Abstract
In developing countries like Bangladesh, livestock is one of the main sources of income. Among several infectious diseases, the Gram-positive bacterium Bacillus anthracis causes a zoonotic disease named anthrax. Animal anthrax outbreaks are a frequently occurring problem in Bangladesh. Our present study aims to molecularly identify and characterize B. anthracis from three districts of Bangladesh by 16S rRNA gene sequencing. B. anthracis was confirmed in soil, meat, and blood samples using PCR. Anthrax-affected soil (n = 128), blood (n = 1), and meat (n = 2) samples were analyzed using PCR. One of the positive samples was randomly chosen for sequencing, and MEGA5 software was used to generate the phylogenetic tree from the sequencing result. A total of 21 (16.40%) soil samples and all of the blood and meat samples were positive for the presence of bacteria, confirmed by PCR. The 16S rRNA gene of B. anthracis Sirajganj-1 was identical to that of other strains. To fulfill the Sustainable Development Goals, it is important to control zoonotic diseases. Our results may help discover the virulent genes of B. anthracis for future investigation and control this zoonotic disease. Also, a proper awareness of vaccination and effective surveillance system is important to eradicate any kind of zoonotic disease in developing nations.
1. Introduction
Bacillus anthracis is a Gram-positive bacterium that is toxigenic, large, rod-shaped, nonmotile, and spore-forming, and is found in the soil [1]. There is a possibility that highly resistant endospores of this bacterium might persist in the environment outside of living hosts for decades during the time between outbreaks [2]. Neutral to slightly alkaline soil; calcareous, vegetative, or humic-rich soil; a drought followed by heavy rainfall; a high mean ambient temperature; opening dead animals without burning them; flooding pastures with contaminated water or dumping infected carcasses in streams, ponds, or rivers; grazing animals on infected pastures; contaminated feed; feeding animals uprooted and unwashed grass or water hyacinth; a decreasing vulture population; and slaughtering are all environmental factors that are commonly known to increase the likelihood of anthrax outbreaks [3,4,5]. There is a possibility that B. anthracis spores may remain viable and inactive in soil for a number of years [6]. It is plausible that they might be a source of infection for cattle that are grazing during this time period; however, they do not provide a direct route of infection for humans. The spores of B. anthracis germinate after they have entered the host, and they create bacilli that are encased and capable of generating toxins. There is a rapid death rate among infected animals due to septicemia and toxemia [7,8].
In Bangladesh, there are infrequent outbreaks of anthrax in animals, which are then followed by outbreaks of anthrax in humans [6]. Carlson et al. [9] estimated that between 20,000 and 100,000 cases of anthrax occur annually around the globe. This is due to the fact that an estimated 1.8 billion people live in areas that are ideal for anthrax, with the vast majority of these individuals residing in rural areas in Africa, Europe, and Asia. In addition to having a negative impact on people’s general lives, an anthrax outbreak has both direct and indirect effects on the economy of the nation. The livestock industry is an important aspect of Bangladesh’s agricultural economy and continues to play a significant role in the lives of those who are economically disadvantaged [10]. Due to a lack of proper measurement, the financial impact of anthrax disease is not being adequately addressed. Between 1949 and 1986, Bangladesh had incidences of anthrax in both animals and humans. Between 1980 and October 2010, Bangladesh saw 450 instances of anthrax in animals and 725 cases of the disease in humans. Additionally, there were 1320 occurrences of cutaneous anthrax up to July of 2016 [11].
There is a particularly high incidence of the disease in the districts of Kushtia, Sirajganj, and Pabna in Bangladesh, which are home to the biggest cattle populations in the nation [12]. B. anthracis is capable of producing spores that are resistant to heat and the majority of chemical disinfectants in situations when conditions are poor. These spores can remain viable in soil for decades [13]. Soil is a potential factor that is associated with the survival of spores and the ecological conditions that are present during anthrax epidemics [14]. This is especially true in states that have temperatures that are classified as tropical or subtropical, such as Bangladesh [15]. The use of molecular techniques is the most efficient way for determining the presence of B. anthrasis in soil-borne anthrax in a timely and definitive manner [16]. The identification of the anthrax-causing bacterium and the molecular characterization of its virulence genes are of the utmost importance, as this zoonotic sickness continues to grow. This might contribute to the development of a successful vaccine [10,17,18]. Molecular characterization and determining the existence of vegetative spores or bacteria were the goals of this investigation, which was meant to identify whether or not these organisms were present in clinical specimens or in the open environment.
2. Materials and Methods
2.1. Sample Collection
This research was conducted at the Bangladesh Agricultural University (BAU), Mymensingh-2202, Bangladesh, in the Department of Microbiology and Hygiene, which is part of the Faculty of Veterinary Science (FVS), according to previous researchers protocol [11,19]. A total of 128 soil samples and three clinical specimens were collected in a random fashion from anthrax-positive locations (Figure 1) that were reported or believed to be sites of corpse disposal or burial, animal habitats, and cattle pasturing sites in the study regions. A wide variety of elements, including dirt, animal waste, bones, and fragments of dead bodies, were found in the soil samples that were collected. In order to collect 400 g of surface soil to a depth of up to two feet, a shovel was used. The material was then carefully transferred to plastic bags that were double-layered and tagged. Using a plastic syringe with a capacity of 5 mL, a single blood sample was collected from a cow that was suspected of having anthrax and then transported to the laboratory. Two samples of meat were acquired from cattle that were suspected of having anthrax and were preserved in nutritional broth. These samples were then transported to the laboratory of the BAU Department of Microbiology and Hygiene while preserving regular laboratory conditions.
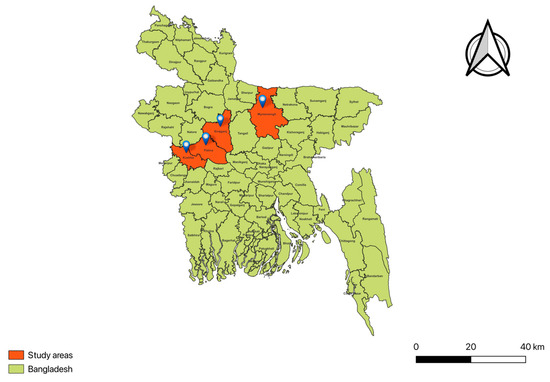
Figure 1.
A geographical map of Bangladesh showing the selected locations of the study areas.
2.2. Cultural Identification
One gram of each soil sample was heated in sterile distilled or deionized water for fifteen minutes in a hot water bath in the laboratory. Following this, 90–100 microliters of the supernatant was plated on selective PLET medium and grown at 37 degrees Celsius for a whole night. When grown on PLET medium, the colonies display cultural characteristics that are typical of B. anthracis. In addition, positive samples were subcultured in a variety of bacteriological media, including nutritional agar, bovine blood agar, and horse blood agar, in order to investigate the characteristics of the colonies. In order to investigate the staining qualities, Gram staining was used [13].
2.3. Extraction of Genomic DNA
Genomic DNA was extracted from each B. anthracis isolate using the boiling method. A single colony of each isolate was diluted with 200 µL of distilled water before being brought to a boil for a duration of ten minutes. Following the boiling process, the samples were put on ice for a brief period of time in order to induce cold shock. At last, centrifugation at 10,000 rpm for ten minutes was carried out. The DNA template for the polymerase chain reaction (PCR) was obtained from the supernatant [20].
2.4. Amplification of Sap, Protective Antigen (PA) of pX01, Capsule (pX02), and 16S rRNA Genes
We employed anthracis-specific primers for PCR (Table 1). A total of 12.5 µL PCR master mix, 2 µL of each primer, 4 µL of template DNA, and 4.5 µL nuclease-free deionized water made up the 25 µL PCR mixture [21]. PCR thermal profiles for Sap, pX01 PA, and pX02 capsule were used from the lab protocol. The 16S rRNA PCR thermal profile was as follows: 2 min for initial denaturation at 95 °C, 30 s for denaturation at 95 °C, 40 s for annealing at 50 °C, 1.5 min for elongation at 72 °C, and 10 min for final extension at 72 °C. The holding temperature was 4 °C. In every instance, the amplified products were electrophoresed in 1.5% agarose gel at a voltage of 60 volts for a duration of one hour. A 1 kb DNA ladder (Promega, San Luis Obispo, CA, USA) was used to evaluate the gel after it had been stained with ethidium bromide. The examination was performed under a UV transilluminator [14].

Table 1.
Primer sequences used in this study.
2.5. Sequencing of PCR Products
In order to purify the PCR product, the PCR Purification Kit was used in accordance with the directions provided by the manufacturer. Electrophoresis was performed on a 1% agarose gel using about 3 µL of the purified PCR product. PCR primers obtained from an offshore commercial sequencing facility (1st BASE, Seri Kembangan, Selangor DE, Malaysia) were used in order to sequence purified PCR products derived from isolates in both orientations. The raw sequencing data were gathered and compiled with the help of MEGA5. The MegAlign and MEGA5 software programs were used in order to align the sequences of the present investigation with the sequences that are accessible in GenBank. ClustalW and MEGA5 were used to perform multiple alignments in order to generate phylogenetic trees.
3. Results and Discussion
Anthrax is thought to be one of the most effective biological warfare agents since its spores are very resistant to natural conditions and may live in the environment for decades. Anthrax has been eradicated in Sweden and, most likely, Austria, the Czech Republic, Denmark, Finland, and Luxembourg [23]. Most of countries are in agreement that to eradicate this disease, early detection is important, and PCR-based detection is a sensitive and accurate approach to do that. Vaccination and mobility restrictions have been the primary approaches utilized in Australia to manage anthrax in pasture-based livestock systems [24], but for proper vaccination, proper isolation and sequencing of that organism is necessary. We know that genes have all the embedded information that is important to make an appropriate vaccine, although many wealthy countries like Canada and the United States employ different methods to control this disease. Canada and the United States have a depopulation program focused on avoiding the spread of anthrax [25]. However, in developing or underdeveloped countries, livestock generate income for many families. That is why depopulation programs are not feasible in many low-income countries as well as in Bangladesh. When it comes to managing and controlling anthrax risk, having a technique that is both quick and sensitive to identify B. anthracis is essential. This is because it allows the early tracking and eradication of the infection source, as well as a prompt diagnosis for administering the proper treatment for the illness.
Preliminary screening was performed on B. anthracis utilizing colony morphologies on PLET agar. Following the appearance of rough, creamy-white colonies that were 1–2 mm in size on PLET agar medium, 21 out of 128 samples were believed to be B. anthracis (Table 2) based on colony morphology. In a blood agar plate, each of the 21 potential B. anthracis isolates produced grey colonies that failed to produce hemolytic activity. Gram staining was performed on the only colony of B. anthracis cultures that was present. Gram staining allowed for the identification of organisms that were Gram-positive, violet, large, rod-shaped, solitary, paired, or long-chained. A distinctive ‘Medusa head’ look was formed by the colonies when they were seen using a microscope with a low power. These colonies exhibited some tailing and noticeable wisps of growth that trailed back towards the parent colony. We employed the heating approach to promote spore germination, as was described in earlier investigations [26]. In this procedure, the samples were heated at temperature–time combinations that were expected to eliminate the majority of vegetative bacteria without damaging the spores. Based on our research, we found that the optimal combination for generating spores is 75 degrees Celsius for fifteen minutes. PLET medium was shown to be acceptable for the isolation of B. anthracis, as was reported in earlier research [26]. Additionally, we discovered that it was able to limit contamination by other closely related bacilli. Other investigations, such as the one conducted by Dragon and Rennie in 2001, have discovered that the use of PLET selective medium is less desirable when compared to the utilization of nonselective media such as sheep blood agar [13].

Table 2.
Prevalence of B. anthracis in examined soil and clinical samples.
The poly-alpha-D-glutamic acid capsule and the tripartite protein toxins that are encoded by the capsule (capA, capB, and capC) and the toxin (pag, cya, and lef) genes on plasmids pXO2 and pXO1, respectively, are the two virulence factors that are responsible for the pathogenic properties expressed by B. anthracis. Genus-specific Sap primers verified 21 isolates as B. anthracis (Figure 2A, Table 2). A total of 11 B. anthracis isolates tested positive for the pX01 gene and 7 for the pX02 gene (Figure 2B,C, Table 3).
Figure 2.
(A) Amplification of Sap gene (639 bp) using specific primer. Lane M: 1 kbp DNA ladder; lane 1–21: positive for Sap gene. (B) Amplification of pX01 gene (596 bp) using specific primer. Lane M: 1 kbp DNA ladder; lane 1–11: positive for pX01 gene. (C) Amplification of pX02 gene (846 bp) using specific primer. Lane M: 1 kbp DNA ladder; Lane 1–7: positive for pX02 gene.

Table 3.
Summary of pX01 and pX02 plasmids in B. anthracis from different locations.
Within the scope of this investigation, there were 21 (16.40%) positive isolates of B. anthracis. The Pabna district, which had the most, was home to forty percent of these individuals. Blood and meat samples demonstrated a detection accuracy of one hundred percent for B. anthracis, which indicates that a blood or meat sample is appropriate for proving the presence of anthrax due to its ability to justify the incidence of the illness. When it comes to the epidemiology of anthrax, the capacity of these spores to stay viable in soil for extended periods of time is a significant component. This is especially true for animals that graze in the area of these corpse burial sites and drink water from adjacent ‘collector’ watering stations. The process by which the bacteria that cause anthrax which present in the soil has been the subject of investigation in a number of research studies. In the north of Canada, in the south of Sudan, and in Isfahan, Iran, research studies that were quite similar to this one were conducted [2,13]. Moazeni-Jula et al. [2] collected sixty samples of soil and discovered nine (15%) of them to be unique. Dragon et al. [13] reported that the theoretical sensitivity for isolating B. anthracis in PLET medium was 10 spores per gram of soil sample. This was reported by the scientific community. All of the work that was performed in this study to isolate and identify B. anthracis from soil samples is consistent with the findings of the “Manual for Laboratory Diagnosis of Anthrax” published by the World Health Organization [27], and the “Basic Laboratory Protocols for the Presumptive Identification of B. anthracis” published by the Centers for Disease Control and Prevention [28].
Utilizing the 16S rRNA gene, forward and reverse primers, and an amplicon size of 1504 base pairs, a commercially available sequencing technique was used to sequence one of the 21 positive isolates. Within the field of phylogeny, the sequence of the 16S rRNA gene has been used extensively as a molecular clock in order to determine the relationships between different types of bacteria. In recent years, it has emerged as a significant method for determining the genus or species of a previously unknown bacterium [22]. When DNA hybridization was performed, it was discovered that the 16S rRNA gene sequences of B. anthracis were quite similar (more than 99%). This finding is confirmed by the fact that the sequences are extremely similar. The 16S rRNA gene of B. anthracis Sirajganj-1 was subjected to phylogenetic analysis, which revealed that the organism is nearly identical to other strains of B. anthracis that are currently in existence (Figure 3).
Figure 3.
Phylogenetic analysis of 16S rRNA gene of Bacillus anthracis (in red box). GenBank accession no. MF353929.
4. Conclusions
This research shows that molecular detection identifies B. anthracis in soil, meat, and clinical samples. The use of these molecular technologies in developing nations, like in Bangladesh’s surveillance systems, might enhance early identification and response, particularly in anthrax-endemic areas. However, poor laboratory equipment, a lack of experienced workers, and logistical issues including cold chain preservation during sample transfer make remote execution difficult. Molecular diagnostics must be integrated into conventional surveillance programs by investing in diagnostic capabilities and field-level training. Finally, an awareness of the zoonotic nature of anthrax, proper vaccination, the treatment of diseased animals by registered veterinarians, and quick molecular detection by PCR may help to reduce the anthrax outbreak in Bangladesh.
Author Contributions
Conceptualization, K.H.M.N.H.N.; methodology, M.M.I.; validation, K.H.M.N.H.N.; formal analysis, M.M.I. and M.A.H.S.; investigation, K.H.M.N.H.N.; resources, K.H.M.N.H.N.; writing—original draft preparation, M.M.I. and M.A.H.S.; writing—review and editing, M.M.I., K.H.M.N.H.N. and M.A.H.S.; visualization, K.H.M.N.H.N. and M.A.H.S.; supervision, K.H.M.N.H.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The experimental procedures and protocols used in this study were approved by Animal Welfare and Experimental Ethics Committee of Bangladesh Agricultural University (approval number AWEEC/BAU/2018(21), approval date 24 December 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Acknowledgments
The authors would like to thank the District Livestock Office, the Upazilla Livestock Office, the Department of Livestock Service, the Department of Microbiology and Hygiene, and all other government body organizations for their technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Turnbull, P.; Quinn, C.; Henderson, I. Bacillus anthracis and Other Bacillus species. In Molecular Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2002; Volume 3, pp. 2011–2031. ISBN 978-0-12-677530-3. [Google Scholar]
- Moazeni Jula, G.R.; Jabbari, A.R.; Malek, B. Isolation of anthrax spores from soil in endemic regions of Isfahan, Iran. Arch. Razi Inst. 2004, 58, 29–38. [Google Scholar]
- Biswas, P.K.; Islam, M.Z.; Shil, S.K.; Chakraborty, R.K.; Ahmed, S.S.U.; Christensen, J.P. Risk factors associated with anthrax in cattle on smallholdings. Epidemiol. Infect. 2012, 140, 1888–1895. [Google Scholar] [CrossRef]
- Hassan, J.; Ahsan, M.; Rahman, M.; Chowdhury, S.; Parvej, M.; Nazir, K. Factors associated with repeated outbreak of anthrax in Bangladesh: Qualitative and quantitative study. J. Adv. Vet. Anim. Res. 2015, 2, 158–164. [Google Scholar] [CrossRef]
- Oaks, J.L.; Gilbert, M.; Virani, M.Z.; Watson, R.T.; Meteyer, C.U.; Rideout, B.A.; Shivaprasad, H.L.; Ahmed, S.; Iqbal Chaudhry, M.J.; Arshad, M.; et al. Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 2004, 427, 630–633. [Google Scholar] [CrossRef]
- Nazir, K.; Hassan, J.; Chowdhury, S.; Rahman, M. Novel multiplex-PCR for rapid detection of Bacillus anthracis spores present in soils of Sirajganj district in Bangladesh. Progress. Agric. 2015, 26, 67–70. [Google Scholar] [CrossRef]
- Sarker, M.S.A.; El Zowalaty, M.E.; Shahid, M.A.H.; Sarker, M.A.; Rahman, M.B.; Järhult, J.D.; Nazir, K.H.M.N.H. Maximization of Livestock Anthrax Vaccination Coverage in Bangladesh: An Alternative Approach. Vaccines 2020, 8, 435. [Google Scholar] [CrossRef]
- Sarker, M.S.A.; Shahid, M.A.H.; Nazir, K.H.M.N.H. Efficaciousness of Sterne 34F-2 strain of Bacillus anthracis vaccine in cattle for anthrax control program in Bangladesh. J. Istanb. Vet. Sci. 2021, 5, 32–38. [Google Scholar] [CrossRef]
- Carlson, C.J.; Kracalik, I.T.; Ross, N.; Alexander, K.A.; Hugh-Jones, M.E.; Fegan, M.; Elkin, B.T.; Epp, T.; Shury, T.K.; Zhang, W.; et al. The global distribution of Bacillus anthracis and associated anthrax risk to humans, livestock and wildlife. Nat. Microbiol. 2019, 4, 1337–1343. [Google Scholar] [CrossRef]
- Zihad, M.; Shahid, M.; Mahmud, M.; Kabir, A.; Kamal, M.; Naim, J.; Hossen, M.; Nazir, K. Molecular detection, antibiogram, and risk factor analysis of Staphylococcus aureus from subclinical mastitis of goats in conventional and organized farms. Vet. Res. Notes 2021, 1, 17–22. [Google Scholar] [CrossRef]
- Islam, M.; Mahmud, M.; Yesmin, S.; Islam, M.; Sarker, M.; Nazir, K. Risk Factors Assessment of Zoonotic Anthrax among the People at Risk (PAR) in Selected Areas of Bangladesh. Asian J. Med. Health 2017, 4, 1–7. [Google Scholar] [CrossRef]
- Farhad, S.; Shahid, M.; Mahmud, M.; Kabir, A.; Das, S.; Rahman, M.; Nazir, K. Molecular detection and antibiogram of Escherichia coli O157 isolated from subclinical mastitis affected cows at Baghabari, Sirajganj. Vet. Res. Notes 2021, 1, 6–11. [Google Scholar] [CrossRef]
- Dragon, D.C.; Rennie, R.P.; Elkin, B.T. Detection of anthrax spores in endemic regions of northern Canada. J. Appl. Microbiol. 2001, 91, 435–441. [Google Scholar] [CrossRef]
- Sarker, M.S.A.; Shahid, M.A.H.; Hoque, M.N.; Sarker, M.A.; Rahman, M.B.; Islam, S.S.; Nazir, K.H.M.N.H. The The Rich Mapping: Be a Supplementary Approach for Anthrax Control at Community Level. J. Adv. Vet. Res. 2021, 11, 41–46. [Google Scholar]
- Sarker, M.S.A.; Shahid, M.A.H.; Rahman, M.B.; Nazir, K.H.M.N.H. An integrated model for anthrax-free zone development in developing countries. J. Infect. Public Health 2023, 16, 141–152. [Google Scholar] [CrossRef]
- Cheun, H.I.; Makino, S.-I.; Watarai, M.; Erdenebaatar, J.; Kawamoto, K.; Uchida, I. Rapid and effective detection of anthrax spores in soil by PCR. J. Appl. Microbiol. 2003, 95, 728–733. [Google Scholar] [CrossRef]
- Khan, M.; Pavel, M.; Keya, A.; Shahid, M.; Ferdausi, T.; Siddique, M.; Hossain, M.; Nazir, K.; Rahman, M.; Rahman, M. Comparative molecular analysis of contemporary isolates of duck plague virus from haor areas of Bangladesh. J. Adv. Biotechnol. Exp. Ther. 2021, 4, 44–52. [Google Scholar] [CrossRef]
- Mahmud, M.M.; Iqbal, M.A.; Shahid, M.A.H.; Das, S.; Kabir, A.; Hossain, M.Z.; Hasan, A.; Raihan, A.; Yadav, R.; Rahman, A.K.M.A. Bovine Foot-and-Mouth-Disease risk factors in Mymensingh district of Bangladesh. Bangladesh J. Vet. Med. 2022, 20, 43–48. [Google Scholar] [CrossRef]
- Ahsan, M.M.; Rahman Khan, M.F.; Rahman, M.B.; Chowdhury, M.Z.H.; Parvej, M.S.; Jahan, M.; Nazir, K.H.M.N.H. Investigation into Bacillus anthracis spore in soil and analysis of environmental parameters related to repeated anthrax outbreak in Sirajganj, Bangladesh. Thai J. Vet. Med. 2013, 43, 449–454. [Google Scholar] [CrossRef]
- Shahid, A.H.; Nazir, K.H.M.N.H.; El Zowalaty, M.E.; Kabir, A.; Sarker, S.A.; Siddique, M.P.; Ashour, H.M. Molecular detection of vancomycin and methicillin resistance in Staphylococcus aureus isolated from food processing environments. One Health 2021, 13, 100276. [Google Scholar] [CrossRef]
- Meghla, N.S.; Mridha, D.; Rana, M.S.; Shahid, M.A.H.; Mahmud, M.M. Isolation, identification and antibiogram of verotoxin producing Escherichia coli from raw salad vegetables at Jashore, Bangladesh. Afr. J. Microbiol. Res. 2021, 15, 401–407. [Google Scholar] [CrossRef]
- Sacchi, C.T.; Whitney, A.M.; Mayer, L.W.; Morey, R.; Steigerwalt, A.; Boras, A.; Weyant, R.S.; Popovic, T. Sequencing of 16S rRNA Gene: A Rapid Tool for Identification of Bacillus anthracis. Emerg. Infect. Dis. 2002, 8, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Schmid, G.; Kaufmann, A. Anthrax in Europe: Its epidemiology, clinical characteristics, and role in bioterrorism. Clin. Microbiol. Infect. 2002, 8, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.J.; Galvin, J.W.; Rubira, R.J.; Condron, R.J.; Bradley, T. Experiences with vaccination and epidemiological investigations on an anthrax outbreak in Australia in 1997. J. Appl. Microbiol. 1999, 87, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Levings, R.L. Emerging and Exotic Zoonotic Disease Preparedness and Response in the United States—Coordination of the Animal Health Component. Zoonoses Public Health 2012, 59, 80–94. [Google Scholar] [CrossRef]
- Chikerema, S.M.; Pfukenyi, D.M.; Hang’ombe, B.M.; L’Abee-Lund, T.M.; Matope, G. Isolation of Bacillus anthracis from soil in selected high-risk areas of Zimbabwe. J. Appl. Microbiol. 2012, 113, 1389–1395. [Google Scholar] [CrossRef]
- WHO. Anthrax in Humans and Animals. Available online: https://www.who.int/publications/i/item/9789241547536 (accessed on 20 April 2024).
- CDC. Human Anthrax Associated with an Epizootic Among Livestock-North Dakota, 2000. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5032a1.htm (accessed on 20 April 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Hellenic Society for Microbiology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).