Abstract
The vaginal microbiome can be perturbed by various intrinsic and extrinsic factors, resulting in a state of dysbiosis that decreases the abundance of commensal lactobacilli and often leads to pathological conditions such as bacterial vaginosis, yeast infections, sexually transmitted infections, and other vaginal disorders. This narrative review explores the molecular and pathophysiological mechanisms of several microbial diseases associated with the dysbiosis of the vaginal microbiome, as well as the efficacy of therapeutic tools for these conditions, such as antibiotic treatment and the use of live biotherapeutic products. A non-systematic, narrative approach was employed. Searches and data extraction were performed using the PubMed and Scopus databases between January and February 2025. All reviewed studies reported vaginal microbiome dysbiosis, with microbial pathogens inducing a specific immune response in the host. Current treatments for vaginal microbiota dysbiosis-related pathologies often result in high relapse and recurrence rates, suggesting microbial resistance and the need for alternative therapeutic strategies. In turn, live biotherapeutic products have demonstrated beneficial effects, restoring microbial balance in dysbiotic conditions. While these findings suggest promising potential for live biotherapeutic products, further rigorous clinical studies are necessary to gain a deeper understanding of the female genital tract ecosystem and to identify novel biomarkers along with their associated health implications. Moreover, the development of new diagnostic and management strategies will facilitate personalized therapeutic approaches. Ultimately, a comprehensive perspective on vaginal care is pivotal, taking into account both microbial and immune dynamics to enhance women’s health outcomes.
1. Introduction
The vaginal microbiome (VM) is a complex and dynamic microenvironment mainly composed of a diverse bacterial community, predominantly lactobacilli, which evolves throughout a woman’s life depending on age, sex hormone levels, and various extrinsic factors [1,2]. Several species of the genus Lactobacillus, including L. crispatus, L. iners, L. paragasseri (formerly L. gasseri), and L. mulieris (formerly L. jensenii), have been reported to be dominant in a healthy vagina [3]. In women of reproductive age, estrogen promotes the proliferation of vaginal epithelial cells, leading to glycogen accumulation, while progesterone induces epithelial cell lysis, facilitating glycogen release [4]. Furthermore, the anaerobic metabolism of glycogen by lactobacilli results in lactic acid production, which lowers the vaginal pH to approximately 4.5, creating an unfavorable ambient for microbial pathogens and preventing the colonization of this ecosystem [5,6]. In addition to acidification, lactobacilli contribute to vaginal defense through multiple mechanisms, including the production of antimicrobial compounds such as bacteriocins, biosurfactants, hydrogen peroxide, arginine deaminase, and co-aggregating molecules, which enhance the epithelial barrier function and also induce anti-inflammatory and immune responses [6,7,8,9]. Additionally, cervical mucus, which is primarily composed of mucins, protects the vaginal mucosa and enhances its barrier function against microbial colonization. Vaginal secretions further contain several proteins that exert antimicrobial activities, such as lactoferrin, lysozyme, α- and β-defensins, elafin, calprotectin, secretory leukocyte protease inhibitor, cathelicidin, and other antimicrobial peptides (AMPs) [10,11,12]. These secretions also contain immune mediators such as cytokines, chemokines, and immunoglobulins [13].
When the vaginal ecosystem is perturbed by various intrinsic (e.g., menstrual cycle, pregnancy, menopause) or extrinsic factors (e.g., diet, smoking, antibiotic treatment, stress, hygiene practices, sexual habits), the composition of the VM changes, leading to a state of dysbiosis characterized by the following: (i) a reduction in the abundance of lactobacilli and an increase in anaerobic bacteria with a decrease in microbial diversity; (ii) the production of amino compounds by the altered bacterial community; (iii) an elevation in vaginal pH above 4.5; and (iv) common pathological conditions such as bacterial vaginosis (BV), yeast infections, sexually transmitted infections (STIs), and other vaginal disturbances [14,15]. In addition, dysbiosis of the VM produces inflammatory responses and abnormal immune responses, all of which can contribute to a variety of female reproductive health problems [16]. Although dysbiosis is a concept that implies an imbalance or maladaptation of bacterial communities in an ecosystem, the concept of what constitutes a normal VM is still controversial, given that the vaginal microbiota is not stable and fluctuates throughout a woman’s life cycle [17]. Figure 1 shows the intrinsic and extrinsic factors influencing VM dysbiosis and several related consequences.
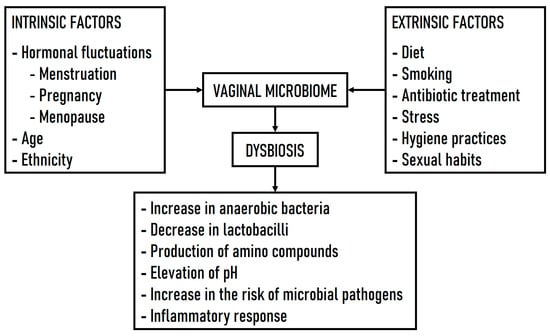
Figure 1.
Intrinsic and extrinsic factors influencing VM dysbiosis and related consequences.
Our understanding of the various types of dysbiosis and their association with urogenital and reproductive disorders has significantly expanded in recent years, driven by the application of molecular techniques. However, the clinical implications of these findings require careful evaluation. Given the complex interplay between microbial communities and host health, synthesizing emerging evidence is essential for refining diagnostic and clinical strategies. In this narrative review, we explore the molecular and pathophysiological mechanisms underlying microbial diseases linked to VM dysbiosis, including BV, aerobic vaginitis (AV), pelvic inflammatory disease (PID), vulvovaginal candidiasis (VVC), and STIs. In addition, we examine antibiotic therapies for these conditions and the use of live biotherapeutic products (LBPs), a novel class of therapeutics with diverse applications, particularly in restoring VM balance.
2. Methods
The present review adopts a non-systematic, narrative approach [18], with the primary aim of evaluating the existing literature on the VM, the pathogenic diseases associated with its dysbiosis, and microbial treatments for these conditions. To provide a comprehensive overview of the current state of research, data were gathered from a range of scientific studies. A search was conducted in the PubMed and Scopus databases to ensure broad access to relevant articles, without any publication date restrictions. No language restrictions were applied, allowing for a wider inclusion of studies, although search terms were limited to English. A combination of keywords related to the VM, dysbiosis-associated diseases, and therapeutic approaches was used, employing Boolean operators to refine the search strategy. Additionally, the reference lists of the relevant studies were reviewed to identify further pertinent articles. The selection process began with an initial retrieval based on predefined search criteria. Each article underwent a two-step screening process to ensure relevance. First, titles and abstracts were reviewed, and studies not directly related to the research focus were excluded. Second, full-text evaluations of the remaining articles were conducted to assess their relevance, particularly in terms of providing substantive information. Inclusion criteria were based on whether studies examined the relationship between VM dysbiosis and genitourinary microbial diseases, as well as treatment approaches for these conditions. Exclusion criteria included the following: (i) articles focusing on the physiological aspects of the female life cycle; (ii) opinion articles, perspective articles, theses, and conference proceedings; and (iii) articles lacking relevant or sufficient data regarding the central topic. Searches and data extraction were performed between January and February 2025. Articles deemed irrelevant based on initial assessments and established criteria were excluded, and only those studies that met all inclusion criteria and provided significant findings relevant to the study’s objectives were retained.
3. Bacterial Vaginosis
BV represents a dysbiosis of the VM, marked by a depletion of lactobacilli, with the exception of L. iners, due to a significant overgrowth of previously minor obligate or facultative anaerobes in the vaginal environment [19], such as Bifidobacterium vaginale (formerly Gardnerella vaginalis), Fannyhessea vaginae (formerly Atopobium vaginae), Ureaplasma urealyticum, Mycoplasma hominis, Prevotella bivia and P. timonensis, Peptoniphilus spp., Megasphaera spp., Mobiluncus spp., Anaerococcus spp., Bacteroides fragilis, Leptotrichia amnionii, Peptostreptococcus spp., Porphyromonas asaccharolytica, Snethia amnii and S. sanguinegens, and several fastidious BV-associated bacteria (BVAB-1 to 3) [15,20,21,22,23]. The latter have recently been taxonomically classified: BVAB-1 assigned to Clostridiales genomosp. BVAB-1, BVAB-2 to the Oscillospiraceae bacterium strain CHIC02, and BVAB-3 to Mageeibacillus indolicus [24]. Advances in molecular technology have allowed the inclusion of non-cultivable bacteria associated with BV, such as the Olegusella massiliensis strain KHD7T, Ezakiella massiliensis strain Marseille P2951T, and Corynebacterium fournierii strain Marseille P2948T [25,26,27].
The BV-associated microbiota forms a biofilm on the vaginal epithelium and produces a cytotoxin that can induce epithelial cell death [28]. In addition, B. vaginale and P. timonensis produce proteolytic enzymes, such as fucosidase and sialidase, which can degrade proteins, as well as decarboxylases that convert amino acids and polyamines [29]. If not degraded, biogenic amines, including polyamines such as agmatine, spermine, spermidine, cadaverine, putrescine, trimethylamine, and tyramine, produce a malodorous (fishy) scent due to an elevation in vaginal pH [30,31]. As a result, cytotoxicity arising from the interaction between organic acids present in the vagina during BV and bacterial polyamines leads to the production of vaginal discharge, which is provoked by the exfoliation of vaginal epithelial cells [32]. Moreover, B. vaginale and other anaerobic bacteria coat the vaginal epithelial cells, leading to the formation of “clue cells”, a distinctive feature of BV [33,34].
3.1. Diagnostic Methods for Bacterial Vaginosis
The diagnosis of BV is challenging due to the absence of a consensus on its definition, the natural variation in the VM across women of different ethnic backgrounds, and its polymicrobial etiology. Two classical methods have been used in the diagnosis of BV: Amsel criteria [35], which are mainly based on real-time clinical criteria, and the Nugent score [36], which consists of the evaluation of bacterial morphotypes on Gram stain. Nevertheless, the currently preferred clinical practice for the diagnosis of BV is Gram stain microscopy, as outlined by the Hay–Ison criteria [37], which is analogous to the Nugent score but easier and faster to use [38]. However, these methods have several shortcomings, such as the interference of other microorganisms in the Gram stain, the subjectivity in the identification of bacterial morphotypes, and the influence of individual skill and experience in the diagnosis and scoring. To avoid certain limitations, Wang et al. [39] presented a proof of concept for a model based on deep learning to quantify Gram staining and automated Nugent score classification.
To address these diagnostic challenges, alternative diagnostic approaches have been explored, such as BD Max vaginal panel, BV multiplex assays, NuSwab R multiplex qPCR, quantitative real-time PCR (qPCR), and SureSwab BV DNA qPCR assay [40,41,42]. The use of these molecular techniques allows for an accurate diagnosis of BV, with sensitivities ranging from 90.5% to 96.7% and specificities ranging from 85.8% to 95% when compared to Amsel criteria and the Nugent score [43]. Table 1 shows the comparison of reference diagnostic methods and molecular methods for the diagnosis of BV (performance data based on Coleman and Gaydos [43]).

Table 1.
Comparison of diagnostic methods for the diagnosis of BV.
Table 1.
Comparison of diagnostic methods for the diagnosis of BV.
| Test Method | Criteria | Advantages | Disadvantages | Performance Data |
|---|---|---|---|---|
| Reference Methods | ||||
| Amsel criteria [35] | Clinical/microscopy-based (wet mount microscopy/phase contrast)
| Rapid point-of-care test |
| Comparison with Nugent score:
|
| Nugent score [36] | Microscopy-based method (Gram stain) 0–3: No indication of BV 4–6: No conclusive indication of BV 7–10: Indication of BV | Objective criteria |
| Comparison with Amsel criteria:
|
| Hay–Ison criteria [37] | Microscopy-based method (Gram-stain) 0: No bacteria1: No indication of BV 2: No conclusive indication of BV 3: Indication of BV 4: Gram-positive cocci | Objective criteria |
| Comparison with Amsel criteria:
|
| Molecular methods | ||||
| FISH [44] | Fluorescence microscopy-based analysis of polymicrobial structures using 16S rRNA probes | Biofilm visualization in situ/dysbiosis visualization |
|
Comparison with reference methods:
|
| Sequencing [45] | Gene sequencing (NGS) | Quantitative determination of the VM |
|
Comparison with reference methods:
|
| Multiplex qPCR [46,47] | Multiplex quantitative PCR | Commercially available automated test, indirect biofilm detection possible |
|
Comparison with reference methods:
|
FISH: fluorescence in situ hybridization; NPV: negative predictive value; PPV: positive predictive value; VM: vaginal microbiome.
Other methods based on the enzymatic detection have also been developed for BV, including the OSOM R BVBlue R test [48] and a tetravalent sialic acid-coated tetraphenylethene luminogen with aggregation-induced emission [49], both designed for the detection of sialidase in vaginal samples. The latter test demonstrates a sensitivity and specificity of 95.40% and 94.94%, respectively, compared to the Amsel method. In addition, a novel immunodetection-based approach, also targeting sialidase, has been developed for BV diagnosis. The nanophotonic operating principle of this biodetection method allows for a more cost-effective, faster, and simpler analysis than the indirect enzyme-linked immunosorbent assay (ELISA). This nanotechnology exhibits high sensitivity and specificity (96.29%) and may offer a rapid diagnostic approach for BV [50].
3.2. Bacterial Vaginosis Biofilm Formation
A biofilm is a structured community of microorganisms encased in a self-produced extracellular matrix, adhering to the surface of epithelial cells. A key characteristic of BV is the formation of a polymicrobial biofilm on vaginal epithelial cells [51]. B. vaginale colonization does not always promote BV [52], indicating that this bacterial species by itself may be necessary but not sufficient for the development of BV. Certain anaerobic bacteria are strongly linked to BV, suggesting that BV is a polymicrobial phenomenon that does not adhere to Koch’s principles [53]. The precise mechanisms underlying biofilm formation in BV remain poorly understood, including the genes involved, communication strategies (e.g., quorum sensing and metabolic communication), and genetic exchange between biofilm-associated bacteria. It remains unclear whether all bacteria present in the BV biofilm play a pathogenic role or are merely a byproduct of biofilm formation [33].
Interestingly, a conceptual model for the pathogenesis of BV has been proposed by Muzny et al. [23], outlining a synergistic relationship between B. vaginale, P. bivia, and F. vaginae [54]. Following sexual exposure to the virulent strains of B. vaginale, vaginal lactobacilli are dislodged and allow the formation of a BV biofilm on the vaginal epithelium [33,55]. Further proteolysis by B. vaginale promotes the growth of P. bivia, producing ammonia derivatives that induce the growth of B. vaginale and increase the biofilm that develops [56]. Both bacterial species then produce sialidase, which degrades the mucin layer of the vaginal epithelium, increasing the adhesion potential of other BV-associated bacteria, such as F. vaginae, to the polymicrobial biofilm [57].
3.3. Immune Response
Symptomatic BV with abnormal vaginal discharge has been linked to host immune factors in marker and transcriptional profiling studies [30]. Innate immunity includes (i) cellular components such as dendritic cells (DCs), macrophages, and neutrophils; (ii) chemical components such as cytokines, damage-associated molecular patterns (DAMPs), AMPs, complement system (C system), and pattern recognition receptors (PRRs); and (iii) physical barriers, such as epithelial cells, mucus, and lactic acid [58].
Microbial surveillance within the female genital tract, for both commensal and pathogenic microorganisms, is primarily achieved through the recognition of microorganisms by PRRs, such as the dectin-1 receptor, toll-like receptors (TLRs), and nucleotide-binding oligomerization domain (NOD). These receptors are present on both the squamous epithelial cells lining the vagina and the columnar cells lining the upper female genital tract [59]. The microbial activation of PRRs triggers cytokine/chemokine signaling cascades, resulting in the secretion of IL-1β, IL-6, and IL-8, as well as tumor necrosis factor-α (TNF-α), which contribute to recruiting or stimulating specialized cells, such as CD4+ helper T cells, NK cells, macrophages, B lymphocytes, and CD8+ cytotoxic T cell lymphocytes [60].
The primary immune defense mechanism triggered during BV pathogenesis is TLR4 ligand-mediated pro-inflammatory signaling through the nuclear factor-κB (NF-κB) pathway [61]. Nevertheless, this pathway is activated when pathogen-associated molecular patterns (PAMPs) bind to PRRs, a process further supported by the C pathways (alternative and lectin pathways) [58,62]. The NF-κB pathway subsequently promotes the secretion of pro-inflammatory cytokines such as IL-1β, which not only stimulates the inflammatory response but also alters the production of secondary pro-inflammatory cytokines/chemokines, specifically TNF-α, IL-6, and IL-8 [63].
The innate immune response varies depending on the pathogen, influenced by the pro-inflammatory cytokines generated during BV pathogenesis in the lower genital tract. The activation of IL-8 typically triggers the activation of neutrophils, which then migrate to the site of infection through chemotaxis and engage in the phagocytosis of the pathogenic bacteria. However, IL-8 levels are not elevated in BV cases, suggesting that BV-associated bacteria exert an inhibitory effect on IL-8 production [64]. In addition to chemotactic cytokines, various AMPs, such as lactoferrin, cathelicidin, and α-defensins, from lactobacilli pre-existing in the vaginal epithelium also induce the endocytic migration of monocytes, macrophages, and neutrophils, leading to inflammation in women with BV [65]. Furthermore, the maturation of DCs is abnormally increased during BV, which enhances antigen presentation to T cells.
Moreover, inflammation is most commonly observed in recurrent BV, which arises due to pathogen overgrowth and increased biofilm production. However, increased pathogenic invasion leads to cellular stress that interferes with the host immune response, shifting the pathogenesis toward infection [58]. The primary mechanism used by BV-associated bacteria to modulate the immune system involves the disruption of host sialic acid uptake, the inhibition of IL-8, and the production of short-chain fatty acids (SCFAs) by bacteria, which negatively impacts the migration of endocytic cell components and exacerbates vaginal inflammation [58,66,67]. In addition, vaginal IgA in women has been shown to be cleaved during BV pathogenesis, resulting in antibody destruction [68]. A recent study reported that specific amino acid metabolites secreted by BV-associated bacteria, including imidazole propionate, may disrupt vaginal epithelial cell function and contribute to inflammation through the mTOR signaling pathway [69]. Figure 2 shows a hypothetical scheme of the immune defense mechanisms involved in the microbial pathogenesis of BV.
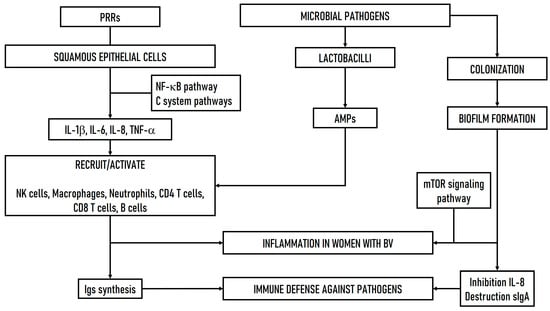
Figure 2.
Hypothetical scheme of the immune defense mechanisms involved in the microbial pathogenesis of BV. PRRs: pattern recognition receptors. C system: complement system. AMPs: antimicrobial peptides. BV: bacterial vaginosis.
4. Other Microbial Pathologies Associated with VM Dysbiosis
It is well known that as VM dysbiosis progresses, women become more susceptible to other microbial infections, including AV [70], PID [71], VVC [72], and STIs [73,74,75,76]. Furthermore, VM dysbiosis may also increase the risk of other non-microbial gynecologic conditions linked to adverse pregnancy outcomes, including preterm birth and miscarriage [77].
4.1. Aerobic Vaginitis
AV is associated with acute pyelonephritis, bacteriuria, lower urinary tract infections, and vaginal inflammation [78]. This pathology is defined by an increased VM of enteric aerobic bacteria that are unrelated to BV, including group B Streptococcus agalactiae (GBS) and S. anginosus, Staphylococcus aureus and S. epidermidis, Escherichia coli, and Enterococcus faecalis [70,79,80]. These aerobic microorganisms induce vaginal mucosal inflammation by disrupting lactobacilli dominance [81], leading to an increased turnover of the superficial epithelial cell layer and potentially causing small erosions, epidermal desquamation, ulceration, and vaginal epithelial atrophy [82]. This pathology occasionally causes morbidity and prevalence in postmenopausal and pregnant women due to reduced estrogen levels and local immunity [83]. The colonization of pregnant women with the pathogenic microorganisms of the AV, such as GBS, is considered a major cause of serious neonatal infections, including pneumonia, meningitis, and neonatal sepsis [84]. In addition to GBS, E. faecalis has also been associated with preterm delivery, low birth weight, and puerperal sepsis, resulting in significant maternal and neonatal morbidity and mortality [85].
4.2. Pelvic Inflammatory Disease
PID is an inflammatory disease of the upper female reproductive tract that may result in serious, long-lasting reproductive complications, such as chronic pelvic pain, ectopic pregnancy, and tubal factor infertility [86,87]. PID may arise due to various microorganisms, such as Mycoplasma genitalium, Chlamydia trachomatis, and Neisseria gonorrhoeae [88,89,90,91,92], ascending from the vagina or cervix to infect upper genital tract structures, likely stemming from the disruption of protective barriers and immune defense mechanisms in the context of inflammation [71]. According to Kerry-Barnard et al. [93], C. trachomatis and N. gonorrhoeae account for up to 30% of PID cases, but the etiology remains unclear in many instances, and it is not known why only a subset of women with genital C. trachomatis infection progress to upper tract involvement. In addition, BV has been implicated in the PID pathogenesis [91] by enhancing susceptibility to STIs and impairing their clearance. Moreover, BV-associated bacteria may contribute via synergistic interactions, promoting the upward spread of STIs [71,94].
A lactobacilli-dominated VM may provide protection against PID, but a VM dominated by anaerobes, such as B. vaginale, may increase susceptibility. In addition, not all lactobacilli species have the same protective effect. Recent studies indicate that D(−)-lactic acid-producing lactobacilli (L. crispatus, L. paragasseri, and L. mulieris) may offer protection against C. trachomatis infection. In contrast, L. iners, which produces L(+)-lactic acid, may offer less protection against pathogen infection. However, no definitive correlation has been found between the VM and the subsequent development of PID [93].
4.3. Vulvovaginal Candidiasis
VVC is one of the most prevalent causes of vulvovaginal itching and discharge worldwide. This condition is characterized by inflammation of the vulval and vaginal epithelium, along with yeast colonization, primarily by Candida albicans. While C. albicans is the causative agent in more than 90% of VVC cases, other non-albicans Candida (NAC) species, including C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis, have also been identified as etiologic agents. However, vaginal symptoms associated with NAC species are frequently noted to be less severe than those seen in VVC caused by C. albicans [72]. In determinate instances, patients may also experience dyspareunia, increased vaginal discharge, dysuria, vaginal soreness, or swelling [95]. Although most women (up to 75%) will experience at least one episode of VVC in their lifetime, some may suffer from recurrent infections. Recurrent VVC, defined as more than three episodes per year, can have a significant impact on quality of life, leading to both physical and psychological symptoms (e.g., anxiety and fear of social interactions). Moreover, it also represents a significant financial burden on both women and the healthcare system [96,97,98].
Several factors are considered to play a role in the development of recurrent VVC. Candida spp. is a commensal yeast present in the vagina of many women, typically migrating from the lower gastrointestinal tract. This colonization may stay without symptoms for extended periods. However, the overgrowth of Candida in the vaginal epithelium can trigger a symptomatic infection that can be impacted by various behavioral aspects, such as sponge/intrauterine device use, sexual activity, and oral contraceptives or hormone replacement therapy [99]. Genetic mutations, such as polymorphisms in TLR2 and mannose-binding lectin 2, have been identified in women with recurrent VVC, increasing their susceptibility to infection. Affected women may also present a genetically determined hyperinflammatory response to Candida colonization and invasion [100]. The influence of host physiological factors in the development of VVC remains unclear. Nevertheless, estrogen undoubtedly plays an important role in determining susceptibility to the disease [101]. Women in their reproductive cycle are most at risk for the onset of the disease, whereas prepubertal girls and postmenopausal women are rarely affected unless they are undergoing hormone replacement therapy [102].
4.4. Susceptibility to Sexually Transmitted Infections
VM dysbiosis and BV may increase the risk of STIs by interacting with mucosal immunity in the female genital tract and altering its protective epithelial barrier [103]. Dysbiosis-associated bacteria such as B. vaginale and Prevotella spp. can disrupt the mucosal barrier via the production of sialidase and host cell lysis, elevating the susceptibility to gynecological infections [104]. The microbial alteration produces a local immune response and inflammation in the vagina characterized by an increase in pro-inflammatory cytokines IL-1α, IL-1β, IL-6, and IL-8 linked with BV-associated bacteria such as Prevotella amnii, Sneathia spp., and Mobiluncus mulieris [61].
4.4.1. Human Immunodeficiency Virus (HIV)
BV contributes to an inflammatory state and disrupts barriers in the vaginal tract, allowing HIV infectious particles to cross the genital epithelium through tears in the squamous epithelium or transcytosis across the endocervical cell monolayer. This process ultimately leads to an infection of underlying CD4+ target cells in the submucosa [105], where viral replication occurs. These infected cells then propagate the viral infection further [106].
Recently, women with non-lactobacilli-dominant VM have been reported to be at a higher risk for the sexual transmission of HIV [107]. BV is more common and persistent in HIV-infected women, especially those with compromised immune systems [108]. L. crispatus has been associated with a reduced inflammatory state, the clearance of BV-associated bacteria, and reduced HIV risk. In contrast, L. iners has been suggested to provide less HIV protection, possibly because this species produces a more intermediate transition to dysbiosis [109].
4.4.2. Human Papillomavirus (HPV)
Similarly to other STIs, the dysbiosis of VM or BV increases the risk of HPV infection and persistence [110,111]. The sialidase genes of F. vaginae and B. vaginale, and the dominance of Snethia spp. in the VM, have been suggested as biomarkers of HPV persistent in women with BV [112,113]. In addition, a VM dominated by L. iners or by anaerobic microorganisms may be a risk factor for persistent HPV infection, due to low protective immunity associated with the loss of VM homeostasis produced by commensal lactobacilli and with the disruption of the protective barrier of the cervical epithelium caused by anaerobic microorganisms [114]. These events promote HPV entry into host cells, facilitating the subsequent integration of the HPV virus into the host DNA and promoting cell division that supports viral replication [73]. In contrast, a VM dominated by L. crispatus protects against HPV persistence and subsequent disease development, and a VM dominated by L. paragasseri is associated with faster HPV clearance [114,115].
4.4.3. Trichomonas Vaginalis Infection
The VM plays a pivotal role in influencing susceptibility to T. vaginalis infection, thereby increasing the risk of trichomoniasis in women with BV or a Nugent score greater than 3 [116]. The VM dysbiosis results in a loss of lactobacilli with an elevated pH, establishing conditions that are more conducive to T. vaginalis growth and colonization [117,118]. In addition, several anaerobic genera, such as Prevotella and Sneathia, may mediate susceptibility to trichomoniasis by producing nicotinamide metabolites [119]. Based on these interactions, interventions aimed at reducing BV incidence and promoting a balanced microbiota (eubiosis) could help lower the rates of trichomoniasis [116].
5. Therapeutic Tools
The treatment of BV and other associated microorganisms currently focuses on stopping microbial proliferation and restoring the normal VM [120]. Clinical therapies commonly involve the use of antimicrobial agents with broad-spectrum activity against anaerobic bacteria and protozoa, such as clindamycin (a lincosamide) and the nitroimidazoles (i.e., metronidazole and tinidazole), and/or the use of LBPs such as probiotics, synbiotics, and vaginal microbiota transplantation (VMT) [121,122].
5.1. Antibiotic Therapies
The World Health Organization’s primary treatment recommendation is 500 mg of oral metronidazole twice daily for one week [123], constituting the first-line treatment for BV. Despite being recognized as the standard therapy, metronidazole administration may lead to side effects, including gastrointestinal discomfort, nausea, and vomiting [124]. Other suggested therapeutic options include 300 mg of oral clindamycin twice daily for one week, 100 mg of intravaginal clindamycin ovules daily for five days, the application of 0.75% intravaginal metronidazole gel for five days, or 2% intravaginal clindamycin cream for one week [123]. It is important to note that the topical application of clindamycin may damage latex-based products, such as condoms, and could also cause pseudomembranous colitis [124]. Furthermore, tinidazole has been approved as an alternative therapy, recommended as an oral regimen (either 2 g/day for two days or 1 g/day for five days) when metronidazole and clindamycin are not well tolerated [125].
Other antimicrobial agents have also been used to treat BV, including rifaximin, azithromycin, and ornidazole or secnidazole [20]. When administered topically or orally, these antimicrobials exhibit almost similar efficacy, with cure rates ranging from 58% to 92% after 1 month of treatment [126]. However, these results are transient, leading to recurrence or reinfection at rates greater than 50% within 6–12 months of treatment [122,127], likely because these treatments only temporarily eradicate BV-associated microorganisms, or these bacteria are reintroduced into the vagina by sexual partners [128,129].
A remarkable decrease in antimicrobial efficacy with an increase in relapse and recurrence rates of 50–67% and 6–12 months, respectively, suggest microbial drug-acquired resistance [130]. Among the most commonly detected causative agents of BV, increased antimicrobial resistance has been identified in strains harboring chromosomal or plasmid resistance genes, including drug efflux pumps, such as ermG-mefA-mrsSA genes for clindamycin, nim genes for metronidazole and secnidazole, mph genes and an mefB efflux pump for azithromycin, rpoB gene for rifaximin, tetQ/X genes for tetracyclines, cifA gene for carbapenems, cepA genes for beta-lactams, and a BexA efflux pump for fluoroquinolones [131,132,133]. In addition to resistance genes, biofilm formation in BV, influenced by factors such as sexual practices, douching, and the presence of non-dominant lactobacilli, is primarily responsible for its high recurrence and the transmission of BV-associated STIs [134,135].
The most commonly used antimicrobial treatments for AV consist of kanamycin, meclocycline, and quinolones, which have intrinsic activity against pathogenic bacteria causing disease while potentially minimizing interference with the VM [136]. Similarly, clindamycin, which is used to treat BV, is also employed for AV treatment due to its broad spectrum of activity against multiple aerobic Gram-positive species. However, infection control with clindamycin tends to be temporary and may not target all species associated with AV [137]. In contrast, broad-spectrum antibiotics like carbapenems and clavulanic acid–beta-lactam combinations are highly effective for the rapid, short-term alleviation of severe symptoms in aerobic infections, particularly deep cutaneous vulvovaginitis caused by GBS or methicillin-resistant S. aureus (MRSA) [138].
5.2. Live Biotherapeutic Products
Recently, live biotherapeutic products (LBPs) have arisen as a novel category of therapeutic agents with wide-ranging applications. LBPs can be defined as biological products containing living organisms, such as bacteria, designed to prevent, treat, and cure human diseases or conditions, excluding vaccines [139]. LBPs include probiotics, synbiotics, and VMTs and are involved in complicated biological processes that are not fully understood. Therefore, it is necessary to investigate the intricate mechanisms of action of LBPs on both the human host and the host microbiome, as well as their potential side effects and long-term safety. This is particularly important considering that the target populations are individuals with existing health conditions who may be immunocompromised [140]. The suggested mechanisms of action of LBPs involve inhibiting the growth of pathogenic microorganisms in the vagina [141] or promoting other potentially beneficial processes such as the production of antimicrobial substances (e.g., bacteriocins and hydrogen peroxide) and stimulating the immune system to help maintain bacterial balance within the vaginal tract [142,143,144]. Furthermore, by adhering to the vaginal epithelium, LBPs may prevent pathogenic bacteria from attaching and compete for the same nutrients, thereby limiting their growth [145].
Several species of the family Lactobacillaceae, including the commensal species of the vaginal tract, have been used as probiotics and LBPs. The lactic acid produced by these bacterial species has potential antiviral and bactericidal activity, inhibiting the replication/growth of genital STI pathogens and pathobionts, such as C. trachomatis, N. gonorrhoeae, GBS, HPV, and HIV [146]. Other antimicrobial compounds like hydrogen peroxide-producing enzymes are prevalent in vaginal L. crispatus strains [147], and although the physiological role of this compound on pathogenic microorganisms is controversial [148], vaginal L. crispatus supernatants strongly inhibit C. albicans growth, virulence gene expression, and hyphal formation [149]. In addition, bacteriocins have also been observed in determinate vaginal lactobacilli, such as lactocin 160, which induces transient pore formation by disrupting the chemiosmotic potential on the cytoplasmic membrane of B. vaginale [150], as well as gassericin E, which inhibits the growth of vaginal pathogens [151]. Lactobacilli can also induce host defense against pathogens by forming microcolonies that attach to epithelial cell receptors and form a physical barrier to pathogen attachment [152]. For example, L. paragasseri inhibits T. vaginalis colonization of vaginal epithelial cells via the action of acidity and protonated lactic acid and through a cell surface aggregation-promoting factor [153]. Similarly, L. crispatus harbors genes encoding a fibronectin-binding (LEA) protein and adhesins that have been shown to interfere with the fibronectin-binding and pilus components of B. vaginale [154,155].
LBPs have demonstrated the ability to enhance host innate immune responses. Bacterial exopolysaccharides (EPSs) produced by L. crispatus can enhance the ability of vaginal VK2 cells to produce the anti-candidal human defensin-2 protein and also improve colonization potential [156]. Moreover, the peptidoglycan of vaginal L. crispatus has the ability to stimulate CD207 expression in Langerhans cells, the antigen-presenting dendritic cells in the vagina, thereby potentially reducing the entry of HIV receptors [157].
LBPs have been studied extensively for the treatment of recurrent BV and VVC, but they have not yet become a standard clinical approach due to variations in study size, methodology and application. Currently, several vaginal formulations are available for the treatment of BV, such as the AZO Complete Feminine Balance™ and Jarro-Dophilus® Women, which contain strains of L. crispatus, L. mulieris, L. paragasseri, and Lacticaseibacillus rhamnosus, as well as Bio-K+® Women’s Health, Jarrow Formulas® Fem-Dophilus® 1 Billion, Jarrow Formulas® Fem-Dophilus® 5 Billion, RepHresh™ Pro-B™ Probiotic, and UltraFlora® Women’s, which contain a blend of the most common strains isolated from the urogenital tract, including Limosilactobacillus reuteri and L. rhamnosus [158,159]. Table 2 shows recent clinical trials on the therapeutic use of LBPs for various vaginal pathologies during the period of 2015–2024.

Table 2.
Clinical trials on the therapeutic use of LBPs for various vaginal pathologies during the period of 2015–2024.
Table 2.
Clinical trials on the therapeutic use of LBPs for various vaginal pathologies during the period of 2015–2024.
| Reference | Type of Study | Treatment | Results |
|---|---|---|---|
| Laue et al. [159] | RCT. N = 36 female volunteers aged ≥18 years at reproductive age with stable menstrual cycles or postmenopausal women presenting BV, initially treated with metronidazole. | Fermented dairy drink: Contained pasteurized whole milk and the yogurt starter cultures Streptococcus thermophilus (95%) and Lactobacillus delbrueckii subsp. bulgaricus (5%). The dairy drink was supplemented with L. crispatus strain LbV 88, L. paragasseri strain LbV 150N, L. mulieris strain LbV 116, and L. rhamnosus strain LbV96 at a concentration of each strain of 1 × 107 CFU/mL for 4 weeks. | After 4 weeks, Amsel scores, discharge and odor, and Nugent scores decreased in the verum group compared to the control group. Supplemental consumption of yogurt containing these probiotic strains improved the recovery rate and symptoms of BV and tended to improve the vaginal microbial pattern. |
| Heczko et al. [160] | RCT. N = 241 (18–50 years) females with diagnoses of R-BV and AV with standard metronidazole treatment. | prOVag® oral probiotic capsules: L. paragasseri strain 57C, Limosilactobacillus fermentum strain 57A, and Lactiplantibacillus plantarum strain 57B, at a concentration of 108 CFU/capsule for 3 months. | prOVag extended the time to clinical relapse of BV/AV symptoms by up to 51% compared to placebo. AV recurrence was delayed by up to 76%. Probiotic use also reduced and maintained a low vaginal pH and Nugent score, and increased vaginal Lactobacillus counts compared to standard treatment. |
| Pendharkar et al. [161] | Observational. N = 50 (18–55 years) females with diagnoses of BV or R-VVC and treated with clindamycin/metronidazole (C/M) and fluconazole. | EcoVag® oral probiotic capsules: L. paragasseri strain DSM 14,869 and L. rhamnosus strain DSM 14870, at a concentration of 108 CFU/strain/capsule for 6–12 months. | In trial I, BV was treated with C/M followed by 5 days of EcoVag® capsules. Trial II included three groups: BV with prolonged C/M and EcoVag®, R-VVC with extended fluconazole and EcoVag®, and fluconazole treatment alone. In trial I, the 6-month cure rate for BV was 50%, and in trial II, both the 6- and 12-month cure rates were 67%. For VVC, women receiving fluconazole and EcoVag® had cure rates of 100% and 89% at 6 and 12 months, respectively, while those receiving fluconazole only had cure rates of 100% and 70%. Lactobacilli were associated with BV cure in both trials, while EcoVag® strains were significant only in trial II. |
| Davar et al. [162] | RCT. N = 59 (mean age 32.3 years) females with diagnosis of R-VVC and treated, initially treated with fluconazole. | Pro-Digest probiotic oral capsules: Lactobacillus acidophilus (7.5 × 109 CFU/capsule, Bifidobacterium bifidum (6 × 109 CFU/capsule), and B. longum (1:5 × 109 CFU/capsule) for 6 months. | The results showed that taking probiotics without antifungal drugs could be highly effective in treating VVC, resulting in a lower recurrence rate as well. |
| Cohen et al. [163] | RCT. N = 228 (18–45 years) females with untreated BV (asymptomatic or symptomatic). | Lactin-V probiotic capsules: L. crispatus strain CTV-05, at 2 × 109 CFU/dose for 10 weeks. | Recurrence of BV at week 12 was lower in the Lactin-V group compared to the placebo group. L. crispatus CTV-05 was detected in 79% of Lactin-V participants at the 12-week visit, and adverse events were similar between the Lactin-V and placebo groups. |
| Reznichenko et al. [164] | Randomized prospective. N = 186 (18–45 years) females with symptomatic BV, cured with metronidazole. | Oral probiotic cocktail: L. crispatus strain LMG S-29995, Levilactobacillus brevis, and L. acidophilus in proportions of 60%, 20%, and 20%, respectively. Treatment for 16 weeks. | Oral intake of probiotics can significantly reduce the percentage of recurrences of BV in recently treated women and prolong the time to recurrence of the disease. |
| Zhang et al. [165] | Parallel controlled trial. N = 70 (18–65 years) females with BV and treated with metronidazole. | Oral probiotics: L. rhamnosus strain GR-1 and Limosilactobacillus reuteri strain RC-14, treated orally for 30–90 days as an adjunct to metronidazole. | There was no significant difference in the overall 30-day total cure rate between the adjunctive probiotic group and the metronidazole group. There was also no significant difference in vaginal microbial diversity and structure between the two groups at 0, 30, or 90 days. The probiotic species were rarely detected in either the vaginal microbiota or the fecal microbiota after administration, which may reveal the cause of the non-effectiveness of oral probiotics. |
| Park et al. [166] | RCT. N = 101 (19–50 years) females with a Nugent score of 4–6. | MED-01 oral probiotic capsules: Ligilactobacillus salivarius strain MG242, L. fermentum strain MG901, L. plantarum strain MG989, Lacticaseibacillus paracasei strain MG4272, and L. rhamnosus strain MG4288 at 1.0 × 109 CFU/capsule each. Treatment for 12 weeks. | Quantitative PCR analysis confirmed that L. plantarum was significantly increased in the vagina, whereas harmful bacteria such as Mobiluncus spp., B. vaginale, and F. vaginae were suppressed after 12 weeks of MED-01 supplementation. These results confirmed that MED-01 can be used as a probiotic for the treatment of BV by improving VM. |
| Tomusiak et al. [167] | RCT. N = 376 (18–40 years) females with a Nugent score of 4–6. | inVag(®) vaginal gelatin capsules: contained >109 CFU/capsule with 25% L. fermentum strain 57A, 25% L. plantarum strain 57B, and 50% L. paragasseri strain 57C. Treatment for 3 months. | The probiotic inVag was safely administered to sustainably restore the healthy VM with a significant increase in the abundance of Lactobacillus. |
| Recine et al. [168] | Prospective case–control. N = 250 (mean age 29.4 years) non-pregnant sexually active women with a diagnosis of BV treated with metronidazole. | NORMOGIN® vaginal tablets: L. rhamnosus strain BMX 54 (containing > 104 CFU/tablet) for 7 months. | Probiotic supplementation with vaginal L. rhamnosus appears to be useful in inhibiting bacterial growth, especially after antibiotic therapy. Patients who underwent prophylactic therapy with NORMOGIN had a significantly lower rate of recurrence compared to patients treated with antibiotics alone. |
| Palma et al. [169] | RCT. N = 117 (>18 years) females with a diagnosis of BV or yeast vaginitis associated with HPV-infection. | Probiotic tablets: L. rhamnosus strain BMX 54, at 104 CFU/tablet for 9 months. | Women in the long-term probiotic-use group were twice as likely as those in the short-term probiotic-use group to resolve HPV-related cytological abnormalities. Complete clearance of HPV was observed in 11.6% of short-term probiotic users compared to 31.2% of long-term users. |
| Marcotte et al. [170] | Prospective–exploratory. N = 39 (18–50 years) females with a diagnosis of BV and treated with triple oral antibiotic (cefixime, doxycycline, and metronidazole). | Vaginal probiotic capsules: L. rhamnosus strain DSM 14,870 and L. paragasseri strain DSM 14869, for 190 days. | Supplementation with vaginal probiotic capsules resulted in colonization of the vagina by the Lactobacillus strains contained in the capsules. Low initial cure rates of BV after a single dose of metronidazole, and the probiotic did not improve BV cure rates or alleviate recurrence, which could be due to treatment failure or very limited power of the study. |
| Mändar et al. [171] | RCT. N = 182 (18–50 years) females with a diagnosis of R-BV and R-VVC. | Vaginal and oral probiotic capsules: L. crispatus strains DSM32717, DSM32720, DSM32718, and DSM32716, at 3 × 1010 CFU/strain/capsule for 3 months. | Both oral and vaginal capsules effectively improved symptoms in patients with R-BV, including reducing discharge volume, odor, and itching/irritation. In patients with R-VVC, both types of capsules successfully relieved the main symptoms of discharge volume and itching/irritation. |
| Russo et al. [172] | Clinical trial. N = 48 females positive for C. albicans and symptoms of VVC after clotrimazole therapy. | Oral synbiotic formulation: L. acidophilus strain GLA-14 and L. rhamnosus strain HN001 plus bovine lactoferrin, for 3–6 months. | After clotrimazole treatment, a significant improvement in symptoms was shown in both groups (control and intervention). However, only women treated with the synbiotic formulation showed a significant improvement in itching and discharge at 3 and 6 months. During the 6-month follow-up, there were significantly fewer relapses in the intervention group compared to placebo. |
| Lyra et al. [173] | RCT. N = 50 (18–50 years) women devoid of vaginal complaints (Nugent score 0–3 and vaginal pH < 4.5). | V-Caps HMPC synbiotic capsules: Capsules: contained 1010 CFU of L. acidophilus strain La-14 and L. rhamnosus strain HN001 in a 4:1 ratio, potato maltodextrin as a carrier, and stearate and silicon dioxide as flow agents. Treatment for 3 weeks. | VM remained stable and dominated by lactobacilli throughout the intervention, and vaginal pH remained optimal (pH of 4.0–4.5). Immune markers elafin and human β-defensin 3 were significantly decreased in the verum group but did not correlate with changes in the microbiota. |
| Vicariotto et al. [174] | Prospective–observational. N = 50 (45–65 years) postmenopausal healthy women. | Synbiotic capsules: L. plantarum strain PBS067, Bifidobacterium animalis subsp. lactis strain BL050, and L. rhamnosus strain LRH020, at 3 × 109 CFU/g (1 × 109 CFU/g of each probiotic strain), plus vitamin B3 and maltodextrin during 28 days. | Significant improvements in the VHI score and substantial reductions in inflammatory cytokine levels (IL-6, IL-1β, and TNF-α) were achieved. In addition, the probiotic intervention facilitated the restoration of the VM, evidenced by an increase in the abundance of lactobacilli. |
| Baldacci et al. [175] | Clinical trial. N ≥ 3000 females with R-BV after metronidazole treatment. | NORMOGIN™ synbiotic vaginal tablets: L. rhamnosus strain BMX 54 plus lactose, for 6 months. | NORMOGIN has been shown in a large sample of women enrolled in clinical trials not only to be able to significantly reduce the BV recurrences after the standard of care administration but also to control the vaginal pathobiosis pathway, restoring the physiological eubiosis from dysbiosis. |
| Vivekanandan et al. [176] | RCT. N = 66 (≥40 and ≤65 years) females with BV symptoms. | VagiBIOM synbiotic suppositories: 1–2% L. crispatus strain Bi16, L. paragasseri strain Bi19, Heyndrickxia coagulans (formerly Bacillus coagulans) strain Bi34, L. acidophilus strain Bi14, coconut oil fatty acids, prebiotic complex (0.05–2%), hyaluronic acid (0.1–0.3%), silica gel (0.1–0.3%), and lactic acid (0.01–0.025%). Treatment for 4 weeks. | After 4 weeks of intervention with VagiBIOM or a placebo, the mean scores for vaginal pH, VAS itch, and Nugent total score were significantly reduced from the baseline. Compared to the baseline scores, the VHI scores improved significantly after 28 days of intervention. In addition, VagiBIOM improved vaginal lactobacilli diversity. |
| Lev-Sagie et al. [177] | Exploratory. Recipients N = 5 (18–50 years) females with a diagnosis of R-BV. | VMT: Donors N = 2, one was non-sexually active for 8 years and other was in a 25-year monogamous relationship. Donors had a negative history of vaginal symptoms and underwent an examination to verify the absence of BV. Follow-up at 5–21 months after VMT. | Of the five patients treated, VMT was associated with complete long-term remission, defined as significant improvements in symptoms and reconstitution of a Lactobacillus-dominated VM in four patients to the end of follow-up. No adverse effects were observed in any of the five women. Notably, remission in three patients required repeated VMT, including a donor change in one patient, to achieve a durable clinical response. |
| Wrønding et al. [178] | Case study. Recipient N = 1 (30 years) females with vaginal microbiome dysbiosis. | VMT: Transfer of cervicovaginal secretions from a healthy donor with a Lactobacillus-dominant vaginal microbiome. | After one VMT, there was a complete shift in microbiome composition to 81.2% L. crispatus and 9% L. mulieris with concomitant resolution of vaginal symptoms. |
| Oerlemans et al. [179] | Interventional. N = 20 (18–50 years) females with a diagnosis of VVC and treatment with fluconazole. | Probiotic gel: L. plantarum strain WCFS1, Lactiplantibacillus pentosus strain KCA1, and L. rhamnosus strain GG, at 109–1010 CFU/g for 4 weeks. | Forty-five percent of women using the probiotic gel experienced symptom relief without the need for additional fluconazole medication, and their fungal levels were similar to those of women treated with fluconazole. Fluconazole alone reduced the abundance of vaginal lactobacilli. |
| Yoshikata et al. [180] | RCT. N = 70 (20–40 years) females with atrophic vaginitis, BV, and genitourinary symptoms. | Foaming wash (Delicate Softwash®), feminine cream (Delicate Softgelcream®) and vaginal gel (Inner gel®): L. rhamnosus strain vitaP1 and L. plantarum strain KCTC3108. Treatment for 4 weeks. | Vaginal pH and pathogenic microorganisms were reduced in both treatment groups (post- and premenopausal women) compared to the control group, which was more significant in the postmenopausal group. Genitourinary symptoms improved significantly in 60% of premenopausal women and 81.3% of postmenopausal women compared to the control group. |
| Shen et al. [181] | Human clinical trial. N = 50 (18–55 years) females with diagnosis of BV. | Postbiotic gel: L. paracasei strain ProSci-92 and L. rhamnosus strain ProSci-109, at 3 × 109 CFU/g of each probiotic strain, plus 5% of skim milk powder and 3% full-fat soybean. Treatment for 1 week. | The results showed that the application of the synbiotic gel improved the symptoms of BV, as indicated by an improvement in the abnormalities of the patients’ vaginal secretions. After application of the gel, the relative abundance of vaginal lactobacilli increased compared to baseline. Significant negative correlations were found between lactobacilli and potential vaginal pathogens (including Gardnerella, Prevotella, and Fannyhessea), as well as vaginal secretion abnormalities. |
AV: aerobic vaginitis; CFUs: colony-forming units; BV: bacterial vaginosis; HPV: human papillomavirus; R-BV: recurrent bacterial vaginosis; RCT: randomized controlled trial; R-VVC: recurrent vulvovaginal candidiasis; VAS: visual analog scale; VHI: vaginal health index; VM: vaginal microbiome; VMT: vaginal microbiome transplantation; VVC: vulvovaginal candidiasis.
As shown in Table 2, eight of the twenty-three studies (34.8%) were based on the oral use of probiotics [159,160,161,162,163,164,165,166], five studies (21.7%) used vaginal probiotics [167,168,169,170,171], three studies (13.0%) employed oral synbiotics [172,173,174], two studies (8.7%) used vaginal synbiotics [175,176], two studies were based on VMT [177,178], and the remaining three studies involved the use of probiotic and postbiotic gels [179,180,181].
6. Discussion
In the eubiosis state, VM is dominated by Lactobacillus species, which play a critical role in vaginal health. When the bacterial composition of VM changes, resulting in a dysbiosis state, it increases the incidence of pathological processes such as BV, yeast infections, STIs, and urinary tract infections [14,15]. In addition, dysbiosis of the VM produces inflammatory responses and abnormal immune responses, all of which can contribute to a variety of female reproductive health problems [182]. Moreover, a mother’s VM significantly influences the microbial colonization of the vaginally delivered infants [183]. Therefore, infected mothers have a higher probability of transmitting pathogens to neonates. Conversely, infants born by C-section acquire microbial taxa similar to those present on the mother’s skin [184].
Risk factors for VM can be divided into two groups: (i) those inherent to the human condition or non-modifiable factors, such as age, hormonal levels, and ethnicity, which are related to the eubiosis or dysbiosis of VM; and (ii) those related to environmental or social components, referred to as modifiable factors, such as diet, probiotic intake, smoking, stress, antibiotic use, hygiene practices, contraceptive use, and sexual habits, which are currently associated with VM dysbiosis [14,185,186]. A high Nugent score in the VM has been associated with a high risk of STIs, preterm birth, adverse pregnancy outcomes, and postpartum endometritis [185,187,188]. Furthermore, Srinivasan et al. [189] suggested that different bacterial species are associated with each of the four Amsel clinical signs for the diagnosis of BV (Table 1).
This review highlights the relationship between the dysbiosis of the VM and the incidence of several microbial pathologies affecting the vaginal tract, such as BV, AV, PID, VVC, and STIs, as well as the pathophysiological concerns linked to these diseases. In addition, the use of therapeutic tools, such as antimicrobials and LBPs, has been widely studied. For decades, chemotherapeutic agents have been employed as the primary treatment option. However, several recurrent diseases and drug-acquired pathogenic strains have recently emerged, and antibiotic therapy has not led to long-term freedom from symptoms [28]. Therefore, the use of LBPs is justified due to their lower risk of use as “generally regarded as safe” (GRAS) microorganisms and their positive risk–benefit ratio compared to antibiotic use in patients affected by recurrent vaginal infections. Moreover, another advantage of LBPs is that they contain native VM strains that are more competitive and better adapted to their ecological niche. However, larger clinical cohorts are still needed; long-term effects are only reported in a limited number of studies, and few have been carried out without the administration of antibiotics. Thus, the effects and mechanisms of action of these potential LBP candidates should be further explored. More recently, several studies have used VMT as an LBP, defined as the replacement of the infected patient’s VM with vaginal discharge containing microbiota from a healthy donor [190], restoring the balance of the patient’s VM and improving her overall health [191]. This approach follows a similar principle to fecal microbiota transplantation, where the introduction of a healthy microbiota aims to re-establish microbial equilibrium in the recipient, potentially contributing to improved health outcomes beyond physiological effects [192]. At present, the procedure is considered experimental, with limited availability. Since VMT involves the transfer of biological material between individuals, it raises several ethical concerns. These concerns include the careful selection and screening of donors, ensuring informed consent and maintaining privacy and confidentiality throughout the process.
Nevertheless, a major obstacle in the advancement of LBPs lies in the limited understanding of the complex interplay between the host, the microbiota, and environmental influences [193]. Oral administration remains the predominant route for LBP delivery, largely due to its safety and ease of use. Nevertheless, the human gastrointestinal tract presents numerous physiological barriers that can alter LBP functionality. These include gastric and bile acids, diverse enzymes, resident microbiota, immune cells, mucosal and epithelial regeneration, peristaltic motion, and the chemical environment [194]. Therefore, identifying optimal delivery strategies and dosage formulations for therapeutic and prophylactic purposes remains an essential avenue for future research [195]. Outcome variability is frequently observed across LBP clinical trials, reflecting the highly individualized nature of the human microbiome and its multifactorial responsiveness to microbial interventions. Such variability is influenced by patient-specific parameters, LBP composition and mechanism of action, and the particular disease being targeted [140]. Within this context, omics-based technologies offer valuable tools for elucidating clinical efficacy, enabling the modeling of interactions among LBPs, diet, and host microbiota to predict health-related outcomes.
The pharmaceutical development of LBPs differs fundamentally from the conventional use of probiotics as foods or dietary supplements. This distinction is rooted in differences in target populations: LBPs are intended for individuals with specific medical conditions, unlike the generally healthy consumers of traditional probiotics. Regulatory requirements also diverge significantly, especially concerning manufacturing standards, administration protocols, dosage regimens, and the nature of clinical validation. As noted by Pot and Vandenplas [196], dosage effects vary according to the strain and its therapeutic purpose, reflecting a degree of rational design. However, crucial variables such as administration timing and dietary influences are often inadequately studied. The formulation matrix and application of protective technologies can markedly impact strain viability and therapeutic effectiveness. Strain selection should be guided by clinical relevance, prioritizing human-derived and food-sourced organisms supported by robust efficacy data [197]. Furthermore, the translational pipeline for LBPs faces substantial economic and logistical barriers. The development and production of LBPs involve technically demanding and resource-intensive processes that require specialized facilities and trained personnel. These factors contribute to elevated production costs and may restrict patient access, particularly for those with rare or chronic diseases [198]. To promote equitable access, supportive measures such as public funding, innovative financial frameworks, and differential pricing models are critically needed.
Despite the pivotal role of the VM in maintaining the health of the female genital tract, it is often overlooked in clinical settings. Given the increasing evidence of how vaginal dysbiosis contributes to a range of microbial and non-microbial gynecological conditions, it is clear that the vagina deserves more focused attention, not only in research and clinical practice but also from individuals themselves, who must prioritize its care. In this context, knowledge about the vagina and the potential risks to which it is exposed should be widely disseminated among the population. For instance, it is common to subject this delicate organ to various potentially harmful and detrimental factors, such as unprotected sexual practices [199], improper or excessive washing [200], and the increasing negative influence of certain social trends, particularly among younger individuals [201,202,203]. For instance, the growing trend of douching, which is often mistakenly believed to promote hygiene, can disrupt the natural microbial ecosystem, leading to an increased risk of infections and dysbiosis [204]. Moreover, the use of certain lubricants, especially those that are not pH-balanced or contain harmful chemicals, may also pose a threat to the delicate balance of the VM [205,206]. In this respect, raising awareness about the subtle indicators of vaginal health, such as unusual changes in odor, color, or consistency of vaginal discharge, could help individuals recognize early signs of imbalance and seek timely intervention. Therefore, the intricate balance of the vaginal ecosystem, governed by a complex interplay of microbial communities and host immune responses, must be better understood to inform effective therapeutic strategies. While current treatments provide some relief, the recurrent nature of these conditions suggests that existing approaches are insufficient to address the root causes of dysbiosis. This underlines the importance of developing more targeted therapies that not only restore microbial balance but also account for individual variability in immune responses and other factors such as hormonal fluctuations. As recent findings suggest promising results from LBPs, future research should explore their long-term impact on vaginal health. Ultimately, a more holistic and personalized approach to vaginal care is essential, considering both microbial and immune dynamics to improve women’s health outcomes.
Within this discussion, it is also important to highlight the impact of diet on the composition of the VM, which has been recognized as an influential external factor. While not inherently determined, the VM can be supported by healthy dietary practices that promote a Lactobacillus-dominated environment. Diets rich in vitamins A, C, D, and E, β-carotene, and minerals such as calcium and zinc have been positively associated with vaginal health, including a reduction in the prevalence of BV and HPV [185]. Indigestible plant fibers and complex carbohydrates, which contribute to the production of SCFAs, lower the pH in the gut, thereby influencing microbial populations and promoting the production of beneficial metabolites. A fiber-rich, plant-based diet fosters the growth of beneficial gut bacteria, and reducing meat consumption in favor of plant-based alternatives has demonstrated significant overall health benefits [207]. Although this mechanism has been primarily studied in the gut, it is conceivable that SCFAs, through their systemic effects, could also influence the VM, since dietary intake, along with the use of probiotics and hormone replacement therapies, may impact its composition [185]. Emerging evidence further suggests that SCFAs play a pivotal role in the cross-talk between the gut and other systems, such as the gut–vagina–bladder axis, potentially influencing conditions like urinary tract infections [208]. Therefore, plant-based diets offer a sustainable strategy for maintaining microbiota stability and promoting overall health, not only in contemporary health practices but also in future dietary approaches. In this regard, the presence of lactic acid bacteria in fermented vegetables offers a valuable source of probiotics, with the health-promoting properties of these foods attributed to their bioactive compounds, which facilitate various physiological processes, including immune modulation, anti-inflammatory effects, and the inhibition of pathogenic microorganisms [209]. Nevertheless, these considerations remain to be further explored, and more rigorous studies are needed to validate the potential mechanisms linking diet, microbiota, and vaginal health.
As with all studies of this kind, the present review has several limitations. (i) Many of the currently available diagnostic tests for BV were developed primarily in premenopausal women. Due to the shift in the VM that occurs in the hypoestrogenic state in postmenopausal women, the diagnosis of BV in this population may be challenging. (ii) It is important to note that the studies included in this review focus on bacterial communities. However, recent advances in the study of the vaginal mycobiome and vaginal viral communities suggest that these microorganisms may play a significant role in the evolution toward VM homeostasis. (iii) Several of the studies reviewed have cross-sectional or exploratory designs with small numbers of cases, making it challenging to infer a causal relationship between the factors studied. (iv) The majority of the studies did not consider the influence of the extrinsic factors on VM, such as stress, antibiotic use, hygiene practices, contraceptive use, or sexual habits, which may affect the susceptibility of the host to microbial pathogens. (v) Few studies have evaluated the host’s immune response to microbial pathogens or changes in the VM. (vi) Specific studies on the effects of therapeutic tools have not been performed. Taking into account the patient’s age and hormonal levels, this limitation reduces the reliability of the results obtained, which could be improved by the application of analytical tools such as structural equation modeling and multistate Markov models. Lastly, (vii) given that the primary focus of this review is on diseases associated with VM dysbiosis, it does not explore in depth the intrinsic and extrinsic factors that contribute to this condition.
7. Conclusions
VM dysbiosis increases susceptibility to various microbial infections and non-microbial gynecologic conditions. The immune response during the pathogenesis of BV and of other VM dysbiosis-related diseases involves complex interactions between innate immune cells, cytokines, and pattern recognition receptors, contributing to inflammation, immune dysregulation, and altered epithelial function. Current treatments for VM dysbiosis-related pathologies often result in high relapse and recurrence rates, suggesting microbial resistance and the need for alternative therapeutic strategies. In turn, LBPs have demonstrated beneficial effects, restoring microbial balance in dysbiotic conditions. Nevertheless, while these findings suggest promising potential for LBPs, further rigorous clinical studies are necessary to gain a deeper understanding of the female genital tract ecosystem and to identify novel biomarkers along with their associated health implications. Moreover, the development of new diagnostic and management strategies will facilitate personalized therapeutic approaches. Ultimately, a comprehensive perspective on vaginal care is pivotal, taking into account both microbial and immune dynamics to enhance women’s health outcomes.
Author Contributions
Conceptualization, A.B.-R. and J.J.B.; Investigation, J.J.B.; Writing—Original Draft Preparation, J.J.B.; Writing—Review and Editing, A.B.-R.; Supervision, J.J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| VM | Vaginal microbiome |
| AMPs | Antimicrobial peptides |
| BV | Bacterial vaginosis |
| STIs | Sexually transmitted infections |
| AV | Aerobic vaginitis |
| PID | Pelvic inflammatory disease |
| VVC | Vulvovaginal candidiasis |
| LBPs | Live biotherapeutic products |
| qPCR | Quantitative real-time PCR |
| HIV | Human immunodeficiency virus |
| HPV | Human papillomavirus |
| DCs | Dendritic cells |
| DAMPs | Damage-associated molecular patterns |
| C system | Complement system |
| PRRs | Pattern recognition receptors |
| TLRs | Toll-like receptors |
| NOD | Nucleotide-binding oligomerization domain |
| TNF-α | Tumor necrosis factor-α |
| PAMPs | Pathogen-associated molecular patterns |
| NF-κB | Nuclear factor-κB |
| GBS | Group B Streptococcus agalactiae |
| SCFAs | Short-chain fatty acids |
| RCT | Randomized controlled trial |
| VMT | Vaginal microbiota transplantation |
| CFUs | Colony-forming units |
| R-BV | Recurrent bacterial vaginosis |
| R-VVC | Recurrent vulvovaginal candidiasis |
| VAS | Visual analog scale |
| VHI | Vaginal health index |
References
- Smith, S.B.; Ravel, J. The vaginal microbiota, host defense, and reproductive physiology. J. Physiol. 2017, 595, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, S.; Greenbaum, G.; Moran-Gilad, J.; Weintraub, A.Y. Ecological dynamics of the vaginal microbiome in relation to health and disease. Am. J. Obstet. Gynecol. 2019, 220, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O.; et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Forney, L.J.; Ravel, J. Vaginal microbiome: Rethinking health and disease. Annu. Rev. Microbiol. 2012, 66, 371–389. [Google Scholar] [CrossRef]
- Pekmezovic, M.; Mogavero, S.; Naglik, J.R.; Hube, B. Host-Pathogen Interactions during Female Genital Tract Infections. Trends Microbiol. 2019, 27, 982–996. [Google Scholar] [CrossRef]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact. 2020, 19, 203. [Google Scholar] [CrossRef]
- Christensen, A.O.; Li, G.; Young, C.H.; Snow, B.; Khan, S.A.; DeVore, S.B.; Edwards, S.; Bouma, G.J.; Navratil, A.M.; Cherrington, B.D.; et al. Peptidylarginine deiminase enzymes and citrullinated proteins in female reproductive physiology and associated diseases. Biol. Reprod. 2022, 107, 1395–1410. [Google Scholar] [CrossRef]
- Fuochi, V.; Cardile, V.; Petronio Petronio, G.; Furneri, P.M. Biological properties and production of bacteriocins-like-inhibitory substances by Lactobacillus sp. strains from human vagina. J. Appl. Microbiol. 2019, 126, 1541–1550. [Google Scholar] [CrossRef]
- Miko, E.; Barakonyi, A. The Role of Hydrogen-Peroxide (H2O2) Produced by Vaginal Microbiota in Female Reproductive Health. Antioxidants 2023, 12, 1055. [Google Scholar] [CrossRef]
- Vagios, S.; Mitchell, C.M. Mutual Preservation: A Review of Interactions Between Cervicovaginal Mucus and Microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 676114. [Google Scholar] [CrossRef]
- Vornhagen, J.; Quach, P.; Santana-Ufret, V.; Alishetti, V.; Brokaw, A.; Armistead, B.; Qing Tang, H.; MacDonald, J.W.; Bammler, T.K.; Adams Waldorf, K.M.; et al. Human Cervical Mucus Plugs Exhibit Insufficiencies in Antimicrobial Activity Towards Group B Streptococcus. J. Infect. Dis. 2018, 217, 1626–1636. [Google Scholar] [CrossRef] [PubMed]
- Yarbrough, V.L.; Winkle, S.; Herbst-Kralovetz, M.M. Antimicrobial peptides in the female reproductive tract: A critical component of the mucosal immune barrier with physiological and clinical implications. Hum. Reprod. Update 2015, 21, 353–377. [Google Scholar] [CrossRef] [PubMed]
- Gutzeit, O.; Gulati, A.; Izadifar, Z.; Stejskalova, A.; Rhbiny, H.; Cotton, J.; Budnik, B.; Shahriar, S.; Goyal, G.; Junaid, A.; et al. Cervical mucus in linked human Cervix and Vagina Chips modulates vaginal dysbiosis. NPJ Womens Health 2025, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Holdcroft, A.M.; Ireland, D.J.; Payne, M.S. The Vaginal Microbiome in Health and Disease—What Role Do Common Intimate Hygiene Practices Play? Microorganisms 2023, 11, 298. [Google Scholar] [CrossRef]
- Kalia, N.; Singh, J.; Kaur, M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: A critical review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 5. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, W.; Yuan, Y.; Zhu, W.; Shang, A. Vaginal microecological characteristics of women in different physiological and pathological period. Front. Cell. Infect. Microbiol. 2022, 12, 959793. [Google Scholar] [CrossRef]
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.; Zhong, X.; Koenig, S.S.; Fu, L.; Ma, Z.S.; Zhou, X.; et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 2012, 4, 132ra52. [Google Scholar] [CrossRef]
- Sukhera, J. Narrative Reviews: Flexible, Rigorous, and Practical. J. Grad. Med. Educ. 2022, 14, 414–417. [Google Scholar] [CrossRef]
- Zheng, N.; Guo, R.; Wang, J.; Zhou, W.; Ling, Z. Contribution of Lactobacillus iners to Vaginal Health and Diseases: A Systematic Review. Front. Cell. Infect. Microbiol. 2021, 11, 792787. [Google Scholar] [CrossRef]
- Abou Chacra, L.; Fenollar, F.; Diop, K. Bacterial Vaginosis: What Do We Currently Know? Front. Cell. Infect. Microbiol. 2022, 11, 672429. [Google Scholar] [CrossRef]
- Carter, K.A.; Fischer, M.D.; Petrova, M.I.; Balkus, J.E. Epidemiologic Evidence on the Role of Lactobacillus iners in Sexually Transmitted Infections and Bacterial Vaginosis: A Series of Systematic Reviews and Meta-Analyses. Sex. Transm. Dis. 2023, 50, 224–235. [Google Scholar] [CrossRef]
- Lev-Sagie, A.; De Seta, F.; Verstraelen, H.; Ventolini, G.; Lonnee-Hoffmann, R.; Vieira-Baptista, P. The Vaginal Microbiome: II. Vaginal Dysbiotic Conditions. J. Low. Genit. Tract Dis. 2022, 26, 79–84. [Google Scholar] [CrossRef]
- Muzny, C.A.; Taylor, C.M.; Swords, W.E.; Tamhane, A.; Chattopadhyay, D.; Cerca, N.; Schwebke, J.R. An Updated Conceptual Model on the Pathogenesis of Bacterial Vaginosis. J. Infect. Dis. 2019, 220, 1399–1405. [Google Scholar] [CrossRef]
- Osei Sekyere, J.; Oyenihi, A.B.; Trama, J.; Adelson, M.E. Species-Specific Analysis of Bacterial Vaginosis-Associated Bacteria. Microbiol. Spectr. 2023, 11, e0467622. [Google Scholar] [CrossRef]
- Diop, K.; Bretelle, F.; Raoult, D.; Fenollar, F. ‘Corynebacterium fournierii’, a new bacterial species isolated from the vaginal sample of a patient with bacterial vaginosis. New Microbes New Infect. 2017, 18, 6–7. [Google Scholar] [CrossRef]
- Diop, K.; Diop, A.; Bretelle, F.; Cadoret, F.; Michelle, C.; Richez, M.; Cocallemen, J.F.; Raoult, D.; Fournier, P.E.; Fenollar, F. Olegusella massiliensis gen. nov., sp. nov., strain KHD7T, a new bacterial genus isolated from the female genital tract of a patient with bacterial vaginosis. Anaerobe 2017, 44, 87–95. [Google Scholar] [CrossRef]
- Diop, K.; Raoult, D.; Bretelle, F.; Fenollar, F. “Ezakiella massiliensis” sp. nov., a new bacterial species isolated from human female genital tract. New Microbes New Infect. 2017, 15, 16–17. [Google Scholar] [CrossRef]
- Swidsinski, S.; Moll, W.M.; Swidsinski, A. Bacterial Vaginosis—Vaginal Polymicrobial Biofilms and Dysbiosis. Dtsch. Arztebl. Int. 2023, 120, 347–354. [Google Scholar] [CrossRef]
- Segui-Perez, C.; de Jongh, R.; Jonkergouw, R.L.W.; Pelayo, P.; Balskus, E.P.; Zomer, A.; Strijbis, K. Prevotella timonensis degrades the vaginal epithelial glycocalyx through high fucosidase and sialidase activities. mBio 2024, 15, e0069124. [Google Scholar] [CrossRef]
- Mondal, A.S.; Sharma, R.; Trivedi, N. Bacterial vaginosis: A state of microbial dysbiosis. Med. Microecol. 2023, 16, 100082. [Google Scholar] [CrossRef]
- Nelson, T.M.; Borgogna, J.L.; Brotman, R.M.; Ravel, J.; Walk, S.T.; Yeoman, C.J. Vaginal biogenic amines: Biomarkers of bacterial vaginosis or precursors to vaginal dysbiosis? Front. Physiol. 2015, 6, 253. [Google Scholar] [CrossRef] [PubMed]
- Amegashie, C.P.; Gilbert, N.M.; Peipert, J.F.; Allsworth, J.E.; Lewis, W.G.; Lewis, A.L. Relationship between Nugent score and vaginal epithelial exfoliation. PLoS ONE 2017, 12, e0177797. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Cerca, N. Influence of Biofilm Formation by Gardnerella vaginalis and Other Anaerobes on Bacterial Vaginosis. J. Infect. Dis. 2015, 212, 1856–1861. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Loening-Baucke, V.; Swidsinski, S.; Sobel, J.D.; Dörffel, Y.; Guschin, A. Clue Cells and Pseudo Clue Cells in Different Morphotypes of Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 2022, 12, 905739. [Google Scholar] [CrossRef]
- Amsel, R.; Totten, P.A.; Spiegel, C.A.; Chen, K.C.S.; Eschenbach, D.; Holmes, K.K. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 1983, 74, 14–22. [Google Scholar] [CrossRef]
- Nugent, R.P.; Krohn, M.A.; Hillier, S.L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 1991, 29, 297–301. [Google Scholar] [CrossRef]
- Ison, C.A.; Hay, P.E. Validation of a simplified grading of Gram stained vaginal smears for use in genitourinary medicine clinics. Sex. Transm. Infect. 2002, 78, 413–415. [Google Scholar] [CrossRef]
- Sherrard, J.; Wilson, J.; Donders, G.; Mendling, W.; Jensen, J.S. 2018 European (IUSTI/WHO) International Union against sexually transmitted infections (IUSTI) World Health Organisation (WHO) guideline on the management of vaginal discharge. Int. J. STD AIDS. 2018, 29, 1258–1725. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Zhao, M.; Wang, Y.; Bai, H.; Wang, Y.; Rui, C.; Fan, C.; Li, J.; Li, N.; et al. Deep Neural Networks Offer Morphologic Classification and Diagnosis of Bacterial Vaginosis. J. Clin. Microbiol. 2021, 59, e02236-20. [Google Scholar] [CrossRef]
- Cartwright, C.P.; Pherson, A.J.; Harris, A.B.; Clancey, M.S.; Nye, M.B. Multicenter study establishing the clinical validity of a nucleic-acid amplification–based assay for the diagnosis of bacterial vaginosis. Diagn. Microbiol. Infect. Dis. 2018, 92, 173–178. [Google Scholar] [CrossRef]
- Gaydos, C.A.; Beqaj, S.; Schwebke, J.R.; Lebed, J.; Smith, B.; Davis, T.E.; Fife, K.H.; Nyirjesy, P.; Spurrell, T.; Furgerson, D.; et al. Clinical Validation of a Test for the Diagnosis of Vaginitis. Obstet. Gynecol. 2017, 130, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, D.W.; Smith, W.L.; Chadwick, S.G.; Toner, G.; Mordechai, E.; Adelson, M.E.; Aguin, T.J.; Sobel, J.D.; Gygax, S.E. Development and Validation of a Highly Accurate Quantitative Real-Time PCR Assay for Diagnosis of Bacterial Vaginosis. J. Clin. Microbiol. 2016, 54, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.S.; Gaydos, C.A. Molecular Diagnosis of Bacterial Vaginosis: An Update. J. Clin. Microbiol. 2018, 56, e00342-18. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef]
- Shipitsyna, E.; Roos, A.; Datcu, R.; Hallén, A.; Fredlund, H.; Jensen, J.S.; Engstrand, L.; Unemo, M. Composition of the vaginal microbiota in women of reproductive age—Sensitive and specific molecular diagnosis of bacterial vaginosis is possible? PLoS ONE 2013, 8, e60670. [Google Scholar] [CrossRef]
- Breding, K.; Selbing, A.; Farnebäck, M. Diagnosis of Bacterial Vaginosis Using a Novel Molecular Real-Time PCR Test. J. Womens Health Gynecol. 2020, 7, 1–7. [Google Scholar]
- van den Munckhof, E.H.A.; van Sitter, R.L.; Boers, K.E.; Lamont, R.F.; Te Witt, R.; le Cessie, S.; Knetsch, C.W.; van Doorn, L.J.; Quint, W.G.V.; Molijn, A.; et al. Comparison of Amsel criteria, Nugent score, culture and two CE-IVD marked quantitative real-time PCRs with microbiota analysis for the diagnosis of bacterial vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 959–966. [Google Scholar] [CrossRef]
- Madhivanan, P.; Krupp, K.; Li, T.; Ravi, K.; Selezneva, J.; Srinivas, V.; Arun, A.; Klausner, J.D. Performance of BVBlue rapid test in detecting bacterial vaginosis among women in Mysore, India. Infect. Dis. Obstet. Gynecol. 2014, 2014, 908313. [Google Scholar] [CrossRef]
- Liu, G.J.; Wang, B.; Zhang, Y.; Xing, G.W.; Yang, X.; Wang, S. A tetravalent sialic acid-coated tetraphenylethene luminogen with aggregation-induced emission characteristics: Design, synthesis and application for sialidase activity assay, high-throughput screening of sialidase inhibitors and diagnosis of bacterial vaginosis. Chem. Commun. 2018, 54, 10691–10694. [Google Scholar]
- Rodríguez-Nava, C.; Cortés-Sarabia, K.; Avila-Huerta, M.D.; Ortiz-Riaño, E.J.; Estrada-Moreno, A.K.; Alarcón-Romero, L.D.C.; Mata-Ruíz, O.; Medina-Flores, Y.; Vences-Velázquez, A.; Morales-Narváez, E. Nanophotonic Sialidase Immunoassay for Bacterial Vaginosis Diagnosis. ACS Pharmacol. Transl. Sci. 2021, 4, 365–371. [Google Scholar] [CrossRef]
- Swidsinski, A.; Mendling, W.; Loening-Baucke, V.; Ladhoff, A.; Swidsinski, S.; Hale, L.P.; Lochs, H. Adherent biofilms in bacterial vaginosis. Obstet. Gynecol. 2005, 106, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Hickey, R.J.; Forney, L.J. Gardnerella vaginalis does not always cause bacterial vaginosis. J. Infect. Dis. 2014, 210, 1682–1683. [Google Scholar] [CrossRef]
- Kumar, N.; Behera, B.; Sagiri, S.S.; Pal, K.; Ray, S.S.; Roy, S. Bacterial vaginosis: Etiology and modalities of treatment—A brief note. J. Pharm. Bioallied Sci. 2011, 3, 496. [Google Scholar]
- Muzny, C.A.; Blanchard, E.; Taylor, C.M.; Aaron, K.J.; Talluri, R.; Griswold, M.E.; Redden, D.T.; Luo, M.; Welsh, D.A.; Van Der Pol, W.J.; et al. Identification of Key Bacteria Involved in the Induction of Incident Bacterial Vaginosis: A Prospective Study. J. Infect. Dis. 2018, 218, 966–978. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Doerffel, Y.; Loening-Baucke, V.; Swidsinski, S.; Verstraelen, H.; Vaneechoutte, M.; Lemm, V.; Schilling, J.; Mendling, W. Gardnerella biofilm involves females and males and is transmitted sexually. Gynecol. Obstet. Investigat. 2010, 70, 256–263. [Google Scholar] [CrossRef]
- Castro, J.; Machado, D.; Cerca, N. Unveiling the role of Gardnerella vaginalis in polymicrobial Bacterial Vaginosis biofilms: The impact of other vaginal pathogens living as neighbors. ISME J. 2019, 13, 1306–1317. [Google Scholar] [CrossRef]
- Hardy, L.; Jespers, V.; Abdellati, S.; De Baetselier, I.; Mwambarangwe, L.; Musengamana, V.; van de Wijgert, J.; Vaneechoutte, M.; Crucitti, T. A fruitful alliance: The synergy between Atopobium vaginae and Gardnerella vaginalis in bacterial vaginosis-associated biofilm. Sex. Transm. Infect. 2016, 92, 487–491. [Google Scholar] [CrossRef]
- Kalia, N.; Singh, J.; Kaur, M. Immunopathology of Recurrent Vulvovaginal Infections: New Aspects and Research Directions. Front. Immunol. 2019, 10, 2034. [Google Scholar] [CrossRef]
- Gholiof, M.; Adamson-De Luca, E.; Wessels, J.M. The female reproductive tract microbiotas, inflammation, and gynecological conditions. Front. Reprod. Health. 2022, 4, 963752. [Google Scholar] [CrossRef]
- Villa, P.; Cipolla, C.; D’Ippolito, S.; Amar, I.D.; Shachor, M.; Ingravalle, F.; Scaldaferri, F.; Puca, P.; Di Simone, N.; Scambia, G. The interplay between immune system and microbiota in gynecological diseases: A narrative review. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5676–5690. [Google Scholar]
- Anahtar, M.N.; Byrne, E.H.; Doherty, K.E.; Bowman, B.A.; Yamamoto, H.S.; Soumillon, M.; Padavattan, N.; Ismail, N.; Moodley, A.; Sabatini, M.E.; et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015, 42, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Pellis, V.; De Seta, F.; Crovella, S.; Bossi, F.; Bulla, R.; Guaschino, S.; Radillo, O.; Garred, P.; Tedesco, F. Mannose binding lectin and C3 act as recognition molecules for infectious agents in the vagina. Clin. Exp. Immunol. 2005, 139, 120–126. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, R.; Li, M.; Zeng, Z.; Zhang, L.; Liao, Q. The interplay between microbiota, metabolites, immunity during BV. Med. Microecol. 2022, 11, 100049. [Google Scholar] [CrossRef]
- Giraldo, P.C.; de Carvalho, J.B.; do Amaral, R.L.; da Silveira Gonçalves, A.K.; Eleutério, J., Jr.; Guimarães, F. Identification of immune cells by flow cytometry in vaginal lavages from women with vulvovaginitis and normal microflora. Am. J. Reprod. Immunol. 2012, 67, 198–205. [Google Scholar] [CrossRef]
- Aldunate, M.; Srbinovski, D.; Hearps, A.C.; Latham, C.F.; Ramsland, P.A.; Gugasyan, R.; Cone, R.A.; Tachedjian, G. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 2015, 6, 164. [Google Scholar] [CrossRef]
- Delgado-Diaz, D.J.; Tyssen, D.; Hayward, J.A.; Gugasyan, R.; Hearps, A.C.; Tachedjian, G. Distinct Immune Responses Elicited From Cervicovaginal Epithelial Cells by Lactic Acid and Short Chain Fatty Acids Associated with Optimal and Non-optimal Vaginal Microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 446. [Google Scholar] [CrossRef]
- Lewis, W.G.; Robinson, L.S.; Perry, J.; Bick, J.L.; Peipert, J.F.; Allsworth, J.E.; Lewis, A.L. Hydrolysis of secreted sialoglycoprotein immunoglobulin A (IgA) in ex vivo and biochemical models of bacterial vaginosis. J. Biol. Chem. 2012, 287, 2079–2089. [Google Scholar] [CrossRef]
- Berard, A.R.; Brubaker, D.K.; Birse, K.; Lamont, A.; Mackelprang, R.D.; Noël-Romas, L.; Perner, M.; Hou, X.; Irungu, E.; Mugo, N.; et al. Vaginal epithelial dysfunction is mediated by the microbiome, metabolome, and mTOR signaling. Cell Rep. 2023, 42, 112474. [Google Scholar] [CrossRef]
- Donders, G.G.G.; Bellen, G.; Grinceviciene, S.; Ruban, K.; Vieira-Baptista, P. Aerobic vaginitis: No longer a stranger. Res. Microbiol. 2017, 168, 845–858. [Google Scholar] [CrossRef]
- Turpin, R.; Tuddenham, S.; He, X.; Klebanoff, M.A.; Ghanem, K.G.; Brotman, R.M. Bacterial Vaginosis and Behavioral Factors Associated with Incident Pelvic Inflammatory Disease in the Longitudinal Study of Vaginal Flora. J. Infect. Dis. 2021, 224, S137–S144. [Google Scholar] [CrossRef]
- Willems, H.M.E.; Ahmed, S.S.; Liu, J.; Xu, Z.; Peters, B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi. 2020, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Norenhag, J.; Hu, Y.O.O.; Brusselaers, N.; Fransson, E.; Ährlund-Richter, A.; Guðnadóttir, U.; Angelidou, P.; Zha, Y.; Hamsten, M.; et al. Vaginal microbiota and human papillomavirus infection among young Swedish women. NPJ Biofilms Microbiomes 2020, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Cherenack, E.M.; Broedlow, C.A.; Klatt, N.R. The vaginal microbiome and HIV transmission dynamics. Curr. Opin. HIV AIDS. 2024, 19, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Lokken, E.M.; Balkus, J.E.; Kiarie, J.; Hughes, J.P.; Jaoko, W.; Totten, P.A.; McClelland, R.S.; Manhart, L.E. Association of Recent Bacterial Vaginosis with Acquisition of Mycoplasma genitalium. Am. J. Epidemiol. 2017, 186, 194–201. [Google Scholar] [CrossRef]
- Santella, B.; Schettino, M.T.; Franci, G.; De Franciscis, P.; Colacurci, N.; Schiattarella, A.; Galdiero, M. Microbiota and HPV: The role of viral infection on vaginal microbiota. J. Med. Virol. 2022, 94, 4478–4484. [Google Scholar] [CrossRef]
- Gudnadottir, U.; Debelius, J.W.; Du, J.; Hugerth, L.W.; Danielsson, H.; Schuppe-Koistinen, I.; Fransson, E.; Brusselaers, N. The vaginal microbiome and the risk of preterm birth: A systematic review and network meta-analysis. Sci. Rep. 2022, 12, 7926. [Google Scholar] [CrossRef]
- Kaambo, E.; Africa, C.; Chambuso, R.; Passmore, J.S. Vaginal Microbiomes Associated with Aerobic Vaginitis and Bacterial Vaginosis. Front. Public Health 2018, 6, 78. [Google Scholar] [CrossRef]
- Tao, Z.; Zhang, L.; Zhang, Q.; Lv, T.; Chen, R.; Wang, L.; Huang, Z.; Hu, L.; Liao, Q. The Pathogenesis of Streptococcus anginosus in Aerobic Vaginitis. Infect. Drug Resist. 2019, 12, 3745–3754. [Google Scholar] [CrossRef]
- Wang, C.; Fan, A.; Li, H.; Yan, Y.; Qi, W.; Wang, Y.; Han, C.; Xue, F. Vaginal bacterial profiles of aerobic vaginitis: A case-control study. Diagn. Microbiol. Infect. Dis. 2020, 96, 114981. [Google Scholar] [CrossRef]
- Fan, A.; Yue, Y.; Geng, N.; Zhang, H.; Wang, Y.; Xue, F. Aerobic vaginitis and mixed infections: Comparison of clinical and laboratory findings. Arch. Gynecol. Obstet. 2013, 287, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, E.F.M.; Wuyts, S.; Bellen, G.; Wittouck, S.; De Boeck, I.; Ruban, K.; Allonsius, C.N.; van den Broek, M.F.L.; Donders, G.G.G.; Lebeer, S. The Dwindling Microbiota of Aerobic Vaginitis, an Inflammatory State Enriched in Pathobionts with Limited TLR Stimulation. Diagnostics. 2020, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Stika, C.S. Atrophic vaginitis. Dermatol. Ther. 2010, 23, 514–522. [Google Scholar] [CrossRef]
- Krauss-Silva, L.; Almada-Horta, A.; Alves, M.B.; Camacho, K.G.; Moreira, M.E.; Braga, A. Basic vaginal pH, bacterial vaginosis and aerobic vaginitis: Prevalence in early pregnancy and risk of spontaneous preterm delivery, a prospective study in a low socioeconomic and multiethnic South American population. BMC Pregnancy Childbirth 2014, 4, 107. [Google Scholar] [CrossRef]
- Valeriano, V.D.; Lahtinen, E.; Hwang, I.C.; Zhang, Y.; Du, J.; Schuppe-Koistinen, I. Vaginal dysbiosis and the potential of vaginal microbiome-directed therapeutics. Front. Microbiomes 2024, 3, 1363089. [Google Scholar] [CrossRef]
- den Heijer, C.D.J.; Hoebe, C.J.P.A.; Driessen, J.H.M.; Wolffs, P.; van den Broek, I.V.F.; Hoenderboom, B.M.; Williams, R.; de Vries, F.; Dukers-Muijrers, N.H.T.M. Chlamydia trachomatis and the Risk of Pelvic Inflammatory Disease, Ectopic Pregnancy, and Female Infertility: A Retrospective Cohort Study Among Primary Care Patients. Clin. Infect. Dis. 2019, 69, 1517–1525. [Google Scholar] [CrossRef]
- Hoenderboom, B.M.; van Benthem, B.H.B.; van Bergen, J.E.A.M.; Dukers-Muijrers, N.H.T.M.; Götz, H.M.; Hoebe, C.J.P.A.; Hogewoning, A.A.; Land, J.A.; van der Sande, M.A.B.; Morré, S.A.; et al. Relation between Chlamydia trachomatis infection and pelvic inflammatory disease, ectopic pregnancy and tubal factor infertility in a Dutch cohort of women previously tested for chlamydia in a chlamydia screening trial. Sex. Transm. Infect. 2019, 95, 300–306. [Google Scholar]
- Price, M.J.; Ades, A.E.; Welton, N.J.; Simms, I.; Macleod, J.; Horner, P.J. Proportion of Pelvic Inflammatory Disease Cases Caused by Chlamydia trachomatis: Consistent Picture from Different Methods. J. Infect. Dis. 2016, 214, 617–624. [Google Scholar] [CrossRef]
- Haggerty, C.L.; Taylor, B.D. Mycoplasma genitalium: An emerging cause of pelvic inflammatory disease. Infect. Dis. Obstet. Gynecol. 2011, 2011, 959816. [Google Scholar] [CrossRef]
- Xu, S.X.; Gray-Owen, S.D. Gonococcal Pelvic Inflammatory Disease: Placing Mechanistic Insights Into the Context of Clinical and Epidemiological Observations. J. Infect. Dis. 2021, 224, S56–S63. [Google Scholar] [CrossRef]
- Ravel, J.; Moreno, I.; Simón, C. Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am. J. Obstet. Gynecol. 2021, 224, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Tamarelle, J.; Thiébaut, A.C.M.; de Barbeyrac, B.; Bébéar, C.; Ravel, J.; Delarocque-Astagneau, E. The vaginal microbiota and its association with human papillomavirus, Chlamydia trachomatis, Neisseria gonorrhoeae and Mycoplasma genitalium infections: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2019, 25, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Kerry-Barnard, S.; Zhou, L.; Phillips, L.; Furegato, M.; Witney, A.A.; Sadiq, S.T.; Oakeshott, P. Vaginal microbiota in ethnically diverse young women who did or did not develop pelvic inflammatory disease: Community-based prospective study. Sex. Transm. Infect. 2022, 98, 503–509. [Google Scholar] [CrossRef]
- Taylor, B.D.; Darville, T.; Haggerty, C.L. Does bacterial vaginosis cause pelvic inflammatory disease? Sex. Transm. Dis. 2013, 40, 117–122. [Google Scholar] [CrossRef]
- Nyirjesy, P.; Brookhart, C.; Lazenby, G.; Schwebke, J.; Sobel, J.D. Vulvovaginal Candidiasis: A Review of the Evidence for the 2021 Centers for Disease Control and Prevention of Sexually Transmitted Infections Treatment Guidelines. Clin. Infect. Dis. 2022, 74, S162–S168. [Google Scholar] [CrossRef]
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018, 18, e339–e347. [Google Scholar] [CrossRef]
- Fukazawa, E.I.; Witkin, S.S.; Robial, R.; Vinagre, J.G.; Baracat, E.C.; Linhares, I.M. Influence of recurrent vulvovaginal candidiasis on quality of life issues. Arch. Gynecol. Obstet. 2019, 300, 647–650. [Google Scholar] [CrossRef]
- Neal, C.M.; Martens, M.G. Clinical challenges in diagnosis and treatment of recurrent vulvovaginal candidiasis. SAGE Open Med. 2022, 10, 20503121221115201. [Google Scholar] [CrossRef]
- Sobel, J.D. Recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2016, 214, 15–21. [Google Scholar] [CrossRef]
- Rosati, D.; Bruno, M.; Jaeger, M.; Ten Oever, J.; Netea, M.G. Recurrent Vulvovaginal Candidiasis: An Immunological Perspective. Microorganisms 2020, 8, 144. [Google Scholar] [CrossRef]
- Hong, E.; Dixit, S.; Fidel, P.L.; Bradford, J.; Fischer, G. Vulvovaginal candidiasis as a chronic disease: Diagnostic criteria and definition. J. Low. Genit. Tract Dis. 2014, 18, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Yeater, K.M.; Hoyer, L.L. Cellular and molecular biology of Candida albicans estrogen response. Eukaryot. Cell 2006, 5, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Velloza, J.; Heffron, R. The Vaginal Microbiome and its Potential to Impact Efficacy of HIV Pre-exposure Prophylaxis for Women. Curr. HIV/AIDS Rep. 2017, 14, 153–160. [Google Scholar] [CrossRef]
- France, M.; Alizadeh, M.; Brown, S.; Ma, B.; Ravel, J. Towards a deeper understanding of the vaginal microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef]
- Shen, R.; Richter, H.E.; Smith, P.D. Interactions between HIV-1 and mucosal cells in the female reproductive tract. Am. J. Reprod. Immunol. 2014, 71, 608–617. [Google Scholar] [CrossRef]
- Hladik, F.; Sakchalathorn, P.; Ballweber, L.; Lentz, G.; Fialkow, M.; Eschenbach, D.; McElrath, M.J. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 2007, 26, 257–270. [Google Scholar] [CrossRef]
- Wang, Y.; Noël-Romas, L.; Perner, M.; Knodel, S.; Molatlhegi, R.; Hoger, S.; Birse, K.; Zuend, C.F.; McKinnon, L.R.; Burgener, A.D. Non-Lactobacillus-Dominant and Polymicrobial Vaginal Microbiomes Are More Common in Younger South African Women and Predictive of Increased Risk of Human Immunodeficiency Virus Acquisition. Clin. Infect. Dis. 2023, 76, 1372–1381. [Google Scholar] [CrossRef]
- Schellenberg, J.J.; Card, C.M.; Ball, T.B.; Mungai, J.N.; Irungu, E.; Kimani, J.; Jaoko, W.; Wachihi, C.; Fowke, K.R.; Plummer, F.A. Bacterial vaginosis, HIV serostatus and T-cell subset distribution in a cohort of East African commercial sex workers: Retrospective analysis. AIDS 2012, 26, 387–393. [Google Scholar] [CrossRef]
- Armstrong, E.; Kaul, R. Beyond bacterial vaginosis: Vaginal lactobacilli and HIV risk. Microbiome 2021, 9, 239. [Google Scholar] [CrossRef]
- Mitra, A.; MacIntyre, D.A.; Marchesi, J.R.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: What do we know and where are we going next? Microbiome 2016, 4, 58. [Google Scholar] [CrossRef]
- Wang, Y.; Thakur, R.; Shen, Q.; He, Y.; Chen, C. Influences of vaginal microbiota on human papillomavirus infection and host immune regulation: What we have learned? Decoding Infect. Transm. 2023, 1, 100002. [Google Scholar] [CrossRef]
- Di Paola, M.; Sani, C.; Clemente, A.M.; Iossa, A.; Perissi, E.; Castronovo, G.; Tanturli, M.; Rivero, D.; Cozzolino, F.; Cavalieri, D.; et al. Characterization of cervico-vaginal microbiota in women developing persistent high-risk Human Papillomavirus infection. Sci. Rep. 2017, 7, 10200. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, S.; Lee, H.; Song, Y.M.; Lee, K.; Han, M.J.; Sung, J.; Ko, G. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS ONE 2013, 8, e63514. [Google Scholar] [CrossRef]
- Mei, L.; Wang, T.; Chen, Y.; Wei, D.; Zhang, Y.; Cui, T.; Meng, J.; Zhang, X.; Liu, Y.; Ding, L.; et al. Dysbiosis of vaginal microbiota associated with persistent high-risk human papilloma virus infection. J. Transl. Med. 2022, 20, 12. [Google Scholar] [CrossRef]
- Alimena, S.; Davis, J.; Fichorova, R.N.; Feldman, S. The vaginal microbiome: A complex milieu affecting risk of human papillomavirus persistence and cervical cancer. Curr. Probl. Cancer. 2022, 46, 100877. [Google Scholar] [CrossRef]
- Balkus, J.E.; Richardson, B.A.; Rabe, L.K.; Taha, T.E.; Mgodi, N.; Kasaro, M.P.; Ramjee, G.; Hoffman, I.F.; Abdool Karim, S.S. Bacterial vaginosis and the risk of Trichomonas vaginalis acquisition among HIV-1-negative women. Sex. Transm. Dis. 2014, 41, 123–128. [Google Scholar] [CrossRef]
- Chiu, S.F.; Huang, P.J.; Cheng, W.H.; Huang, C.Y.; Chu, L.J.; Lee, C.C.; Lin, H.C.; Chen, L.C.; Lin, W.N.; Tsao, C.H.; et al. Vaginal Microbiota of the Sexually Transmitted Infections Caused by Chlamydia trachomatis and Trichomonas vaginalis in Women with Vaginitis in Taiwan. Microorganisms 2021, 9, 1864. [Google Scholar] [CrossRef]
- Margarita, V.; Fiori, P.L.; Rappelli, P. Impact of Symbiosis Between Trichomonas vaginalis and Mycoplasma hominis on Vaginal Dysbiosis: A Mini Review. Front. Cell. Infect. Microbiol. 2020, 10, 179. [Google Scholar] [CrossRef]
- Jarrett, O.D.; Srinivasan, S.; Richardson, B.A.; Fiedler, T.; Wallis, J.M.; Kinuthia, J.; Jaoko, W.; Mandaliya, K.; Fredricks, D.N.; McClelland, R.S. Specific Vaginal Bacteria Are Associated with an Increased Risk of Trichomonas vaginalis Acquisition in Women. J. Infect. Dis. 2019, 220, 1503–1510. [Google Scholar] [CrossRef]
- Marrazzo, J.M.; David, M.; Heather, W.D.; Schulte, J.D.O.; Sobel, J.D.; Hillier, S.L.; Deal, C.; Fredricks, D.N. Bacterial vaginosis: Identifying research gaps proceedings of a workshop sponsored by DHHS/NIH/NIAID. Sex. Transm. Dis. 2010, 37, 732–744. [Google Scholar] [CrossRef]
- Abbe, C.; Mitchell, C.M. Bacterial vaginosis: A review of approaches to treatment and prevention. Front. Reprod. Health 2023, 5, 1100029. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.S.; Sobel, J.D. Current Treatment of Bacterial Vaginosis—Limitations and Need for Innovation. J. Infect. Dis. 2016, 214, S14–S20. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for the Management of Symptomatic Sexually Transmitted Infections; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Machado, D.; Castro, J.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J.; Cerca, N. Bacterial Vaginosis Biofilms: Challenges to Current Therapies and Emerging Solutions. Front. Microbiol. 2016, 6, 1528. [Google Scholar] [CrossRef] [PubMed]
- Schwebke, J.R.; Desmond, R.A. Tinidazole vs metronidazole for the treatment of bacterial vaginosis. Am. J. Obstet. Gynecol. 2011, 204, 211.e1–211.e2116. [Google Scholar] [CrossRef]
- Donders, G.G.G.; Zodzika, J.; Rezeberga, D. Treatment of bacterial vaginosis: What we have and what we miss. Expert. Opin. Pharmacother. 2014, 15, 645–657. [Google Scholar] [CrossRef]
- Bilardi, J.; Walker, S.; McNair, R.; Mooney-Somers, J.; Temple-Smith, M.; Bellhouse, C.; Fairley, C.; Chen, M.; Bradshaw, C. Women’s Management of Recurrent Bacterial Vaginosis and Experiences of Clinical Care: A Qualitative Study. PLoS ONE 2016, 11, e0151794. [Google Scholar] [CrossRef]
- Bradshaw, C.S.; Brotman, R.M. Making inroads into improving treatment of bacterial vaginosis—Striving for long-term cure. BMC Infect. Dis. 2015, 15, 292. [Google Scholar] [CrossRef]
- Marrazzo, J.M.; Fiedler, T.L.; Srinivasan, S.; Thomas, K.K.; Liu, C.; Ko, D.; Xie, H.; Saracino, M.; Fredricks, D.N. Extravaginal reservoirs of vaginal bacteria as risk factors for incident bacterial vaginosis. J. Infect. Dis. 2012, 205, 1580–1588. [Google Scholar] [CrossRef]
- Muzny, C.A.; Sobel, J.D. The Role of Antimicrobial Resistance in Refractory and Recurrent Bacterial Vaginosis and Current Recommendations for Treatment. Antibiotics 2022, 11, 500. [Google Scholar] [CrossRef]
- DuPont, H.L. The potential for development of clinically relevant microbial resistance to rifaximin-α: A narrative review. Clin. Microbiol. Rev. 2023, 36, e0003923. [Google Scholar] [CrossRef]
- Shaskolskiy, B.; Dementieva, E.; Leinsoo, A.; Runina, A.; Vorobyev, D.; Plakhova, X.; Kubanov, A.; Deryabin, D.; Gryadunov, D. Drug Resistance Mechanisms in Bacteria Causing Sexually Transmitted Diseases and Associated with Vaginosis. Front. Microbiol. 2016, 7, 747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Song, J.J.; Zeng, S.H.; Huang, Y.L.; Luo, J.J.; Guo, W.L.; Li, X.Y. Plasmid-mediated azithromycin resistance in non-typhoidal Salmonella recovered from human infections. J. Antimicrob. Chemother. 2024, 79, 2688–2697. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Parvaiz, F.; Manzoor, S. Bacterial vaginosis: An insight into the prevalence, alternative treatments regimen and it’s associated resistance patterns. Microb. Pathog. 2019, 127, 21–30. [Google Scholar] [CrossRef]
- Nourbakhsh, F.; Nasrollahzadeh, M.S.; Tajani, A.S.; Soheili, V.; Hadizadeh, F. Bacterial biofilms and their resistance mechanisms: A brief look at treatment with natural agents. Folia Microbiol. 2022, 67, 535–554. [Google Scholar] [CrossRef]
- Tempera, G.; Furneri, P.M. Management of aerobic vaginitis. Gynecol. Obstet. Investig. 2010, 70, 244–249. [Google Scholar] [CrossRef]
- Sousa, L.G.V.; Pereira, S.A.; Cerca, N. Fighting polymicrobial biofilms in bacterial vaginosis. Microb. Biotechnol. 2023, 16, 1423–1437. [Google Scholar] [CrossRef]
- Donders, G.G.; Ruban, K.; Bellen, G. Selecting anti-microbial treatment of aerobic vaginitis. Curr. Infect. Dis. Rep. 2015, 17, 477. [Google Scholar] [CrossRef]
- FDA. Early Clinical Trials with Live Biotherapeutic Products: Chemistry, Manufacturing, and Control Information; Food Drug and Administration: College Park, MD, USA, 2016.
- Cordaillat-Simmons, M.; Rouanet, A.; Pot, B. Live biotherapeutic products: The importance of a defined regulatory framework. Exp. Mol. Med. 2020, 52, 1397–1406. [Google Scholar] [CrossRef]
- Amabebe, E.; Bhatnagar, N.; Kamble, N.; Reynolds, S.; Anumba, D.O. Exploring the antimicrobial properties of vaginal Lactobacillus crispatus against preterm birth-associated bacteria. Reprod. Fertil. 2022, 3, L6–L8. [Google Scholar] [CrossRef]
- Lagenaur, L.A.; Hemmerling, A.; Chiu, C.; Miller, S.; Lee, P.P.; Cohen, C.R.; Parks, T.P. Connecting the Dots: Translating the Vaginal Microbiome into a Drug. J. Infect. Dis. 2021, 223, S296–S306. [Google Scholar] [CrossRef]
- López-Moreno, A.; Aguilera, M. Vaginal Probiotics for Reproductive Health and Related Dysbiosis: Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 1461. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hugerth, L.W.; Schuppe-Koistinen, I.; Du, J. The right bug in the right place: Opportunities for bacterial vaginosis treatment. NPJ Biofilms Microbiomes 2022, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Lehtoranta, L.; Ala-Jaakkola, R.; Laitila, A.; Maukonen, J. Healthy Vaginal Microbiota and Influence of Probiotics Across the Female Life Span. Front. Microbiol. 2022, 13, 819958. [Google Scholar] [CrossRef] [PubMed]
- Muzny, C.A.; Łaniewski, P.; Schwebke, J.R.; Herbst-Kralovetz, M.M. Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr. Opin. Infect. Dis. 2020, 33, 59–65. [Google Scholar] [CrossRef]
- van der Veer, C.; Hertzberger, R.Y.; Bruisten, S.M.; Tytgat, H.L.P.; Swanenburg, J.; de Kat Angelino-Bart, A.; Schuren, F.; Molenaar, D.; Reid, G.; de Vries, H.; et al. Comparative genomics of human Lactobacillus crispatus isolates reveals genes for glycosylation and glycogen degradation: Implications for in vivo dominance of the vaginal microbiota. Microbiome 2019, 7, 49. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Yang, E.; Yan, L.; Li, T.; Zhuang, H. Antimicrobial Compounds Produced by Vaginal Lactobacillus crispatus Are Able to Strongly Inhibit Candida albicans Growth, Hyphal Formation and Regulate Virulence-Related Gene Expressions. Front. Microbiol. 2017, 8, 564. [Google Scholar] [CrossRef]
- Turovskiy, Y.; Ludescher, R.D.; Aroutcheva, A.A.; Faro, S.; Chikindas, M.L. Lactocin 160, a Bacteriocin Produced by Vaginal Lactobacillus rhamnosus, Targets Cytoplasmic Membranes of the Vaginal Pathogen, Gardnerella vaginalis. Probiotics Antimicrob. Proteins 2009, 1, 67–74. [Google Scholar] [CrossRef]
- Maldonado-Barragán, A.; Caballero-Guerrero, B.; Martín, V.; Ruiz-Barba, J.L.; Rodríguez, J.M. Purification and genetic characterization of gassericin E, a novel co-culture inducible bacteriocin from Lactobacillus gasseri EV1461 isolated from the vagina of a healthy woman. BMC Microbiol. 2016, 16, 37. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C. Host interactions of probiotic bacterial surface molecules: Comparison with commensals and pathogens. Nat. Rev. Microbiol. 2010, 8, 171–184. [Google Scholar] [CrossRef]
- Pradines, B.; Domenichini, S.; Lievin-Le Moal, V. Adherent Bacteria and Parasiticidal Secretion Products of Human Cervicovaginal Microbiota-Associated Lactobacillus gasseri Confer Non-Identical Cell Protection against Trichomonas vaginalis-Induced Cell Detachment. Pharmaceuticals 2022, 15, 1350. [Google Scholar] [CrossRef] [PubMed]
- Edelman, S.M.; Lehti, T.A.; Kainulainen, V.; Antikainen, J.; Kylväjä, R.; Baumann, M.; Westerlund-Wikström, B.; Korhonen, T.K. Identification of a high-molecular-mass Lactobacillus epithelium adhesin (LEA) of Lactobacillus crispatus ST1 that binds to stratified squamous epithelium. Microbiology 2012, 158, 1713–1722. [Google Scholar] [CrossRef]
- Ojala, T.; Kankainen, M.; Castro, J.; Cerca, N.; Edelman, S.; Westerlund-Wikström, B.; Paulin, L.; Holm, L.; Auvinen, P. Comparative genomics of Lactobacillus crispatus suggests novel mechanisms for the competitive exclusion of Gardnerella vaginalis. BMC Genom. 2014, 15, 1070. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, G.; Molinaro, A.; Cimini, D.; De Castro, C.; Valli, V.; De Gregorio, V.; De Rosa, M.; Schiraldi, C. Lactobacillus crispatus L1: High cell density cultivation and exopolysaccharide structure characterization to highlight potentially beneficial effects against vaginal pathogens. BMC Microbiol. 2014, 14, 137. [Google Scholar] [CrossRef]
- Song, J.; Lang, F.; Zhao, N.; Guo, Y.; Zhang, H. Vaginal Lactobacilli Induce Differentiation of Monocytic Precursors Toward Langerhans-like Cells: In Vitro Evidence. Front. Immunol. 2018, 9, 2437. [Google Scholar] [CrossRef]
- Hummelen, R.; Changalucha, J.; Butamanya, N.L.; Cook, A.; Habbema, J.D.; Reid, G. Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 to prevent or cure bacterial vaginosis among women with HIV. Int. J. Gynaecol. Obstet. 2010, 111, 245–248. [Google Scholar] [CrossRef]
- Laue, C.; Papazova, E.; Liesegang, A.; Pannenbeckers, A.; Arendarski, P.; Linnerth, B.; Domig, K.J.; Kneifel, W.; Petricevic, L.; Schrezenmeir, J. Effect of a yoghurt drink containing Lactobacillus strains on bacterial vaginosis in women—A double-blind, randomised, controlled clinical pilot trial. Benef. Microbes 2018, 9, 35–50. [Google Scholar] [CrossRef]
- Heczko, P.B.; Tomusiak, A.; Adamski, P.; Jakimiuk, A.J.; Stefański, G.; Mikołajczyk-Cichońska, A.; Suda-Szczurek, M.; Strus, M. Supplementation of standard antibiotic therapy with oral probiotics for bacterial vaginosis and aerobic vaginitis: A randomised, double-blind, placebo-controlled trial. BMC Womens Health 2015, 15, 115. [Google Scholar] [CrossRef]
- Pendharkar, S.; Brandsborg, E.; Hammarström, L.; Marcotte, H.; Larsson, P.G. Vaginal colonisation by probiotic lactobacilli and clinical outcome in women conventionally treated for bacterial vaginosis and yeast infection. BMC Infect. Dis. 2015, 15, 255. [Google Scholar] [CrossRef]
- Davar, R.; Nokhostin, F.; Eftekhar, M.; Sekhavat, L.; Bashiri Zadeh, M.; Shamsi, F. Comparing the Recurrence of Vulvovaginal Candidiasis in Patients Undergoing Prophylactic Treatment with Probiotic and Placebo During the 6 Months. Probiotics Antimicrob. Proteins 2016, 8, 130–133. [Google Scholar] [CrossRef]
- Cohen, C.R.; Wierzbicki, M.R.; French, A.L.; Morris, S.; Newmann, S.; Reno, H.; Green, L.; Miller, S.; Powell, J.; Parks, T.; et al. Randomized Trial of Lactin-V to Prevent Recurrence of Bacterial Vaginosis. N. Engl. J. Med. 2020, 382, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Reznichenko, H.; Henyk, N.; Maliuk, V.; Khyzhnyak, T.; Tynna, Y.; Filipiuk, I.; Veresniuk, N.; Zubrytska, L.; Quintens, J.; Richir, K.; et al. Oral Intake of Lactobacilli Can Be Helpful in Symptomatic Bacterial Vaginosis: A Randomized Clinical Study. J. Low. Genit. Tract Dis. 2020, 24, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lyu, J.; Ge, L.; Huang, L.; Peng, Z.; Liang, Y.; Zhang, X.; Fan, S. Probiotic Lacticaseibacillus rhamnosus GR-1 and Limosilactobacillus reuteri RC-14 as an Adjunctive Treatment for Bacterial Vaginosis Do Not Increase the Cure Rate in a Chinese Cohort: A Prospective, Parallel-Group, Randomized, Controlled Study. Front. Cell. Infect. Microbiol. 2021, 11, 669901. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Lee, E.S.; Park, S.T.; Jeong, S.Y.; Yun, Y.; Kim, Y.; Jeong, Y.; Kang, C.H.; Choi, H.J. Efficacy and Safety of MED-01 Probiotics on Vaginal Health: A 12-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 331. [Google Scholar] [CrossRef]
- Tomusiak, A.; Strus, M.; Heczko, P.B.; Adamski, P.; Stefański, G.; Mikołajczyk-Cichońska, A.; Suda-Szczurek, M. Efficacy and safety of a vaginal medicinal product containing three strains of probiotic bacteria: A multicenter, randomized, double-blind, and placebo-controlled trial. Drug Des. Devel. Ther. 2015, 9, 5345–5354. [Google Scholar] [CrossRef]
- Recine, N.; Palma, E.; Domenici, L.; Giorgini, M.; Imperiale, L.; Sassu, C.; Musella, A.; Marchetti, C.; Muzii, L.; Panici, P.B. Restoring vaginal microbiota: Biological control of bacterial vaginosis. A prospective case-control study using Lactobacillus rhamnosus BMX 54 as adjuvant treatment against bacterial vaginosis. Arch. Gynecol. Obstet. 2016, 293, 101–107. [Google Scholar] [CrossRef]
- Palma, E.; Recine, N.; Domenici, L.; Giorgini, M.; Pierangeli, A.; Panici, P.B. Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: A promising solution against HPV-infection. BMC Infect. Dis. 2018, 18, 13. [Google Scholar] [CrossRef]
- Marcotte, H.; Larsson, P.G.; Andersen, K.K.; Zuo, F.; Mikkelsen, L.S.; Brandsborg, E.; Gray, G.; Laher, F.; Otwombe, K. An exploratory pilot study evaluating the supplementation of standard antibiotic therapy with probiotic lactobacilli in south African women with bacterial vaginosis. BMC Infect. Dis. 2019, 19, 824. [Google Scholar] [CrossRef]
- Mändar, R.; Sõerunurk, G.; Štšepetova, J.; Smidt, I.; Rööp, T.; Kõljalg, S.; Saare, M.; Ausmees, K.; Le, D.D.; Jaagura, M.; et al. Impact of Lactobacillus crispatus-containing oral and vaginal probiotics on vaginal health: A randomised double-blind placebo controlled clinical trial. Benef. Microbes 2023, 14, 143–152. [Google Scholar] [CrossRef]
- Russo, R.; Superti, F.; Karadja, E.; De Seta, F. Randomised clinical trial in women with Recurrent Vulvovaginal Candidiasis: Efficacy of probiotics and lactoferrin as maintenance treatment. Mycoses 2019, 62, 328–335. [Google Scholar] [CrossRef]
- Lyra, A.; Ala-Jaakkola, R.; Yeung, N.; Datta, N.; Evans, K.; Hibberd, A.; Lehtinen, M.J.; Forssten, S.D.; Ibarra, A.; Pesonen, T.; et al. A Healthy Vaginal Microbiota Remains Stable during Oral Probiotic Supplementation: A Randomised Controlled Trial. Microorganisms 2023, 11, 499. [Google Scholar] [CrossRef] [PubMed]
- Vicariotto, F.; Malfa, P.; Viciani, E.; Dell’Atti, F.; Squarzanti, D.F.; Marcante, A.; Castagnetti, A.; Ponchia, R.; Governini, L.; De Leo, V. Efficacy of Lactiplantibacillus plantarum PBS067, Bifidobacterium animalis subsp. lactis BL050, and Lacticaseibacillus rhamnosus LRH020 in the Amelioration of Vaginal Microbiota in Post-Menopausal Women: A Prospective Observational Clinical Trial. Nutrients 2024, 16, 402. [Google Scholar]
- Baldacci, F.; Baldacci, M.; Bertini, M. Lactobacillus rhamnosus BMX 54 + Lactose, A Symbiotic Long-Lasting Vaginal Approach to Improve Women’s Health. Int. J. Womens Health 2020, 12, 1099–1104. [Google Scholar] [CrossRef]
- Vivekanandan, V.; Khan, Z.H.; Venugopal, G.; Musunuru, B.; Mishra, P.; Srivastava, S.; Ramadass, B.; Subhadra, B. VagiBIOM Lactobacillus suppository improves vaginal health index in perimenopausal women with bacterial vaginosis: A randomized control trial. Sci. Rep. 2024, 14, 3317. [Google Scholar] [CrossRef]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef]
- Wrønding, T.; Vomstein, K.; Bosma, E.F.; Mortensen, B.; Westh, H.; Heintz, J.E.; Mollerup, S.; Petersen, A.M.; Ensign, L.M.; DeLong, K.; et al. Antibiotic-free vaginal microbiota transplant with donor engraftment, dysbiosis resolution and live birth after recurrent pregnancy loss: A proof of concept case study. EClinicalMedicine 2023, 61, 102070. [Google Scholar] [CrossRef]
- Oerlemans, E.F.M.; Bellen, G.; Claes, I.; Henkens, T.; Allonsius, C.N.; Wittouck, S.; van den Broek, M.F.L.; Wuyts, S.; Kiekens, F.; Donders, G.G.G.; et al. Impact of a lactobacilli-containing gel on vulvovaginal candidosis and the vaginal microbiome. Sci. Rep. 2020, 10, 7976. [Google Scholar] [CrossRef]
- Yoshikata, R.; Yamaguchi, M.; Mase, Y.; Tatsuyuki, A.; Myint, K.Z.Y.; Ohta, H. Evaluation of the efficacy of Lactobacillus-containing feminine hygiene products on vaginal microbiome and genitourinary symptoms in pre- and postmenopausal women: A pilot randomized controlled trial. PLoS ONE 2022, 17, e0270242. [Google Scholar] [CrossRef]
- Shen, X.; Xu, L.; Zhang, Z.; Yang, Y.; Li, P.; Ma, T.; Guo, S.; Kwok, L.Y.; Sun, Z. Postbiotic gel relieves clinical symptoms of bacterial vaginitis by regulating the vaginal microbiota. Front. Cell. Infect. Microbiol. 2023, 13, 1114364. [Google Scholar] [CrossRef]
- Al-Nasiry, S.; Ambrosino, E.; Schlaepfer, M.; Morré, S.A.; Wieten, L.; Voncken, J.W.; Spinelli, M.; Mueller, M.; Kramer, B.W. The Interplay Between Reproductive Tract Microbiota and Immunological System in Human Reproduction. Front. Immunol. 2020, 11, 378. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Neurodevelopmental Disorders Associated with Gut Microbiome Dysbiosis in Children. Children 2024, 11, 796. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Ruiz, A.; Borrego, J.J. Microbial Dysbiosis in the Skin Microbiome and Its Psychological Consequences. Microorganisms 2024, 12, 1908. [Google Scholar] [CrossRef]
- Barrientos-Durán, A.; Fuentes-López, A.; de Salazar, A.; Plaza-Díaz, J.; García, F. Reviewing the Composition of Vaginal Microbiota: Inclusion of Nutrition and Probiotic Factors in the Maintenance of Eubiosis. Nutrients 2020, 12, 419. [Google Scholar] [CrossRef]
- Morsli, M.; Gimenez, E.; Magnan, C.; Salipante, F.; Huberlant, S.; Letouzey, V.; Lavigne, J.P. The association between lifestyle factors and the composition of the vaginal microbiota: A review. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 1869–1881. [Google Scholar] [CrossRef]
- Allsworth, J.E.; Peipert, J.F. Severity of bacterial vaginosis and the risk of sexually transmitted infection. Am. J. Obstet. Gynecol. 2011, 205, 113.e1–113.e6. [Google Scholar] [CrossRef]
- Lamont, R.F.; Taylor-Robinson, D. The role of bacterial vaginosis, aerobic vaginitis, abnormal vaginal flora and the risk of preterm birth. BJOG 2010, 117, 119–120. [Google Scholar] [CrossRef]
- Srinivasan, S.; Hoffman, N.G.; Morgan, M.T.; Matsen, F.A.; Fiedler, T.L.; Hall, R.W.; Ross, F.J.; McCoy, C.O.; Bumgarner, R.; Marrazzo, J.M.; et al. Bacterial communities in women with bacterial vaginosis: High resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS ONE 2012, 7, e37818. [Google Scholar] [CrossRef]
- DeLong, K.; Bensouda, S.; Zulfiqar, F.; Zierden, H.C.; Hoang, T.M.; Abraham, A.G.; Coleman, J.S.; Cone, R.A.; Gravitt, P.E.; Hendrix, C.W.; et al. Conceptual Design of a Universal Donor Screening Approach for Vaginal Microbiota Transplant. Front. Cell. Infect. Microbiol. 2019, 9, 306. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, J.; Zhang, G. Vaginal microbiota transplantation is a truly opulent and promising edge: Fully grasp its potential. Front. Cell. Infect. Microbiol. 2024, 14, 1280636. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Fecal Microbiota Transplantation as a Tool for Therapeutic Modulation of Neurological and Mental Disorders. SciBase Neurol. 2024, 2, 1018. [Google Scholar] [CrossRef]
- Rouanet, A.; Bolca, S.; Bru, A.; Claes, I.; Cvejic, H.; Girgis, H.; Harper, A.; Lavergne, S.N.; Mathys, S.; Pane, M.; et al. Live Biotherapeutic Products, A Road Map for Safety Assessment. Front. Med. 2020, 7, 237. [Google Scholar] [CrossRef] [PubMed]
- Heavey, M.K.; Durmusoglu, D.; Crook, N.; Anselmo, A.C. Discovery and delivery strategies for engineered live biotherapeutic products. Trends Biotechnol. 2022, 40, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Ducarmon, Q.R.; Kuijper, E.J.; Olle, B. Opportunities and Challenges in Development of Live Biotherapeutic Products to Fight Infections. J. Infect. Dis. 2021, 223, S283–S289. [Google Scholar] [CrossRef]
- Pot, B.; Vandenplas, Y. Factors that influence clinical efficacy of live biotherapeutic products. Eur. J. Med. Res. 2021, 26, 40. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Wong, S.; Yu, J.; Lee, Y.Y.; Terauchi, J.; Lai, H.C.; Luo, J.C.; Kao, C.Y.; Yu, S.L.; Liou, J.M.; et al. Development of live biotherapeutic products: A position statement of Asia-Pacific Microbiota Consortium. Gut 2025, 74, 706–713. [Google Scholar] [CrossRef]
- Dreher-Lesnick, S.M.; Stibitz, S.; Carlson, P.E., Jr. U.S. Regulatory Considerations for Development of Live Biotherapeutic Products as Drugs. Microbiol. Spectr. 2017, 5, 10.1128. [Google Scholar] [CrossRef]
- Nakra, N.A.; Madan, R.P.; Buckley, N.; Huber, A.M.; Freiermuth, J.L.; Espinoza, L.; Walsh, J.; Parikh, U.M.; Penrose, K.J.; Keller, M.J.; et al. Loss of innate host defense following unprotected vaginal sex. J. Infect. Dis. 2016, 213, 840–847. [Google Scholar] [CrossRef]
- Sabo, M.C.; Balkus, J.E.; Richardson, B.A.; Srinivasan, S.; Kimani, J.; Anzala, O.; Schwebke, J.; Feidler, T.L.; Fredricks, D.N.; McClelland, R.S. Association between vaginal washing and vaginal bacterial concentrations. PLoS ONE 2019, 14, e0210825. [Google Scholar] [CrossRef]
- Michala, L. The Adolescent and Genital Dissatisfaction. Clin. Obstet. Gynecol. 2020, 63, 528–535. [Google Scholar] [CrossRef]
- Moulton, L.J.; Jernigan, A.M. Management of Retained Genital Piercings: A Case Report and Review. Case Rep. Obstet. Gynecol. 2017, 2017, 2402145. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A. A current overview on adolescent alcohol misuse and its potential negative impacts. Alcohol. Drug Addict./Alkohol. Narkomania 2025, 37, 205–231. [Google Scholar] [CrossRef]
- Aslan, E.; Bechelaghem, N. To ‘douche’ or not to ‘douche’: Hygiene habits may have detrimental effects on vaginal microbiota. J. Obstet. Gynaecol. 2018, 38, 678–681. [Google Scholar] [CrossRef]
- Łaniewski, P.; Owen, K.A.; Khnanisho, M.; Brotman, R.M.; Herbst-Kralovetz, M.M. Clinical and Personal Lubricants Impact the Growth of Vaginal Lactobacillus Species and Colonization of Vaginal Epithelial Cells: An in Vitro Study. Sex. Transm. Dis. 2021, 48, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, E.M.; Łaniewski, P.; Herbst-Kralovetz, M.M.; Brotman, R.M. Personal and Clinical Vaginal Lubricants: Impact on Local Vaginal Microenvironment and Implications for Epithelial Cell Host Response and Barrier Function. J. Infect. Dis. 2019, 220, 2009–2018. [Google Scholar] [CrossRef]
- Jovandaric, M.Z.; Jovanović, K.; Raus, M.; Babic, S.; Igic, T.; Kotlica, B.; Milicevic, S. The Significance of Plant Nutrition in the Creation of the Intestinal Microbiota—Prevention of Chronic Diseases: A Narrative Review. Medicina 2024, 60, 1969. [Google Scholar] [CrossRef]
- Yang, H.J.; Kim, D.S.; Lee, K.W.; Kim, Y.H. The Urinary Microbiome; Axis Crosstalk and Short-Chain Fatty Acid. Diagnostics 2022, 12, 3119. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; González-Domenech, C.M.; Borrego, J.J. The Role of Fermented Vegetables as a Sustainable and Health-Promoting Nutritional Resource. Appl. Sci. 2024, 14, 10853. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Hellenic Society for Microbiology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).