The Trend of Long Pentraxin 3 and Other Inflammatory Serum Markers in the 30 Days After Total Hip Arthroplasty
Abstract
1. Introduction
2. Materials and Methods
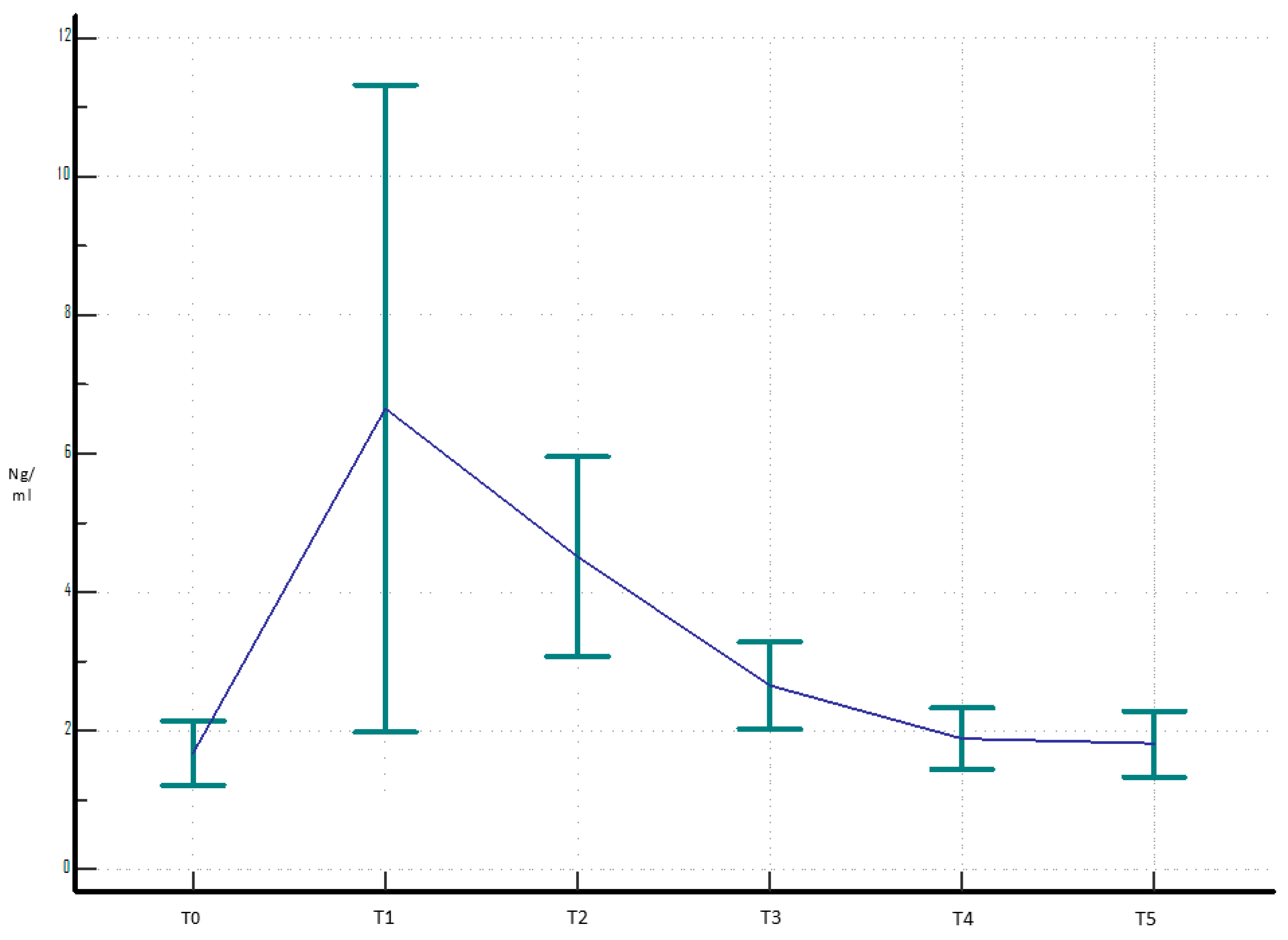
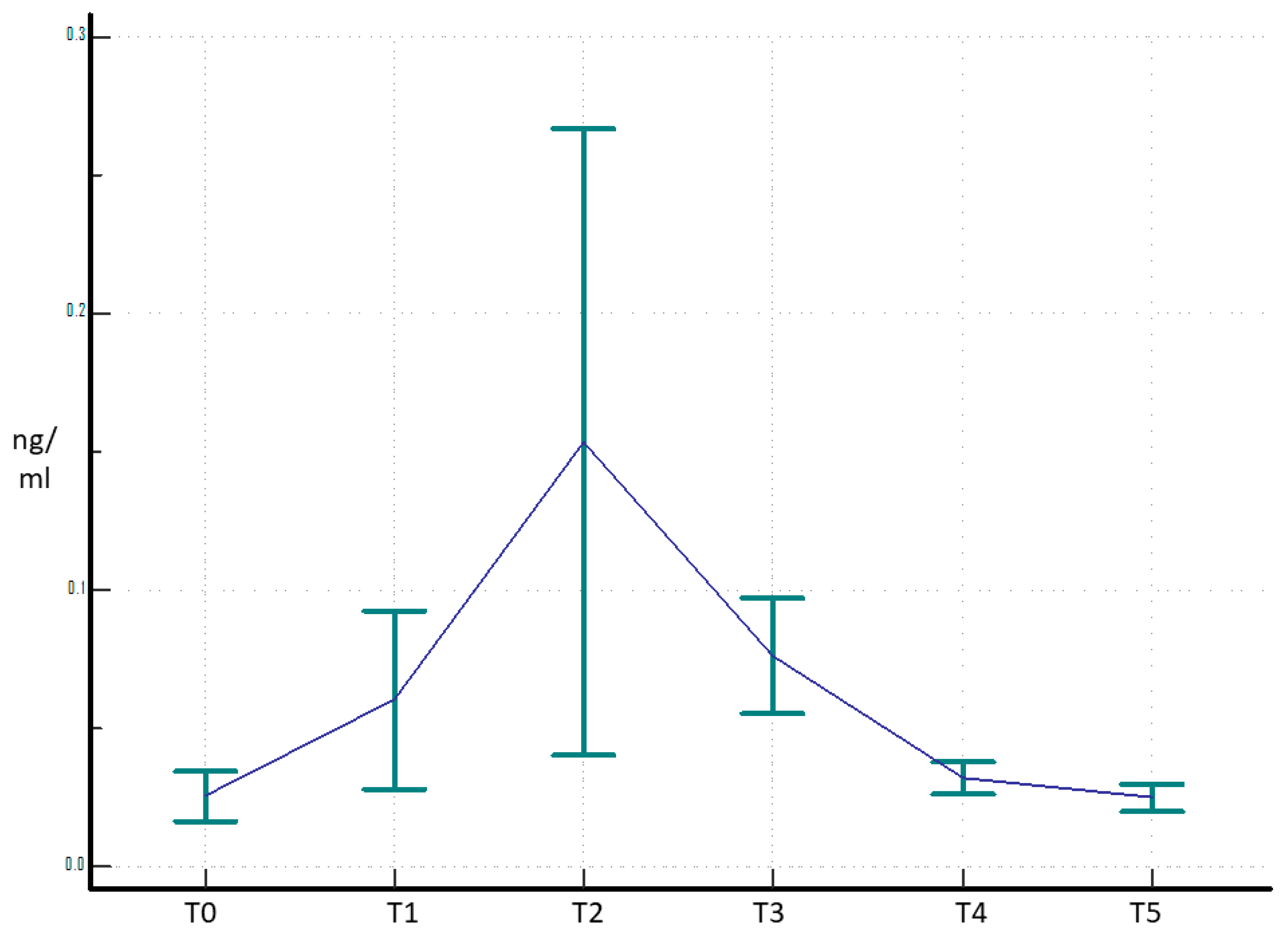
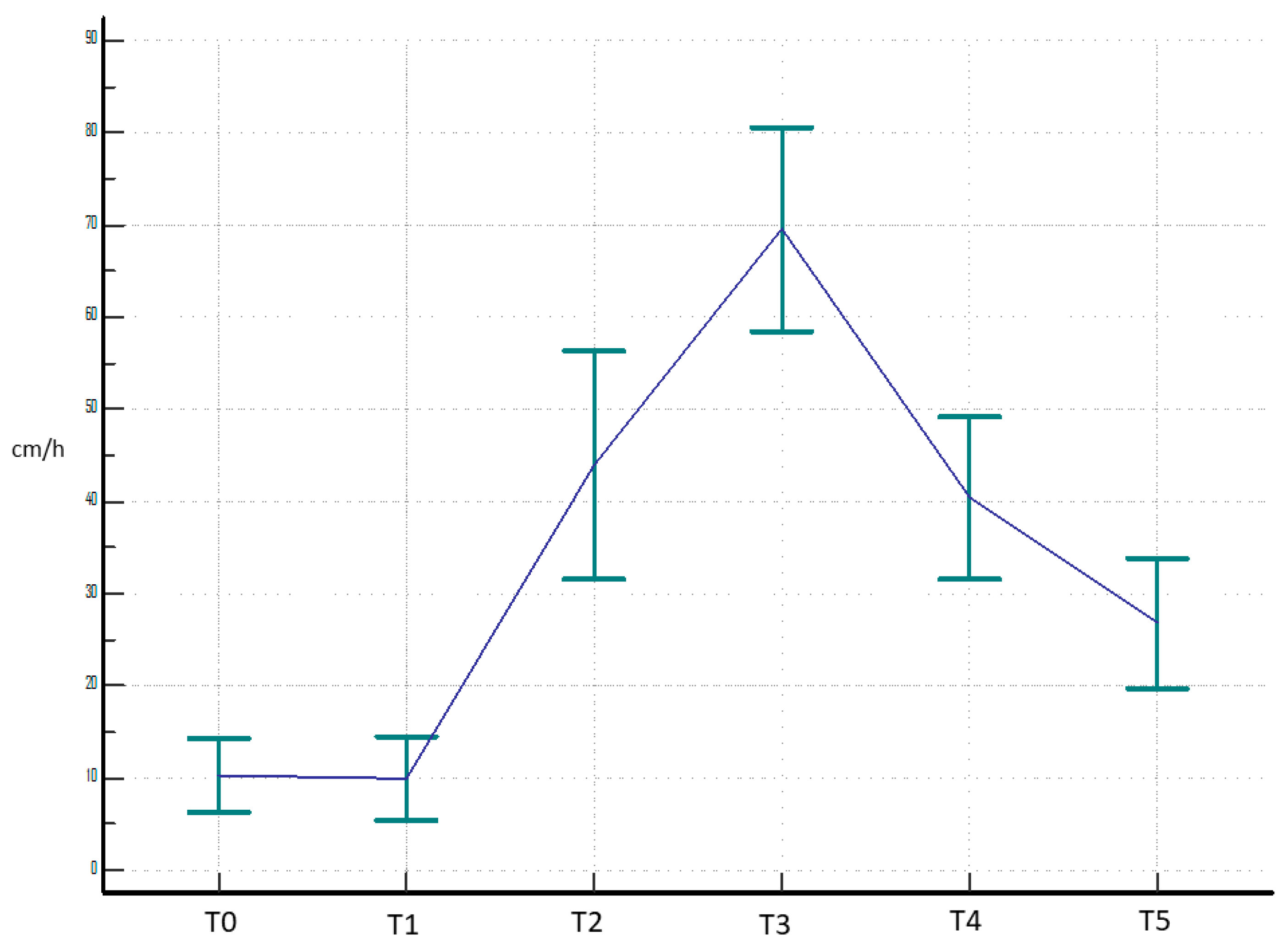
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PTX3 | Long Pentraxin 3 |
| THA | Total Hip Arthroplasty |
| CRP | C-Reactive Protein |
| ESR | Erythrocyte Sedimentation Rate |
| OA | Osteoarthritis |
| PJI | Periprosthetic Joint Infection |
| IL-6 | Interleukin-6 |
| BMI | Body Mass Index |
| EDTA | Ethylenediaminetetraacetic acid |
| ANOVA | Analysis of Variance |
| TNF-α | Tumor Necrosis Factor |
References
- Saracco, M.; Fidanza, A.; Necozione, S.; Maccauro, G.; Logroscino, G. Could Short Stems THA Be a Good Bone-Saving Option Even in Obese Patients? J. Clin. Med. 2022, 11, 7114. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, S.; Al-Mansoori, A.A.; Chand, M.R.; Villa, J.M.; Suarez, J.C.; Patel, P.D. What Is the Optimal Criteria to Use for Detecting Periprosthetic Joint Infections Before Total Joint Arthroplasty? J. Arthroplast. 2018, 33, S201–S204. [Google Scholar] [CrossRef] [PubMed]
- Ghirardelli, S.; Touloupakis, G.; Antonini, G.; Violante, B.; Fidanza, A.; Indelli, P.F. Debridement, antibiotic, pearls, irrigation and retention of the implant and other local strategies on hip periprosthetic joint infections. Minerva Orthop 2022, 73, 4. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Beswick, A.D.; Peters, T.J.; Gooberman-Hill, R.; Whitehouse, M.R.; Blom, A.W.; Moore, A.J.; Hills, R.K. Health Care Needs and Support for Patients Undergoing Treatment for Prosthetic Joint Infection following Hip or Knee Arthroplasty: A Systematic Review. PLoS ONE 2017, 12, e0169068. [Google Scholar] [CrossRef]
- Iannotti, F.; Prati, P.; Fidanza, A.; Iorio, R.; Ferretti, A.; Pèrez Prieto, D.; Kort, N.; Violante, B.; Pipino, G.; Schiavone Panni, A.; et al. Prevention of Periprosthetic Joint Infection (PJI): A Clinical Practice Protocol in High-Risk Patients. Trop. Med. Infect. Dis. 2020, 5, 186. [Google Scholar] [CrossRef]
- Nelson, S.B.; Pinkney, J.A.; Chen, A.F.; Tande, A.J. Periprosthetic Joint Infection: Current Clinical Challenges. Clin. Infect. Dis. 2023, 77, e34–e45. [Google Scholar] [CrossRef]
- Parvizi, J.; Zmistowski, B.; Berbari, E.F.; Bauer, T.W.; Springer, B.D.; Della Valle, C.J.; Garvin, K.L.; Mont, M.A.; Wongworawat, M.D.; Zalavras, C.G. New Definition for Periprosthetic Joint Infection: From the Workgroup of the Musculoskeletal Infection Society. Clin. Orthop. 2011, 469, 2992–2994. [Google Scholar] [CrossRef]
- Shohat, N.; Bauer, T.; Buttaro, M.; Budhiparama, N.; Cashman, J.; Della Valle, C.J.; Drago, L.; Gehrke, T.; Marcelino Gomes, L.S.; Goswami, K.; et al. Hip and Knee Section, What is the Definition of a Periprosthetic Joint Infection (PJI) of the Knee and the Hip? Can the Same Criteria be Used for Both Joints?: Proceedings of International Consensus on Orthopedic Infections. J. Arthroplast. 2019, 34, S325–S327. [Google Scholar] [CrossRef]
- McNally, M.; Sousa, R.; WouthuyzenBakker, M.; Chen, A.F.; Soriano, A.; Vogely, H.C.; Clauss, M.; Higuera, C.A.; Trebše, R. The EBJIS definition of periprosthetic joint infection: A practical guide for clinicians. Bone Jt. J. 2021, 103-B, 18–25. [Google Scholar] [CrossRef]
- Luppi, V.; Regis, D.; Sandri, A.; Magnan, B. Diagnosis of periprosthetic hip infection: A clinical update. Acta Biomed. Atenei Parm. 2023, 94, e2023095. [Google Scholar] [CrossRef]
- Logroscino, G.; Fidanza, A.; Giusti, I.; Poppa, G.; Troianello, L.; Calafiore, F.; Saracco, M.; Avigni, R.; Leone, R.; Mantovani, A.; et al. Pentraxin 3, a new biomarker for the diagnosis and management of PJI in primary and revision hip arthroplasty. Acta Biomed. Atenei Parm. 2023, 94, e2023100. [Google Scholar] [CrossRef]
- Kunes, P.; Holubcova, Z.; Kolackova, M.; Krejsek, J. Pentraxin 3(PTX 3): An Endogenous Modulator of the Inflammatory Response. Mediat. Inflamm. 2012, 2012, 920517. [Google Scholar] [CrossRef] [PubMed]
- Erenler, A.K.; Yücel, O.; Baydın, A. Clinical Use of Pentraxin 3: A Review. Direct Res. J. Health Pharmacol. (DRJHP) 2017, 5, 7–13. [Google Scholar]
- Garlanda, C.; Jaillon, S.; Doni, A.; Bottazzi, B.; Mantovani, A. PTX3, a humoral pattern recognition molecule at the interface between microbe and matrix recognition. Curr. Opin. Immunol. 2016, 38, 39–44. [Google Scholar] [CrossRef]
- Loppini, M.; Di Maio, M.; Avigni, R.; Leone, R.; Inforzato, A.; Grappiolo, G.; Mantovani, A.; Bottazzi, B. Long Pentraxin 3 as a New Biomarker for Diagnosis of Hip and Knee Periprosthetic Joint Infections. J. Clin. Med. 2023, 12, 1055. [Google Scholar] [CrossRef]
- Hurler, L.; Mescia, F.; Bergamaschi, L.; Cambridge Institute of Therapeutic Immunology and Infectious Disease-National Institute of Health Research (CITIID-NIHR) COVID BioResource Collaboration; Kajdácsi, E.; Sinkovits, G.; Cervenak, L.; Prohászka, Z.; Lyons, P.A.; Toonen, E.J.M. sMR and PTX3 levels associate with COVID-19 outcome and survival but not with Long COVID. iScience 2024, 27, 110162. [Google Scholar] [CrossRef]
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-linked immunosorbent assay for the quantitative/qualitative analysis of plant secondary metabolites. J. Nat. Med. 2018, 72, 32–42. [Google Scholar] [CrossRef]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Evans, J.T.; Evans, J.P.; Walker, R.W.; Blom, A.W.; Whitehouse, M.R.; Sayers, A. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet 2019, 393, 647–654. [Google Scholar] [CrossRef]
- Parisi, T.J.; Konopka, J.F.; Bedair, H.S. What is the Long-term Economic Societal Effect of Periprosthetic Infections After THA? A Markov Analysis. Clin. Orthop 2017, 475, 1891–1900. [Google Scholar] [CrossRef]
- Sigmund, I.K.; Puchner, S.E.; Windhager, R. Serum Inflammatory Biomarkers in the Diagnosis of Periprosthetic Joint Infections. Biomedicines 2021, 9, 1128. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Strina, D.; Possetti, V.; Leone, R.; Valentino, S.; Chiappetta, K.; Loppini, M.; Mantovani, A.; Bottazzi, B.; Asselta, R. Inforzato Interleukin-1β Polymorphisms Are Genetic Markers of Susceptibility to Periprosthetic Joint Infection in Total Hip and Knee Arthroplasty. Genes 2024, 15, 596. [Google Scholar] [CrossRef] [PubMed]
- Doni, A.; Michela, M.; Bottazzi, B.; Peri, G.; Valentino, S.; Polentarutti, N.; Garlanda, C.; Mantovani, A. Mantovani; Regulation of PTX3, a key component of humoral innate immunity in human dendritic cells: Stimulation by IL-10 and inhibition by IFN-γ. J. Leukoc. Biol. 2006, 79, 797. [Google Scholar] [CrossRef]
- Chen, H.; Li, T.; Yan, S.; Liu, M.; Liu, K.; Zhang, H.; Gao, M.; Xiao, X. Pentraxin-3 Is a Strong Biomarker of Sepsis Severity Identification and Predictor of 90-Day Mortality in Intensive Care Units via Sepsis 3.0 Definitions. Diagnostics 2021, 11, 1906. [Google Scholar] [CrossRef] [PubMed]
- Davoudian, S.; Piovani, D.; Desai, A.; Mapelli, S.N.; Leone, R.; Sironi, M.; Valentino, S.; Silva-Gomes, R.; Stravalaci, M.; Asgari, F.; et al. A cytokine/PTX3 prognostic index as a predictor of mortality in sepsis. Front. Immunol. 2022, 13, 979232. [Google Scholar] [CrossRef]
- Parente, R.; Sobacchi, C.; Bottazzi, B.; Mantovani, A.; Grčevic, D.; Inforzato, A. The Long Pentraxin PTX3 in Bone Homeostasis and Pathology. Front. Immunol. 2019, 10, 2628. [Google Scholar] [CrossRef]


| Fever | Local Sign of Infection | Catheter | Additional Venous Access | Infection |
|---|---|---|---|---|
| No 45 (90%) | No 48 (96%) | No 38 (76%) | No 47 (94%) | No 50 (100%) |
| Yes 5 (10,%) | Yes 2(4%) | Yes 12 (24%) | Yes 3 (6%) | Yes 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Hellenic Society for Microbiology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fidanza, A.; Marinucci, V.; Vitale, L.; Poppa, G.; Giusti, I.; Necozione, S.; Logroscino, G. The Trend of Long Pentraxin 3 and Other Inflammatory Serum Markers in the 30 Days After Total Hip Arthroplasty. Acta Microbiol. Hell. 2025, 70, 7. https://doi.org/10.3390/amh70010007
Fidanza A, Marinucci V, Vitale L, Poppa G, Giusti I, Necozione S, Logroscino G. The Trend of Long Pentraxin 3 and Other Inflammatory Serum Markers in the 30 Days After Total Hip Arthroplasty. Acta Microbiologica Hellenica. 2025; 70(1):7. https://doi.org/10.3390/amh70010007
Chicago/Turabian StyleFidanza, Andrea, Valeria Marinucci, Lorenzo Vitale, Giuseppina Poppa, Ilaria Giusti, Stefano Necozione, and Giandomenico Logroscino. 2025. "The Trend of Long Pentraxin 3 and Other Inflammatory Serum Markers in the 30 Days After Total Hip Arthroplasty" Acta Microbiologica Hellenica 70, no. 1: 7. https://doi.org/10.3390/amh70010007
APA StyleFidanza, A., Marinucci, V., Vitale, L., Poppa, G., Giusti, I., Necozione, S., & Logroscino, G. (2025). The Trend of Long Pentraxin 3 and Other Inflammatory Serum Markers in the 30 Days After Total Hip Arthroplasty. Acta Microbiologica Hellenica, 70(1), 7. https://doi.org/10.3390/amh70010007






