Altered iso- and oxo-Fecal Bile Acid Concentrations in Dogs with Chronic Enteropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Working Solutions and Standards
2.3. Animals and Fecal Samples
2.4. Sample Extraction Protocol for Bile Acid Analysis
2.5. LC-MS/MS Analysis
2.6. Post-Acquisition Data Processing
2.7. Method Optimization and Validation
2.7.1. Carryover and Selectivity
2.7.2. Stability
2.7.3. Fecal Volume Optimization and Dilution
2.7.4. Spiking
2.7.5. Intra- and Inter-Day Precision
2.8. Statistical Analysis
3. Results
3.1. Method Optimization and Validation
3.1.1. Carryover and Selectivity
3.1.2. Stability
3.1.3. Fecal Volume Optimization and Dilution
3.1.4. Spiking
3.1.5. Intra- and Inter-Day Precision
3.2. Fecal Bile Acid Concentrations in Healthy Dogs and Dogs with Chronic Enteropathy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiorucci, S.; Carino, A.; Baldoni, M.; Santucci, L.; Costanzi, E.; Graziosi, L.; Distrutti, E.; Biagioli, M. Bile acid signaling in inflammatory bowel diseases. Dig. Dis. Sci. 2021, 66, 674–693. [Google Scholar] [CrossRef] [PubMed]
- Heinken, A.; Ravcheev, D.A.; Baldini, F.; Heirendt, L.; Fleming, R.M.T.; Thiele, I. Systematic assessment of secondary bile acid metabolism in gut microbes reveals distinct metabolic capabilities in inflammatory bowel disease. Microbiome 2019, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef]
- Batta, A.; Salen, G.; Arora, R.; Shefer, S.; Batta, M.; Person, A. Side chain conjugation prevents bacterial 7-dehydroxylation of bile acids. J. Biol. Chem. 1990, 265, 10925–10928. [Google Scholar] [CrossRef]
- Rowe, J.C.; Winston, J.A. Collaborative metabolism: Gut microbes play a key role in canine and feline bile acid metabolism. Vet. Sci. 2024, 11, 94. [Google Scholar] [CrossRef]
- Lan, K.; Su, M.; Xie, G.; Ferslew, B.C.; Brouwer, K.L.R.; Rajani, C.; Liu, C.; Jia, W. Key role for the 12-hydroxy group in the negative ion fragmentation of unconjugated c24 bile acids. Anal. Chem. 2016, 88, 7041–7048. [Google Scholar] [CrossRef]
- Fleishman, J.S.; Kumar, S. Bile acid metabolism and signaling in health and disease: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 97. [Google Scholar] [CrossRef]
- Wang, H.; Chen, J.; Hollister, K.; Sowers, L.C.; Forman, B.M. Endogenous bile acids are ligands for the nuclear receptor fxr/bar. Mol. Cell 1999, 3, 543–553. [Google Scholar] [CrossRef]
- Jadhav, K.; Xu, Y.; Xu, Y.; Li, Y.; Xu, J.; Zhu, Y.; Adorini, L.; Lee, Y.K.; Kasumov, T.; Yin, L.; et al. Reversal of metabolic disorders by pharmacological activation of bile acid receptors TGR5 and FXR. Mol. Metab. 2018, 9, 131–140. [Google Scholar] [CrossRef]
- Copple, B.L.; Li, T. Pharmacology of bile acid receptors: Evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol. Res. 2016, 104, 9–21. [Google Scholar] [CrossRef]
- Jergens, A.E.; Heilmann, R.M. Canine chronic enteropathy-current state-of-the-art and emerging concepts. Front. Vet. Sci. 2022, 9, 923013. [Google Scholar] [CrossRef] [PubMed]
- Bromke, M.A.; Krzystek-Korpacka, M. Bile acid signaling in inflammatory bowel disease. Int. J. Mol. Sci. 2021, 22, 9096. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liu, Y.; Zhou, X.; Navaneethan, U.; Shen, B.; Guo, B. An lc-esi-ms method for the quantitative analysis of bile acids composition in fecal materials. Biomed. Chromatogr. 2012, 26, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Choucair, I.; Nemet, I.; Li, L.; Cole, M.A.; Skye, S.M.; Kirsop, J.D.; Fischbach, M.A.; Gogonea, V.; Brown, J.M.; Tang, W.H.W.; et al. Quantification of bile acids: A mass spectrometry platform for studying gut microbe connection to metabolic diseases. J. Lipid Res. 2020, 61, 159–177. [Google Scholar] [CrossRef]
- Comito, R.; Porru, E.; Interino, N.; Conti, M.; Terragni, R.; Gotti, R.; Candela, M.; Simoni, P.; Roda, A.; Fiori, J. Metabolic bile acid profile impairments in dogs affected by chronic inflammatory enteropathy. Metabolites 2023, 13, 980. [Google Scholar] [CrossRef]
- Dewaele, D.; Annaert, P.; Hoeben, E. Lc-ms/ms analysis of bile acids in in vitro samples. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2019; Volume 1981, pp. 15–23. [Google Scholar] [CrossRef]
- Farhat, Z.; Sampson, J.N.; Hildesheim, A.; Safaeian, M.; Porras, C.; Cortés, B.; Herrero, R.; Romero, B.; Vogtmann, E.; Sinha, R.; et al. Reproducibility, temporal variability, and concordance of serum and fecal bile acids and short chain fatty acids in a population-based study. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1875–1883. [Google Scholar] [CrossRef]
- Franco, P.; Porru, E.; Fiori, J.; Gioiello, A.; Cerra, B.; Roda, G.; Caliceti, C.; Simoni, P.; Roda, A. Identification and quantification of oxo-bile acids in human faeces with liquid chromatography–mass spectrometry: A potent tool for human gut acidic sterolbiome studies. J. Chromatogr. A 2019, 1585, 70–81. [Google Scholar] [CrossRef]
- Gómez, C.; Stücheli, S.; Kratschmar, D.V.; Bouitbir, J.; Odermatt, A. Development and validation of a highly sensitive lc-ms/ms method for the analysis of bile acids in serum, plasma, and liver tissue samples. Metabolites 2020, 10, 282. [Google Scholar] [CrossRef]
- Hagio, M.; Matsumoto, M.; Fukushima, M.; Hara, H.; Ishizuka, S. Improved analysis of bile acids in tissues and intestinal contents of rats using lc/esi-ms. J. Lipid Res. 2009, 50, 173–180. [Google Scholar] [CrossRef]
- Herstad, K.M.V.; Rønning, H.T.; Bakke, A.M.; Moe, L.; Skancke, E. Changes in the faecal bile acid profile in dogs fed dry food vs high content of beef: A pilot study. Acta Vet. Scand. 2018, 60, 29. [Google Scholar] [CrossRef]
- Humbert, L.; Maubert, M.A.; Wolf, C.; Duboc, H.; Mahé, M.; Farabos, D.; Seksik, P.; Mallet, J.M.; Trugnan, G.; Masliah, J.; et al. Bile acid profiling in human biological samples: Comparison of extraction procedures and application to normal and cholestatic patients. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 899, 135–145. [Google Scholar] [CrossRef] [PubMed]
- John, C.; Werner, P.; Worthmann, A.; Wegner, K.; Tödter, K.; Scheja, L.; Rohn, S.; Heeren, J.; Fischer, M. A liquid chromatography-tandem mass spectrometry-based method for the simultaneous determination of hydroxy sterols and bile acids. J. Chromatogr. A 2014, 1371, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Krautbauer, S.; Liebisch, G. Lc-ms/ms analysis of bile acids. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1730, pp. 103–110. [Google Scholar] [CrossRef]
- Lee, J.W.; Cowley, E.S.; Wolf, P.G.; Doden, H.L.; Murai, T.; Caicedo, K.Y.O.; Ly, L.K.; Sun, F.; Takei, H.; Nittono, H.; et al. Formation of secondary allo-bile acids by novel enzymes from gut firmicutes. Gut Microbes 2022, 14, 2132903. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Tan, X.; Wang, W.; Zeng, W.; Gui, L.; Su, M.; Liu, C.; Jia, W.; Xu, L.; Lan, K. Species differences of bile acid redox metabolism: Tertiary oxidation of deoxycholate is conserved in preclinical animals. Drug Metab. Dispos. 2020, 48, 499–507. [Google Scholar] [CrossRef]
- Liu, Y.; Song, Q.; Zheng, J.; Li, J.; Zhao, Y.; Li, C.; Song, Y.; Tu, P. Sensitive profiling of phenols, bile acids, sterols, and eicosanoids in mammalian urine by large volume direct injection-online solid phase extraction-ultra high performance liquid chromatography-polarity switching tandem mass spectrometry. RSC Adv. 2016, 6, 81826–81837. [Google Scholar] [CrossRef]
- Marion, S.; Desharnais, L.; Studer, N.; Dong, Y.; Notter, M.D.; Poudel, S.; Menin, L.; Janowczyk, A.; Hettich, R.L.; Hapfelmeier, S.; et al. Biogeography of microbial bile acid transformations along the murine gut. J. Lipid Res. 2020, 61, 1450–1463. [Google Scholar] [CrossRef]
- Muto, A.; Takei, H.; Unno, A.; Murai, T.; Kurosawa, T.; Ogawa, S.; Iida, T.; Ikegawa, S.; Mori, J.; Ohtake, A.; et al. Detection of δ4-3-oxo-steroid 5β-reductase deficiency by lc-esi-ms/ms measurement of urinary bile acids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 900, 24–31. [Google Scholar] [CrossRef]
- Odermatt, A.; Da Cunha, T.; Penno, C.A.; Chandsawangbhuwana, C.; Reichert, C.; Wolf, A.; Dong, M.; Baker, M.E. Hepatic reduction of the secondary bile acid 7-oxolithocholic acid is mediated by 11β-hydroxysteroid dehydrogenase 1. Biochem. J. 2011, 436, 621–629. [Google Scholar] [CrossRef]
- Porru, E.; Scicchitano, D.; Interino, N.; Tavella, T.; Candela, M.; Roda, A.; Fiori, J. Analysis of fecal bile acids and metabolites by high resolution mass spectrometry in farm animals and correlation with microbiota. Sci. Rep. 2022, 12, 2866. [Google Scholar] [CrossRef]
- Prinville, V.; Ohlund, L.; Sleno, L. Targeted analysis of 46 bile acids to study the effect of acetaminophen in rat by lc-ms/ms. Metabolites 2020, 10, 26. [Google Scholar] [CrossRef]
- Reiter, S.; Dunkel, A.; Metwaly, A.; Panes, J.; Salas, A.; Haller, D.; Hofmann, T. Development of a highly sensitive ultra-high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry quantitation method for fecal bile acids and application on crohn’s disease studies. J. Agric. Food Chem. 2021, 69, 5238–5251. [Google Scholar] [CrossRef] [PubMed]
- Riethorst, D.; Mols, R.; Duchateau, G.; Tack, J.; Brouwers, J.; Augustijns, P. Characterization of human duodenal fluids in fasted and fed state conditions. J. Pharm. Sci. 2016, 105, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Sangaraju, D.; Shi, Y.; Van Parys, M.; Ray, A.; Walker, A.; Caminiti, R.; Milanowski, D.; Jaochico, A.; Dean, B.; Liang, X. Robust and comprehensive targeted metabolomics method for quantification of 50 different primary, secondary, and sulfated bile acids in multiple biological species (human, monkey, rabbit, dog, and rat) and matrices (plasma and urine) using liquid chromatography high resolution mass spectrometry (lc-hrms) analysis. J. Am. Soc. Mass. Spectrom. 2021, 32, 2033–2049. [Google Scholar] [CrossRef] [PubMed]
- Sarafian, M.H.; Lewis, M.R.; Pechlivanis, A.; Ralphs, S.; McPhail, M.J.W.; Patel, V.C.; Dumas, M.-E.; Holmes, E.; Nicholson, J.K. Bile acid profiling and quantification in biofluids using ultra-performance liquid chromatography tandem mass spectrometry. Anal. Chem. 2015, 87, 9662–9670. [Google Scholar] [CrossRef]
- Sato, Y.; Atarashi, K.; Plichta, D.R.; Arai, Y.; Sasajima, S.; Kearney, S.M.; Suda, W.; Takeshita, K.; Sasaki, T.; Okamoto, S.; et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 2021, 599, 458–464. [Google Scholar] [CrossRef]
- Scherer, M.; Gnewuch, C.; Schmitz, G.; Liebisch, G. Rapid quantification of bile acids and their conjugates in serum by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 3920–3925. [Google Scholar] [CrossRef]
- Shafaei, A.; Rees, J.; Christophersen, C.T.; Devine, A.; Broadhurst, D.; Boyce, M.C. Extraction and quantitative determination of bile acids in feces. Anal. Chim. Acta 2021, 1150, 338224. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kaneko, R.; Nomura, M.; Naito, H.; Kitamori, K.; Nakajima, T.; Ogawa, T.; Hattori, H.; Seno, H.; Ishii, A. Simple and rapid quantitation of 21 bile acids in rat serum and liver by uplc-ms-ms: Effect of high fat diet on glycine conjugates of rat bile acids. Nagoya J. Med. Sci. 2013, 75, 57–71. [Google Scholar]
- Tagliacozzi, D.; Mozzi, A.F.; Casetta, B.; Bertucci, P.; Bernardini, S.; Di Ilio, C.; Urbani, A.; Federici, G. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: A simple and rapid one-step method. Clin. Chem. Lab. Med. 2003, 41, 1633–1641. [Google Scholar] [CrossRef]
- Wang, S.; Martins, R.; Sullivan, M.C.; Friedman, E.S.; Misic, A.M.; El-Fahmawi, A.; De Martinis, E.C.P.; O’Brien, K.; Chen, Y.; Bradley, C.; et al. Diet-induced remission in chronic enteropathy is associated with altered microbial community structure and synthesis of secondary bile acids. Microbiome 2019, 7, 126. [Google Scholar] [CrossRef]
- Wang, T.; Lin, S.; Liu, R.; Li, H.; Liu, Z.; Xu, H.; Li, Q.; Bi, K. Acute lung injury therapeutic mechanism exploration for chinese classic prescription qingzao jiufei decoction by uflc-ms/ms quantification of bile acids, fatty acids and eicosanoids in rats. J. Pharm. Biomed. Anal. 2020, 189, 113463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Yang, J.; Ren, F.; Li, Y. Quantitative profiling of bile acids in feces of humans and rodents by ultra-high-performance liquid chromatography–quadrupole time-of-flight mass spectrometry. Metabolites 2022, 12, 633. [Google Scholar] [CrossRef] [PubMed]
- Jergens, A.E.; Schreiner, C.A.; Frank, D.E.; Niyo, Y.; Ahrens, F.E.; Eckersall, P.D.; Benson, T.J.; Evans, R. A scoring index for disease activity in canine inflammatory bowel disease. J. Vet. Intern. Med. 2003, 17, 291–297. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Ich Guideline m10 on Bioanalytical Method Validation and Study Sample Analysis. 2019. Available online: https://www.ema.europa.eu/ (accessed on 7 October 2024).
- Fernández-Metzler, C.; Ackermann, B.; Garofolo, F.; Arnold, M.E.; DeSilva, B.; Gu, H.; Laterza, O.; Mao, Y.; Rose, M.; Vazvaei-Smith, F.; et al. Biomarker assay validation by mass spectrometry. Aaps J. 2022, 24, 66. [Google Scholar] [CrossRef]
- Morlacchi, P.; Ramos, R.J.F.; Deckers, C.; Zhao, L.; Sartain, M.; Cuthbertson, D.; Cross, J.R. (Eds.) Mp 595 deciphering the microbiome: A targeted lc/ms/ms method for the comprehensive analysis of bile acids in biological samples. In Proceedings of the ASMS, Anaheim, CA, USA, 2–6 June 2024. [Google Scholar]
- Bile Acids Malabsorption Panel, Serum and Feces Mayo Foundation for Medical Education and Research2024. Available online: https://www.mayocliniclabs.com/test-catalog/overview/619919#Specimen (accessed on 11 May 2024).
- Neuberger-Castillo, L.; Ammerlaan, W.; Betsou, F. Fitness for purpose of stabilized stool samples for bile acid metabolite analyses. Sci. Rep. 2021, 11, 7904. [Google Scholar] [CrossRef]
- Strasberg, S.M.; Grossman, J.G.; Sullivan, R.B.; Stoentcheva, T.; Worley, L.A. The hydrocholeretic bile acid dehydrocholic acid is a safe and moderately effective treatment for recurrent acute cholangitis due to biliary stasis. HPB 2019, 21, S176–S177. [Google Scholar] [CrossRef]
- Reiter, S.; Dunkel, A.; Dawid, C.; Hofmann, T. Targeted lc-ms/ms profiling of bile acids in various animal tissues. J. Agric. Food Chem. 2021, 69, 10572–10580. [Google Scholar] [CrossRef]
- Zheng, J.J.; Shields, E.E.; Snow, K.J.; Nelson, D.M.; Olah, T.V.; Reily, M.D.; Robertson, D.G.; Shipkova, P.A.; Stryker, S.A.; Xin, B.; et al. The utility of stable isotope labeled (SIL) analogues in the bioanalysis of endogenous compounds by lc-ms applied to the study of bile acids in a metabolomics assay. Anal. Biochem. 2016, 503, 71–78. [Google Scholar] [CrossRef]
- Shiffka, S.J.; Kane, M.A.; Swaan, P.W. Planar bile acids in health and disease. Biochim. Biophys. Acta Biomembr. 2017, 1859, 2269–2276. [Google Scholar] [CrossRef]
- Tadano, T.; Kanoh, M.; Kondoh, H.; Matsumoto, M.; Mimura, K.; Kanoh, Y.; Sakamoto, K.; Kamano, T. Kinetic analysis of bile acids in the feces of colorectal cancer patients by gas chromatography-mass spectrometry (gc-ms). Rinsho byori. Jpn. J. Clin. Pathol. 2007, 55, 417–427. [Google Scholar]
- Makino, I.; Sjövall, J.; Norman, A.; Strandvik, B. Excretion of 3beta-hydroxy-5-cholenoic and 3a-hydroxy-5a-cholanoic acids in urine of infants with biliary atresia. FEBS Lett. 1971, 15, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.D.; Myers, C.J.; Harris, S.C.; Kakiyama, G.; Lee, I.-K.; Yun, B.-S.; Matsuzaki, K.; Furukawa, M.; Min, H.-K.; Bajaj, J.S.; et al. Bile acid 7α-dehydroxylating gut bacteria secrete antibiotics that inhibit clostridium difficile: Role of secondary bile acids. Cell Chem. Biol. 2019, 26, 27–34.e24. [Google Scholar] [CrossRef] [PubMed]
- Takei, H.; Narushima, S.; Suzuki, M.; Kakiyama, G.; Sasaki, T.; Murai, T.; Yamashiro, Y.; Nittono, H. Characterization of long-chain fatty acid-linked bile acids: A major conjugation form of 3β-hydroxy bile acids in feces. J. Lipid Res. 2022, 63, 100275. [Google Scholar] [CrossRef] [PubMed]
- Jover, A.; Meijide, F.; Rodríguez Núñez, E.; Vázquez Tato, J.; Castiñeiras, A.; Hofmann, A.F.; Ton-nu, H.T. Crystal structure of 3β,12α-dihydroxy-5β-cholan-24-oic acid (iso-deoxycholic acid). J. Mol. Struct. 2000, 523, 299–307. [Google Scholar] [CrossRef]
- Thanissery, R.; Winston, J.A.; Theriot, C.M. Inhibition of spore germination, growth, and toxin activity of clinically relevant c. Difficile strains by gut microbiota derived secondary bile acids. Anaerobe 2017, 45, 86–100. [Google Scholar] [CrossRef]
- Campbell, C.; McKenney, P.T.; Konstantinovsky, D.; Isaeva, O.I.; Schizas, M.; Verter, J.; Mai, C.; Jin, W.B.; Guo, C.J.; Violante, S.; et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory t cells. Nature 2020, 581, 475–479. [Google Scholar] [CrossRef]
- Devlin, A.S.; Fischbach, M.A. A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat. Chem. Biol. 2015, 11, 685–690. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Xie, G.; Raufman, J.P.; Hogan, S.; Griffin, T.L.; Packard, C.A.; Chatfield, D.A.; Hagey, L.R.; Steinbach, J.H.; Hofmann, A.F. Human cecal bile acids: Concentration and spectrum. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G256–G263. [Google Scholar] [CrossRef]
- Li, Q.; Larouche-Lebel, É.; Loughran, K.A.; Huh, T.P.; Suchodolski, J.S.; Oyama, M.A. Gut dysbiosis and its associations with gut microbiota-derived metabolites in dogs with myxomatous mitral valve disease. mSystems 2021, 6, 2. [Google Scholar] [CrossRef]
- Giaretta, P.R.; Rech, R.R.; Guard, B.C.; Blake, A.B.; Blick, A.K.; Steiner, J.M.; Lidbury, J.A.; Cook, A.K.; Hanifeh, M.; Spillmann, T.; et al. Comparison of intestinal expression of the apical sodium-dependent bile acid transporter between dogs with and without chronic inflammatory enteropathy. J. Vet. Intern. Med. 2018, 32, 1918–1926. [Google Scholar] [CrossRef]
- Blake, A.B.; Guard, B.C.; Honneffer, J.B.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Altered microbiota, fecal lactate, and fecal bile acids in dogs with gastrointestinal disease. PLoS ONE 2019, 14, 224454. [Google Scholar] [CrossRef] [PubMed]
- Guard, B.C.; Honneffer, J.B.; Jergens, A.E.; Jonika, M.M.; Toresson, L.; Lawrence, Y.A.; Webb, C.B.; Hill, S.; Lidbury, J.A.; Steiner, J.M.; et al. Longitudinal assessment of microbial dysbiosis, fecal unconjugated bile acid concentrations, and disease activity in dogs with steroid-responsive chronic inflammatory enteropathy. J. Vet. Intern. Med. 2019, 33, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.; Weidenmaier, C.; Timberlake, S.; Gura Sadovsky, R. Depletion of key gut bacteria predicts disrupted bile acid metabolism in inflammatory bowel disease. Microbiol. Spectr. 2024, 13, e01999-24. [Google Scholar] [CrossRef] [PubMed]
- AlShawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 2017, 93, fix136. [Google Scholar] [CrossRef]
- Pilla, R.; Guard, B.C.; Blake, A.B.; Ackermann, M.; Webb, C.; Hill, S.; Lidbury, J.A.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Long-term recovery of the fecal microbiome and metabolome of dogs with steroid-responsive enteropathy. Animals 2021, 11, 2498. [Google Scholar] [CrossRef]
- Guzior, D.V.; Quinn, R.A. Review: Microbial transformations of human bile acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef]
- Thomas, C.; Pellicciari, R.; Pruzanski, M.; Auwerx, J.; Schoonjans, K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 2008, 7, 678. [Google Scholar] [CrossRef]
- Jia, W.; Li, Y.; Cheung, K.C.P.; Zheng, X. Bile acid signaling in the regulation of whole body metabolic and immunological homeostasis. Sci. China Life Sci. 2024, 67, 865–878. [Google Scholar] [CrossRef]
- Rhimi, S.; Jablaoui, A.; Hernandez, J.; Mariaule, V.; Akermi, N.; Méric, T.; Mkaouar, H.; Wysocka, M.; Lesner, A.; Borgi, M.A.; et al. Industrial diet intervention modulates the interplay between gut microbiota and host in semi-stray dogs. Anim. Microbiome 2024, 6, 69. [Google Scholar] [CrossRef]
- Eyssen, H.J.; De Pauw, G.; Van Eldere, J. Formation of hyodeoxycholic acid from muricholic acid and hyocholic acid by an unidentified gram-positive rod termed HDCA-1 isolated from rat intestinal microflora. Appl. Env. Microbiol. 1999, 65, 3158–3163. [Google Scholar] [CrossRef]
- Madsen, D.; Beaver, M.; Chang, L.; Bruckner-Kardoss, E.; Wostmann, B. Analysis of bile acids in conventional and germfree rats. J. Lipid Res. 1976, 17, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.Ø.; Julienne, H.; Hyötyläinen, T.; Sen, P.; Fan, Y.; Pedersen, H.K.; Jäntti, S.; Hansen, T.H.; Nielsen, T.; Jørgensen, T.; et al. Conjugated c-6 hydroxylated bile acids in serum relate to human metabolic health and gut clostridia species. Sci. Rep. 2021, 11, 13252. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Y.; Li, R.; Zhang, X.; Chen, T.; Mei, F.; Liu, R.; Chen, M.; Ge, Y.; Hu, H.; et al. Gut microbial metabolite hyodeoxycholic acid targets the tlr4/md2 complex to attenuate inflammation and protect against sepsis. Mol. Ther. 2023, 31, 1017–1032. [Google Scholar] [CrossRef]



| Agilent 1260 Binary Pump | Agilent 1290 Binary Pump | ||||||
|---|---|---|---|---|---|---|---|
| Time (min) | A (%) | B (%) | Flow (mL/min) | Time (min) | A (%) | B (%) | Flow (mL/min) |
| 2.1 | 90 | 10 | 0.30 | 2 | 90 | 10 | 0.4 |
| 2.5 | 90 | 10 | 0.05 | 2.1 | 45 | 55 | 0.4 |
| 7.8 | 90 | 10 | 0.05 | 16 | 18 | 82 | 0.4 |
| 7.9 | 90 | 10 | 0.30 | 16.1 | 1 | 99 | 0.4 |
| 8 | 50 | 50 | 0.30 | 18 | 1 | 99 | 0.4 |
| 9 | 5 | 95 | 0.30 | 18.01 | 90 | 10 | 0.4 |
| 13 | 1 | 99 | 0.30 | 20 | 90 | 10 | 0.4 |
| 18 | 1 | 99 | 0.30 | ||||
| 18.01 | 90 | 10 | 0.30 | ||||
| 20 | 90 | 10 | 0.30 | ||||
| H | CE | |||
|---|---|---|---|---|
| Bile Acid | Median [Min–Max] ng/mg Fecal Dry Matter | Median [Min–Max] ng/mg Fecal Dry Matter | p-Value | q-Value |
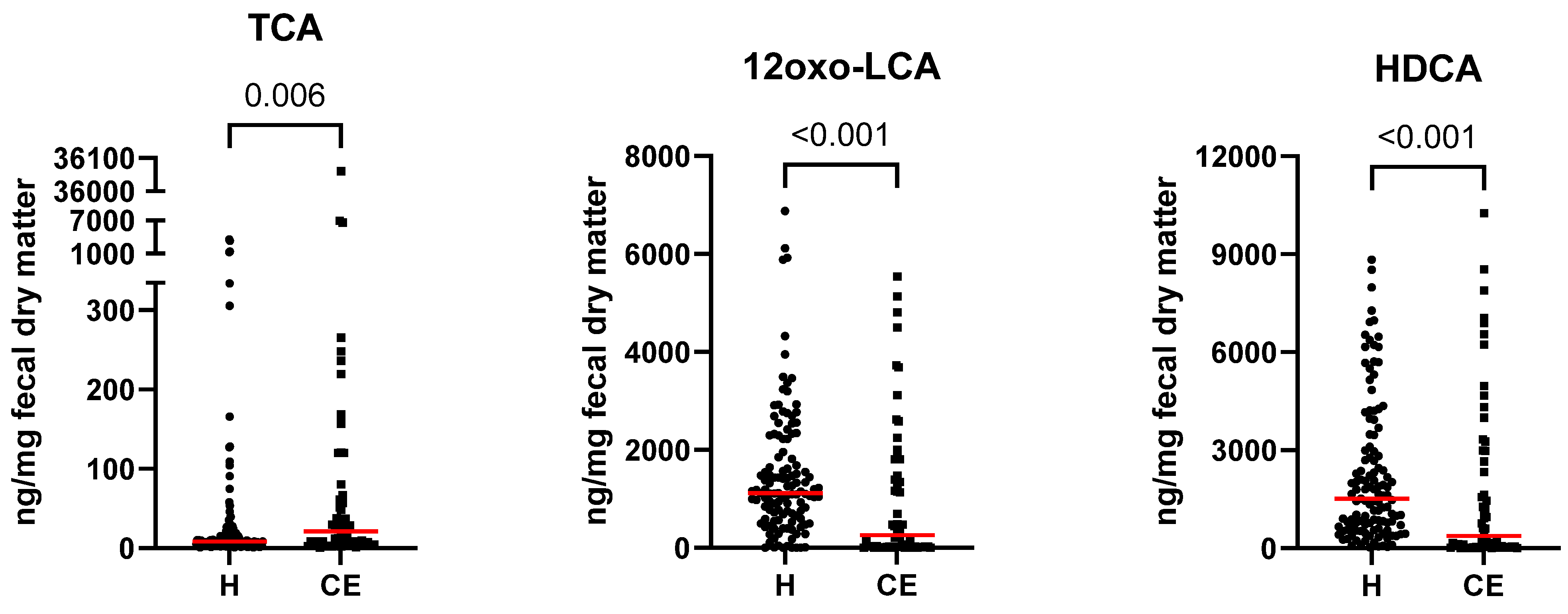
| 12oxo-lithocholic acid (12oxo-LCA) | 1118.50 [0.14–6879.17] | 256.77 [0.02–5539.55] | 0.000 | 0.000 |
| 3oxo-cholic acid (3oxo-CA) | 5.91 [0.05–220.8] | 18.13 [0.14–217.62] | 0.008 | 0.014 |
| 3oxo-chenodeoxycholic acid (3oxo-CDCA) | 4.10 [0.28–185.3] | 3.86 [0.49–78.79] | 0.333 | 0.409 |
| 3oxo-deoxycholic acid (3oxo-DCA) | 471.14 [0.38–5447.33] | 74.99 [0.02–5172.46] | 0.000 | 0.000 |
| 7,12oxo-lithocholic acid (7,12oxo-LCA) | 2.85 [0.15–138.79] | 5.56 [0.04–174.47] | 0.156 | 0.211 |
| 7oxo-deoxycholic acid (7oxo-DCA) | 71.56 [3.36–5577.38] | 676.41 [0.35–9438.78] | 0.000 | 0.000 |
| 7oxo-lithocholic acid (7oxo-LCA) | 36.97 [2.79–224.32] | 67.22 [2.63–301.46] | 0.002 | 0.004 |
| cholic acid (CA) | 81.70 [2.91–24,299.77] | 1308.27 [10.44–32,283.47] | 0.000 | 0.000 |
| chenodeoxycholic acid (CDCA) | 51.03 [1.86–2130.86] | 106.03 [2.65–1983.05] | 0.018 | 0.028 |
| deoxycholic acid (DCA) | 3134.37 [2.10–8831.39] | 1798.97 [1.57–5680.31] | 0.000 | 0.000 |
| glycocholic acid (GCA) | 0.38 [0.02–45.75] | 2.98 [0.02–269.05] | 0.000 | 0.000 |
| glycochenodeoxycholic acid (GCDCA) | 0.30 [0–28.18] | 0.29 [0.01–7.30] | 0.962 | 0.962 |
| glycodeoxycholic acid (GDCA) | 1.73 [0.01–13.78] | 1.82 [0.01–51.40] | 0.704 | 0.761 |
| glycolithocholic acid (GLCA) | 0.45 [0.01–2.99] | 0.18 [0–3.62] | 0.000 | 0.001 |
| glycoursodeoxycholic acid (GUDCA) | 0.03 [0–0.35] | 0.02 [0–1.24] | 0.802 | 0.833 |
| hyocholic acid (HCA) | 1.31 [0.04–25.57] | 0.56 [0.04–24.49] | 0.000 | 0.000 |
| hyodeoxycholic acid (HDCA) | 1517.30 [25.70–8831.39] | 373.08 [0.44–10,256.23] | 0.000 | 0.001 |
| iso-Cholic acid (Iso-CA) | 8.36 [0.37–354.99] | 44.41 [0.84–670.61] | 0.000 | 0.000 |
| iso-Chenodeoxycholic acid (Iso-CDCA) | 308.21 [2.62–797.28] | 39.61 [0.03–1025.65] | 0.000 | 0.000 |
| lithocholic acid (LCA) | 680.73 [2.85–5409.83] | 431.18 [0.21–10,256.23] | 0.034 | 0.051 |
| taurocholic acid (TCA) | 8.24 [0.96–3564.24] | 20.91 [0.74–36,059.81] | 0.006 | 0.011 |
| taurochenodeoxycholic acid (TCDCA) | 1.98 [0.08–454.54] | 2.46 [0.09–1900.07] | 0.189 | 0.243 |
| taurodeoxycholic acid (TDCA) | 14.10 [0.12–775.86] | 18.87 [0.03–1032.81] | 0.502 | 0.589 |
| taurolithocholic acid (TLCA) | 0.98 [0.02–61.17] | 0.95 [0.01–77.58] | 0.704 | 0.761 |
| tauroursodeoxycholic acid (TUDCA) | 0.13 [0–9.47] | 0.61 [0.01–156.45] | 0.000 | 0.000 |
| ursocholic acid (UCA) | 15.36 [0.70–557.75] | 65.37 [0.05–1053.34] | 0.000 | 0.000 |
| ursodeoxycholic acid (UDCA) | 20.55 [1.39–208.06] | 27.83 [1.35–451.24] | 0.126 | 0.179 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blake, A.B.; Toresson, L.C.; Chen, C.-C.; Ishii, P.E.; Phillips, R.K.; Giaretta, P.R.; Cavasin, J.P.; Lidbury, J.A.; Suchodolski, J.S. Altered iso- and oxo-Fecal Bile Acid Concentrations in Dogs with Chronic Enteropathy. Pets 2025, 2, 18. https://doi.org/10.3390/pets2020018
Blake AB, Toresson LC, Chen C-C, Ishii PE, Phillips RK, Giaretta PR, Cavasin JP, Lidbury JA, Suchodolski JS. Altered iso- and oxo-Fecal Bile Acid Concentrations in Dogs with Chronic Enteropathy. Pets. 2025; 2(2):18. https://doi.org/10.3390/pets2020018
Chicago/Turabian StyleBlake, Amanda B., Linda C. Toresson, Chih-Chun Chen, Patricia E. Ishii, Robert Kyle Phillips, Paula R. Giaretta, Joao P. Cavasin, Jonathan A. Lidbury, and Jan S. Suchodolski. 2025. "Altered iso- and oxo-Fecal Bile Acid Concentrations in Dogs with Chronic Enteropathy" Pets 2, no. 2: 18. https://doi.org/10.3390/pets2020018
APA StyleBlake, A. B., Toresson, L. C., Chen, C.-C., Ishii, P. E., Phillips, R. K., Giaretta, P. R., Cavasin, J. P., Lidbury, J. A., & Suchodolski, J. S. (2025). Altered iso- and oxo-Fecal Bile Acid Concentrations in Dogs with Chronic Enteropathy. Pets, 2(2), 18. https://doi.org/10.3390/pets2020018