cats with a disease. Being mainly advertised via online platforms, the survey might have not been accessible for owners that have no access to the internet, presumably being elderly cat-owners. Moreover, as common in studies based on a survey women are over-represented in this study. Furthermore, mainly highly engaged pet owners respond more frequently to pet-related surveys. Finally, the possibility of conditioning favorable behavior due to reward or penalty cannot be completely excluded. Further studies are required to investigate the influence of conditioning by cat-owners and the influence of medication on disease-related behavioral changes. To support our results, research including clinical data and comprehensive owner interviews are requisite.
5. Conclusions
Several behavioral changes originate from an underlying systemic disease, potentially serving as early signs of various diseases. Still, the understanding of the physiological and unphysiological behavior by owners is limited, as subtle changes are often overlooked. Many CDS-related symptoms were seen in aged cats with no diagnosed disease, which may indicate an underlying pathological background. Certain behavioral changes, particularly house-soiling and vocalization for unknown reasons, were more prevalent in the diseased cats prior to diagnosis compared to the healthy cats. Furthermore, behavioral associations found before diagnosis in three of the four examined diseases underscore the potential for early recognition, which enables timely intervention. However, behavioral changes are often perceived infrequently, as they are often unspecific or subtle. To detect abnormal from normal, veterinarians need to be familiar with feline behavioral and clinical changes associated with aging. A comprehensive medical history is crucial for cats, as symptoms may not be consciously perceived, not considered as problematic, disregarded as non-pathological, or dismissed as normal aging. To ensure prolongation and enhancement of the quality of life of elderly cats, early recognition of potential diseases is essential for the administration of adequate therapy and management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pets2020021/s1, File S1: Questionnaire; File S2: Results multiple linear regression; Table S1: Demographic data; Table S2: Risk factors; Table S3: Descriptive behavioral changes, Figure S1: Comparative behavioral changes.
Author Contributions
Conceptualization, J.E. and F.K.; methodology, F.K.; software, J.E.; validation, J.E.; formal analysis, J.E.; investigation, J.E.; resources, F.K.; data curation, J.E.; writing—original draft preparation, J.E.; writing—review and editing, F.K.; visualization, J.E.; supervision, F.K.; project administration, F.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
According to national legislation, ethics approval was not required for this type of study (https://www.rv.hessenrecht.hessen.de/perma?d=jlr-DSIFGHErahmen).
Informed Consent Statement
The collection, storage and use of data from the cats of the participants was made entirely voluntary, and the participants were informed of this fact. Data on cats was collected for this research project and specific free and informed consent was obtained from the participants in accordance with the Declaration of Helsinki.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to the cat owners who participated on the survey.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Institutional Review Board Statement and Informed Consent Statement. This change does not affect the scientific content of the article.
Abbreviations
The following abbreviations are used in this manuscript:
| OA | Osteoarthritis |
| CKD | Chronic kidney disease |
| CDS | Cognitive dysfunction syndrome |
| PCA | Principal component analysis |
References
- IVH and ZZF Germany. The German pet Market: Structure and Sales Data. Available online: https://www.zzf.de/fileadmin/ZZF/Pressemeldungen/2024/2024_04_16_Marktdaten/ZZF_IVH_Der_Deutsche_Heimtiermarkt_2023.pdf (accessed on 1 September 2024). (In German).
- Gunn-Moore, D. Considering older cats. J. Small Anim. Pract. 2006, 47, 430–431. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, D.G.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Longevity and mortality of cats attending primary care veterinary practices in England. J. Feline Med. Surg. 2015, 17, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Bellows, J.; Center, S.; Daristotle, L.; Estrada, A.H.; Flickinger, E.A.; Horwitz, D.F.; Lascelles, B.D.X.; Lepine, A.; Perea, S.; Scherk, M.; et al. Evaluating aging in cats: How to determine what is healthy and what is disease. J. Feline Med. Surg. 2016, 18, 551–570. [Google Scholar] [CrossRef]
- Bellows, J.; Center, S.; Daristotle, L.; Estrada, A.H.; Flickinger, E.A.; Horwitz, D.F.; Lascelles, B.D.X.; Lepine, A.; Perea, S.; Scherk, M.; et al. Aging in cats: Common physical and functional changes. J. Feline Med. Surg. 2016, 18, 533–550. [Google Scholar] [CrossRef]
- Sordo, L.; Breheny, C.; Halls, V.; Cotter, A.; Tørnqvist-Johnsen, C.; Caney, S.M.A.; Gunn-Moore, D.A. Prevalence of Disease and Age-Related Behavioural Changes in Cats: Past and Present. Vet. Sci. 2020, 7, 85. [Google Scholar] [CrossRef]
- Dowgray, N.; Pinchbeck, G.; Eyre, K.; Biourge, V.; Comerford, E.; German, A.J. Aging in Cats: Owner Observations and Clinical Finding in 206 Mature Cats at Enrolment to the Cat Prospective Aging and Welfare Study. Front. Vet. Sci. 2022, 9, 859041. [Google Scholar] [CrossRef] [PubMed]
- Gunn-Moore, D.; Moffat, K.; Christie, L.-A.; Head, E. Cognitive dysfunction and the neurobiology of ageing in cats. J. Small Anim. Pract. 2007, 48, 546–553. [Google Scholar] [CrossRef]
- Sordo, L.; Gunn-Moore, D.A. Cognitive Dysfunction in Cats: Update on Neuropathological and Behavioural Changes Plus Clinical Management. Vet. Rec. 2021, 188, e3. [Google Scholar] [CrossRef]
- Kremendahl, J. Multimorbidity in geriatric cats is often a challenge [article in German]. Kleintier Konkret. 2015, 6, 18–24. [Google Scholar] [CrossRef]
- Hardie, E.M.; Roe, S.C.; Martin, F.R. Radiographic evidence of degenerative joint disease in geriatric cats: 100 cases (1994–1997). J. Am. Vet. Med. Assoc. 2002, 220, 628–632. [Google Scholar] [CrossRef]
- Bennett, D.; Zainal Ariffin, S.M.B.; Johnston, P. Osteoarthritis in the cat: 1. How common is it and how easy to recognise? J. Feline Med. Surg. 2012, 14, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, B.P.; Steagall, P.V. Chronic pain in cats: Recent advances in clinical assessment. J. Feline Med. Surg. 2019, 21, 601–614. [Google Scholar] [CrossRef]
- Reynolds, B.S.; Lefebvre, H.P. Feline CKD: Pathophysiology and risk factors--what do we know? J. Feline Med. Surg. 2013, 15, 3–14. [Google Scholar] [CrossRef]
- Brown, C.A.; Elliott, J.; Schmiedt, C.W.; Brown, S.A. Chronic Kidney Disease in Aged Cats: Clinical Features, Morphology, and Proposed Pathogeneses. Vet. Pathol. 2016, 53, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Marino, C.L.; Lascelles, B.D.X.; Vaden, S.L.; Gruen, M.E.; Marks, S.L. Prevalence and classification of chronic kidney disease in cats randomly selected from four age groups and in cats recruited for degenerative joint disease studies. J. Feline Med. Surg. 2014, 16, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Meeking, S.A. Thyroid disorders in the geriatric patient. Vet. Clin. N. Am. Small Anim. 2005, 35, 635–665. [Google Scholar] [CrossRef]
- van Hoek, I.; Daminet, S. Interactions between thyroid and kidney function in pathological conditions of these organ systems: A review. Gen. Comp. Endocrinol. 2009, 160, 205–215. [Google Scholar] [CrossRef]
- Peterson, M.E.; Kintzer, P.P.; Cavanagh, P.G.; Fox, P.R.; Ferguson, D.C.; Johnson, G.F.; Becker, D.V. Feline hyperthyroidism: Pretreatment clinical and laboratory evaluation of 131 cases. J. Am. Vet. Med. Assoc. 1983, 183, 103–110. [Google Scholar] [CrossRef]
- Broussard, J.D.; Peterson, M.E.; Fox, P.R. Changes in clinical and laboratory findings in cats with hyperthyroidism from 1983 to 1993. J. Am. Vet. Med. Assoc. 1995, 206, 302–305. [Google Scholar] [CrossRef]
- Sassnau, R. Epidemiological investigation on the prevalence of feline hyperthyroidism in an urban population in Germany. Tierärztl. Prax. 2006, 34, 450–457. (In German) [Google Scholar] [CrossRef]
- Wakeling, J.; Melian, C.; Font, A. Evidence for Differing Incidences of Feline Hyperthyroidism in London, UK and Spain. In Proceedings of the 15th ECVIM-CA Congress, Glasgow, UK, 1–3 September 2005; p. 220. [Google Scholar]
- Vaske, H.H.; Schermerhorn, T.; Armbrust, L.; Grauer, G.F. Diagnosis and management of feline hyperthyroidism: Current perspectives. Vet. Med. 2014, 5, 85–96. [Google Scholar] [CrossRef]
- Khare, D.S.; Gupta, D.K.; Shukla, P.C.; Meena, N.S.; Khare, R. Feline hyperthyroidism: An overview. J. Entomol. Zool. Stud. 2018, 6, 418–423. [Google Scholar]
- Milner, R.J.; Channell, C.D.; Levy, J.K.; Schaer, M. Survival times for cats with hyperthyroidism treated with iodine 131, methimazole, or both: 167 cases (1996–2003). J. Am. Vet. Med. Assoc. 2006, 228, 559–563. [Google Scholar] [CrossRef]
- Landsberg, G.M.; Denenberg, S.; Araujo, J.A. Cognitive dysfunction in cats: A syndrome we used to dismiss as ‘old age’. J. Feline Med. Surg. 2010, 12, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Sordo, L.; Martini, A.C.; Houston, E.F.; Head, E.; Gunn-Moore, D. Neuropathology of Aging in Cats and its Similarities to Human Alzheimer’s Disease. Front. Aging 2021, 2, 684607. [Google Scholar] [CrossRef] [PubMed]
- MacQuiddy, B.; Moreno, J.; Frank, J.; McGrath, S. Survey of risk factors and frequency of clinical signs observed with feline cognitive dysfunction syndrome. J. Feline Med. Surg. 2022, 24, e131–e137. [Google Scholar] [CrossRef]
- Casey, R.A.; Bradshaw, J.W. Owner compliance and clinical outcome measures for domestic cats undergoing clinical behavior therapy. J. Vet. Behav. 2008, 3, 114–124. [Google Scholar] [CrossRef]
- Amat, M.; de la Torre, J.L.R.; Fatjó, J.; Mariotti, V.M.; van Wijk, S.; Manteca, X. Potential risk factors associated with feline behaviour problems. Appl. Anim. Behav. Sci. 2009, 121, 134–139. [Google Scholar] [CrossRef]
- Ray, M.; Carney, H.C.; Boynton, B.; Quimby, J.; Robertson, S.; St Denis, K.; Tuzio, H.; Wright, B. 2021 AAFP Feline Senior Care Guidelines. J. Feline Med. Surg. 2021, 23, 613–638. [Google Scholar] [CrossRef]
- Taylor, S.S.; Sparkes, A.H.; Briscoe, K.; Carter, J.; Sala, S.C.; Jepson, R.E.; Reynolds, B.S.; Scansen, B.A. ISFM Consensus Guidelines on the Diagnosis and Management of Hypertension in Cats. J. Feline Med. Surg. 2017, 19, 288–303. [Google Scholar] [CrossRef]
- Morita, S.; Mochizuki, Y.; Matsumoto, I.; Horii, A.; Ohmori, T.; Hirao, D.; Hasegawa, H.; Yoshimura, A.; Baba, T.; Suzuki, S.; et al. Use of amlodipine in the treatment of cats with systemic hypertension in Japan. J. Vet. Med. Sci. 2024, 86, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Acierno, M.J.; Brown, S.; Coleman, A.E.; Jepson, R.E.; Papich, M.; Stepien, R.L.; Syme, H.M. ACVIM consensus statement: Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J. Vet. Intern. Med. 2018, 32, 1803–1822. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, A.M.; McPeake, K.; Affenzeller, N.; Mills, D.S. Common Risk Factors for Urinary House Soiling (Periuria) in Cats and Its Differentiation: The Sensitivity and Specificity of Common Diagnostic Signs. Front. Vet. Sci. 2018, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Lemetayer, J.; Taylor, S. Inflammatory joint disease in cats: Diagnostic approach and treatment. J. Feline Med. Surg. 2014, 16, 547–562. [Google Scholar] [CrossRef]
- Lunn, K.F.; James, K.J. Normal and Abnormal Water Balance: Polyuria and Polydipsia. Compend. Contin. Educ. Vet. 2007, 29, 612–614. [Google Scholar]
- Kobayashi, D.L.; Peterson, M.E.; Graves, T.K.; Lesser, M.; Nichols, C.E. Hypertension in cats with chronic renal failure or hyperthyroidism. Vet. Int. Med. 1990, 4, 58–62. [Google Scholar] [CrossRef]
- Syme, H.M.; Barber, P.J.; Markwell, P.J.; Elliott, J. Prevalence of systolic hypertension in cats with chronic renal failure at initial evaluation. J. Am. Vet. Med. Assoc. 2002, 220, 1799–1804. [Google Scholar] [CrossRef]
- Peterson, M. Hyperthyroidism in cats: What’s causing this epidemic of thyroid disease and can we prevent it? J. Feline Med. Surg. 2012, 14, 804–818. [Google Scholar] [CrossRef]
- Carney, H.C.; Ward, C.R.; Bailey, S.J.; Bruyette, D.; Dennis, S.; Ferguson, D.; Hinc, A.; Rucinsky, A.R. 2016 AAFP Guidelines for the Management of Feline Hyperthyroidism. J. Feline Med. Surg. 2016, 18, 400–416. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).