A Protocol for Fecal Microbiota Transplantation Using Freeze-Dried Capsules: Dosage and Outcomes in 171 Dogs with Chronic Enteropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Populations
2.3. Donors Selection
2.4. FMT Preparation
2.5. FMT Administration Protocol
2.6. Variables and Data Sources
- The clinical response, as assessed through a global clinical evaluation conducted 15 days post-treatment, which classified subjects as either “responders” (R) or “non-responders” (NR) based on their improvement. Subjects exhibiting positive clinical outcomes following fecal microbiota transplantation (FMT) were labeled as responders (R), while those showing no significant improvement were classified as non-responders (NR). This classification was made by veterinarians based on their professional judgment during a clinical examination. The evaluation process involved completing a questionnaire that included the assessment of animal weight, body condition score (BCS), the CIBDAI score, and 15 clinical signs associated with chronic enteropathy (CE);
- The clinical response, calculated as the variation of the mean value of 15 clinical signs of CE (see Table 1 for the complete list): for each indicator, a score ranging from 1 to 5 indicated the rising severity of the related clinical sign in the active phase of the disease;
- The clinical response, calculated as the variation of the CIBDAI score: a score ranging from 0 to 3 indicated the rising severity of the related clinical sign for each indicator included in the CIBDAI, both before and after FMT, and the total composite CIBDAI score was then obtained as the sum of the single scores;
- The type of capsule (size 4 or size 1, renamed from now on as “S” or “L”) received daily by each subject;
- The dose of freeze-dried FMT material administered to each subject daily, expressed as mg/kg body weight (BW); this dose includes fecal material, trehalose, and sodium chloride (see “FMT Preparation and Administration”);
- The dose of freeze-dried fecal material administered to each subject daily, expressed as mg/kg BW, i.e., the quantity of freeze-dried FMT material, discarding trehalose and sodium chloride contribution;
- The quantification of alive bacterial cells administered daily with the FMT treatment, quantified as Active Fluorescent Unit (AFU)/kg BW by flow cytometry analysis;
- The quantification of total bacterial cells administered daily with the FMT treatment, quantified as Total Fluorescent Unit (TFU)/kg BW by flow cytometry analysis.
2.7. Statistical Methods
3. Results
3.1. Animals Included in the Study
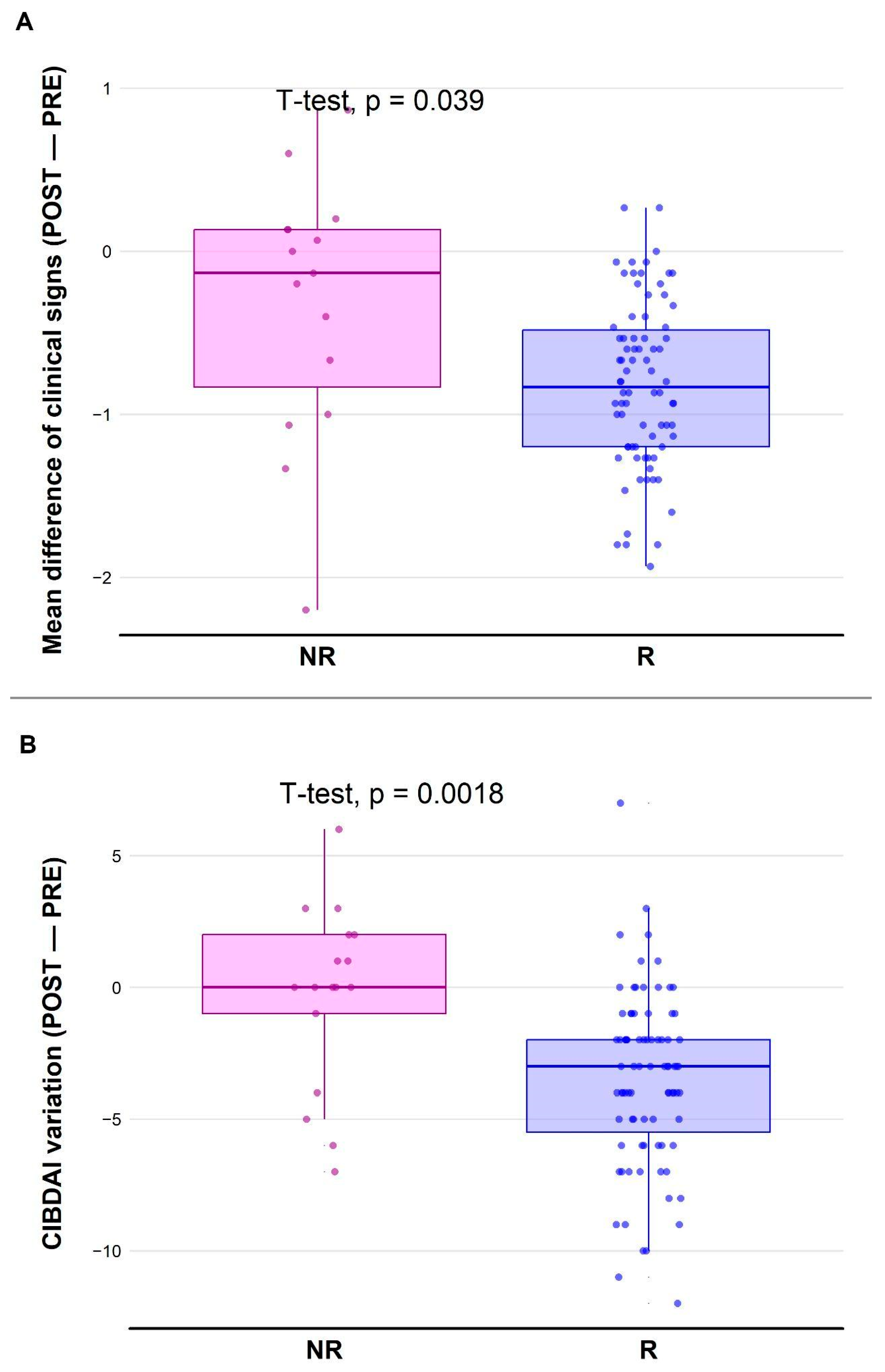
3.2. Coherence of R/NR Labeling with Clinical Signs
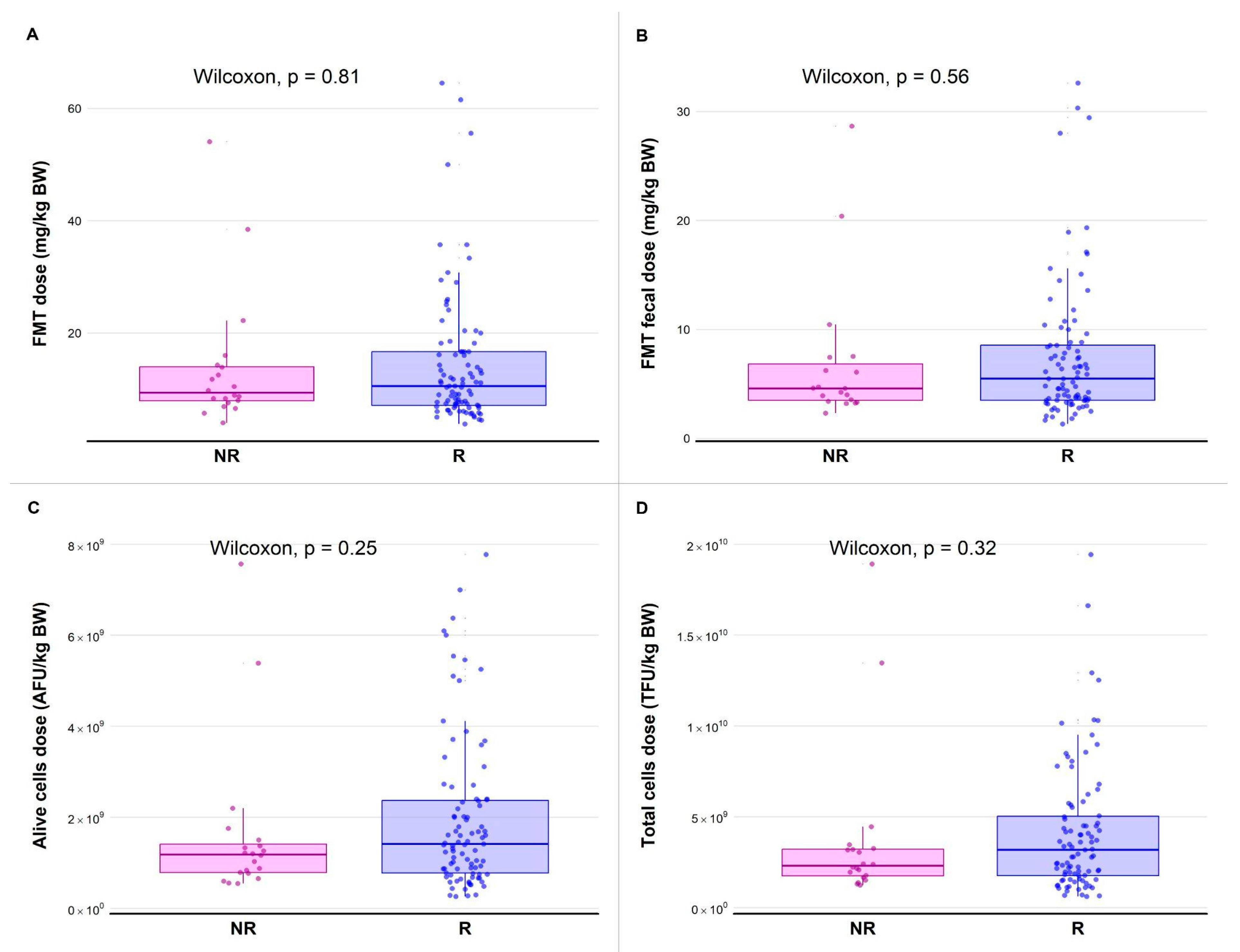
3.3. Administered Dose Quantification
3.4. Clinical Efficacy of Capsule FMT (cFMT)
3.4.1. Relationship Between Capsule Size and Response
3.4.2. Relationship Between Dose and Variation of the Mean of CE Clinical Signs
3.4.3. Relationship Between Dose and Variation of CIBDAI
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khoruts, A.; Sadowsky, M.J. Understanding the mechanisms of faecal microbiota transplantation. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 508–516. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; E Coffin, S.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Hediyal, T.A.; Vichitra, C.; Anand, N.; Bhaskaran, M.; Essa, S.M.; Kumar, P.; Qoronfleh, M.W.; Akbar, M.; Kaul-Ghanekar, R.; Mahalakshmi, A.M.; et al. Protective effects of fecal microbiota transplantation against ischemic stroke and other neurological disorders: An update. Front. Immunol. 2024, 15, 1324018. [Google Scholar] [CrossRef] [PubMed]
- Libertucci, J.; Young, V.B. The role of the microbiota in infectious diseases. Nat. Microbiol. 2019, 4, 35–45. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Frese, S. Nutrition in the Control of Inflammation: Emerging Roles for the Microbiome and Epigenome; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Jung Lee, W.; Lattimer, L.D.N.; Stephen, S.; Borum, M.L.; Doman, D.B. Fecal Microbiota Transplantation: A Review of Emerging Indications Beyond Relapsing Clostridium difficile Toxin Colitis. Gastroenterol. Hepatol. 2015, 11, 24–32. [Google Scholar]
- Xu, H.; Cao, C.; Ren, Y.; Weng, S.; Liu, L.; Guo, C.; Wang, L.; Han, X.; Ren, J.; Liu, Z. Antitumor effects of fecal microbiota transplantation: Implications for microbiome modulation in cancer treatment. Front. Immunol. 2022, 13, 949490. [Google Scholar] [CrossRef]
- Zikou, E.; Koliaki, C.; Makrilakis, K. The Role of Fecal Microbiota Transplantation (FMT) in the Management of Metabolic Diseases in Humans: A Narrative Review. Biomedicines 2024, 12, 1871. [Google Scholar] [CrossRef]
- Niina, A.; Kibe, R.; Suzuki, R.; Yuchi, Y.; Teshima, T.; Matsumoto, H.; Kataoka, Y.; Koyama, H. Improvement in Clinical Symptoms and Fecal Microbiome After Fecal Microbiota Transplantation in a Dog with Inflammatory Bowel Disease. Vet. Med. Res. Rep. 2019, 10, 197–201. [Google Scholar] [CrossRef]
- Chaitman, J.; Gaschen, F. Fecal Microbiota Transplantation in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 219–233. [Google Scholar] [CrossRef]
- Pérez-Accino, J.; Salavati, M.; Glendinning, L.; Salavati Schmitz, S. Effect of a single rectal fecal microbiota transplantation on clinical severity and fecal microbial communities in dogs with chronic inflammatory enteropathy. J. Vet. Intern. Med. 2025, 39, e17264. [Google Scholar] [CrossRef]
- Toresson, L.; Spillmann, T.; Pilla, R.; Ludvigsson, U.; Hellgren, J.; Olmedal, G.; Suchodolski, J.S. Clinical Effects of Faecal Microbiota Transplantation as Adjunctive Therapy in Dogs with Chronic Enteropathies-A Retrospective Case Series of 41 Dogs. Vet. Sci. 2023, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Innocente, G.; Patuzzi, I.; Furlanello, T.; Di Camillo, B.; Bargelloni, L.; Giron, M.C.; Facchin, S.; Savarino, E.; Azzolin, M.; Simionati, B. Machine Learning and Canine Chronic Enteropathies: A New Approach to Investigate FMT Effects. Vet. Sci. 2022, 9, 502. [Google Scholar] [CrossRef]
- Winston, J.A.; Suchodolski, J.S.; Gaschen, F.; Busch, K.; Marsilio, S.; Costa, M.C.; Chaitman, J.; Coffey, E.L.; Dandrieux, J.R.; Gal, A.; et al. Clinical Guidelines for Fecal Microbiota Transplantation in Companion Animals. Adv. Small Anim. Care 2024, 5, 79–107. [Google Scholar] [CrossRef]
- Ademe, M. Benefits of fecal microbiota transplantation: A comprehensive review. J. Infect. Dev. Ctries. 2020, 14, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, M.N.; Shaheen, W.; Oo, Y.H.; Iqbal, T.H. Immunological mechanisms underpinning faecal microbiota transplantation for the treatment of inflammatory bowel disease. Clin. Exp. Immunol. 2020, 199, 24–38. [Google Scholar] [CrossRef]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef]
- Laflamme, D.P. Development and validation of a body condition score system for dogs. Canine Pract. 1997, 22, 10–15. [Google Scholar]
- Jeusette, I.; Greco, D.; Aquino, F.; Detilleux, J.; Peterson, M.; Romano, V.; Torre, C. Effect of breed on body composition and comparison between various methods to estimate body composition in dogs. Res. Vet. Sci. 2010, 88, 227–232. [Google Scholar] [CrossRef]
- Langlois, D.K.; Koenigshof, A.M.; Mani, R. Metronidazole treatment of acute diarrhea in dogs: A randomized double blinded placebo-controlled clinical trial. J. Vet. Intern. Med. 2020, 34, 98–104. [Google Scholar] [CrossRef]
- ISO 19344:2015; Milk and milk products—Starter cultures, probiotics and fermented products—Quantification of lactic acid bacteria by flow cytometry. ISO: Geneva, Sweden, 2015. Available online: https://www.iso.org/standard/64658.html (accessed on 1 February 2025).
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Staley, C.; Hamilton, M.J.; Vaughn, B.P.; Graiziger, C.T.; Newman, K.M.; Kabage, A.J.; Sadowsky, M.J.; Khoruts, A. Successful Resolution of Recurrent Clostridium difficile Infection using Freeze-Dried, Encapsulated Fecal Microbiota; Pragmatic Cohort Study. Am. J. Gastroenterol. 2017, 112, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Ramai, D.; Zakhia, K.; Fields, P.J.; Ofosu, A.; Patel, G.; Shahnazarian, V.; Lai, J.K.; Dhaliwal, A.; Reddy, M.; Chang, S. Fecal Microbiota Transplantation (FMT) with Colonoscopy Is Superior to Enema and Nasogastric Tube While Comparable to Capsule for the Treatment of Recurrent Clostridioides difficile Infection: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2021, 66, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Haifer, C.; Paramsothy, S.; O Kaakoush, N.; Saikal, A.; Ghaly, S.; Yang, T.; Luu, L.D.W.; Borody, T.J.; Leong, R.W. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): A randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2022, 7, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Seekatz, A.M.; Theriot, C.M.; Rao, K.; Chang, Y.-M.; Freeman, A.E.; Kao, J.Y.; Young, V.B. Restoration of short chain fatty acid and bile acid metabolism following fecal microbiota transplantation in patients with recurrent Clostridium difficile infection. Anaerobe 2018, 53, 64–73. [Google Scholar] [CrossRef]

| Clinical Signs | Not at All (1) | Little (2) | Moderate (3) | High (4) | Very High (5) | I Don’t Know |
|---|---|---|---|---|---|---|
| Diarrhea | ||||||
| Mucus in stool | ||||||
| Blood in stool | ||||||
| Vomiting | ||||||
| Reflux | ||||||
| Nausea | ||||||
| Pica | ||||||
| Wight loss | ||||||
| Loss of vitality | ||||||
| Lack of appetite | ||||||
| Restlessness | ||||||
| Itching | ||||||
| Abdominal pain | ||||||
| Flatulence | ||||||
| Borborigmi |
| Age (years) | Mean | 7.41 |
| Median | 6.59 | |
| SD | 4.02 | |
| Min | 1.49 | |
| Max | 17.07 | |
| Duration of disease (weeks) | Mean | 110.5 |
| Median | 77.64 | |
| SD | 95.76 | |
| Min | 5.29 | |
| Max | 541.57 | |
| CIBDAI | Mean | 5.41 |
| Median | 5 | |
| SD | 3.33 | |
| Min | 0 | |
| Max | 16 | |
| Sex (%) | F | 43 |
| M | 57 | |
| Top 5 breeds (%) | Mixed breed dogs | 17.12 |
| German Shepherd | 14.41 | |
| Chihuahua | 5.41 | |
| French Bulldog | 4.5 | |
| Golden Retriever | 3.6 |
| Clinical Sign | Prevalence PRE | Prevalence POST |
|---|---|---|
| Diarrhea | 92.5% | 58.8% |
| Borborygmi | 75.0% | 47.0% |
| Flatulence | 68.0% | 48.0% |
| Mucus in stool | 67.0% | 41.2% |
| Weight loss | 64.8% | 19.6% |
| Nausea | 60.6% | 33.3% |
| Abdominal pain | 58.8% | 25.7% |
| Vomiting | 58.5% | 21.6% |
| Loss of vitality | 57.5% | 20.4% |
| Restlessness | 56.2% | 23.3% |
| Loss of appetite | 51.9% | 26.2% |
| Reflux | 42.9% | 22.2% |
| Blood in stool | 41.9% | 11.8% |
| Itching | 40.6% | 30.4% |
| Pica | 40.4% | 24.5% |
| Capsule Size | NR | R | %R |
| S | 4 | 28 | 87.50% |
| L | 16 | 63 | 79.75% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brugnoli, F.; Simionati, B.; Patuzzi, I.; Lombardi, A.; Giron, M.C.; Savarino, E.; Facchin, S.; Innocente, G. A Protocol for Fecal Microbiota Transplantation Using Freeze-Dried Capsules: Dosage and Outcomes in 171 Dogs with Chronic Enteropathy. Pets 2025, 2, 16. https://doi.org/10.3390/pets2020016
Brugnoli F, Simionati B, Patuzzi I, Lombardi A, Giron MC, Savarino E, Facchin S, Innocente G. A Protocol for Fecal Microbiota Transplantation Using Freeze-Dried Capsules: Dosage and Outcomes in 171 Dogs with Chronic Enteropathy. Pets. 2025; 2(2):16. https://doi.org/10.3390/pets2020016
Chicago/Turabian StyleBrugnoli, Francesca, Barbara Simionati, Ilaria Patuzzi, Angiolella Lombardi, Maria Cecilia Giron, Edoardo Savarino, Sonia Facchin, and Giada Innocente. 2025. "A Protocol for Fecal Microbiota Transplantation Using Freeze-Dried Capsules: Dosage and Outcomes in 171 Dogs with Chronic Enteropathy" Pets 2, no. 2: 16. https://doi.org/10.3390/pets2020016
APA StyleBrugnoli, F., Simionati, B., Patuzzi, I., Lombardi, A., Giron, M. C., Savarino, E., Facchin, S., & Innocente, G. (2025). A Protocol for Fecal Microbiota Transplantation Using Freeze-Dried Capsules: Dosage and Outcomes in 171 Dogs with Chronic Enteropathy. Pets, 2(2), 16. https://doi.org/10.3390/pets2020016






