Combating Malaria with Vaccines: Insights from the One Health Framework
Abstract
1. Introduction
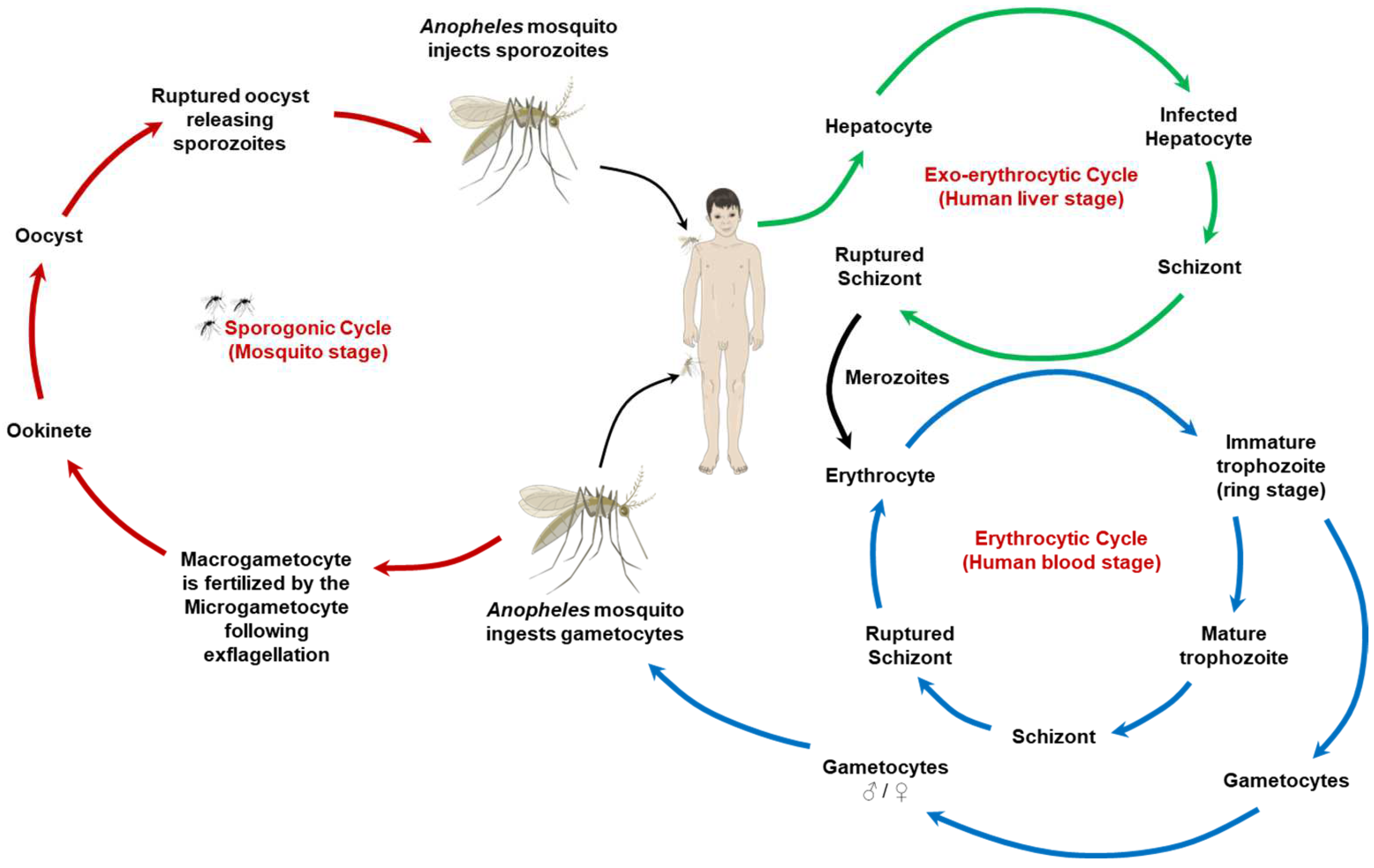
2. The Malaria Parasite and Its Lifecycle: A One Health View
3. Current Landscape of Malaria Vaccine Development
4. One Health Challenges in Malaria Vaccination Program
5. Integrating One Health in Malaria Vaccine Research and Development
6. Ethical, Social, and Economic Considerations in Malaria Vaccination Program
7. Future Directions and Innovations in Malaria Eradication Efforts
8. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Meara, W.; Mangeni, J.; Steketee, R.; Greenwood, B. Changes in the Burden of Malaria in Sub-Saharan Africa. Lancet Infect. Dis. 2010, 10, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Kamau, A.; Mogeni, P.; Okiro, E.A.; Snow, R.W.; Bejon, P. A Systematic Review of Changing Malaria Disease Burden in Sub-Saharan Africa since 2000: Comparing Model Predictions and Empirical Observations. BMC Med. 2020, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2020: 20 Years of Global Progress and Challenges. 2020. Available online: https://www.who.int/publications/i/item/9789240015791 (accessed on 20 March 2024).
- Liu, Q.; Jing, W.; Kang, L.; Liu, J.; Liu, M. Trends of the Global, Regional and National Incidence of Malaria in 204 Countries from 1990 to 2019 and Implications for Malaria Prevention. J. Travel Med. 2021, 28, taab046. [Google Scholar] [CrossRef]
- Hay, S.I.; Guerra, C.A.; Tatem, A.J.; Noor, A.M.; Snow, R.W. The Global Distribution and Population at Risk of Malaria: Past, Present, and Future. Lancet Infect. Dis. 2004, 4, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Wei, L.; Liang, H.; Yan, D.; Zhang, J.; Wang, Z. Trends of the Global, Regional and National Incidence, Mortality, and Disability-Adjusted Life Years of Malaria, 1990–2019: An Analysis of the Global Burden of Disease Study 2019. RMHP 2023, 16, 1187–1201. [Google Scholar] [CrossRef]
- Cohen, S. Development of a Malaria Vaccine. J. R. Soc. Med. 1978, 71, 476–478. [Google Scholar] [CrossRef]
- RTS,S Clinical Trials Partnership. Efficacy and Safety of RTS,S/AS01 Malaria Vaccine with or without a Booster Dose in Infants and Children in Africa: Final Results of a Phase 3, Individually Randomised, Controlled Trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef]
- MacDonald, A.J.; Mordecai, E.A. Amazon Deforestation Drives Malaria Transmission, and Malaria Burden Reduces Forest Clearing. Proc. Natl. Acad. Sci. USA 2019, 116, 22212–22218. [Google Scholar] [CrossRef]
- Prusty, D.; Gupta, N.; Upadhyay, A.; Dar, A.; Naik, B.; Kumar, N.; Prajapati, V.K. Asymptomatic Malaria Infection Prevailing Risks for Human Health and Malaria Elimination. Infect. Genet. Evol. 2021, 93, 104987. [Google Scholar] [CrossRef]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The Importance of Vector Control for the Control and Elimination of Vector-Borne Diseases. PLoS Negl. Trop. Dis. 2020, 14, e0007831. [Google Scholar] [CrossRef]
- Benelli, G.; Beier, J.C. Current Vector Control Challenges in the Fight against Malaria. Acta Trop. 2017, 174, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Zinsstag, J.; Schelling, E.; Waltner-Toews, D.; Tanner, M. From “One Medicine” to “One Health” and Systemic Approaches to Health and Well-Being. Prev. Vet. Med. 2011, 101, 148–156. [Google Scholar] [CrossRef]
- Tsoumani, M.E.; Voyiatzaki, C.; Efstathiou, A. Malaria Vaccines: From the Past towards the mRNA Vaccine Era. Vaccines 2023, 11, 1452. [Google Scholar] [CrossRef]
- Lancet, T. Malaria Vaccines: A Test for Global Health. Lancet 2024, 403, 503. [Google Scholar] [CrossRef]
- Monroe, A.; Williams, N.A.; Ogoma, S.; Karema, C.; Okumu, F. Reflections on the 2021 World Malaria Report and the Future of Malaria Control. Malar. J. 2022, 21, 154. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.K. Evidence and Implications of Mortality Associated with Acute Plasmodium Vivax Malaria. Clin. Microbiol. Rev. 2013, 26, 36–57. [Google Scholar] [CrossRef]
- Mueller, I.; Galinski, M.R.; Baird, J.K.; Carlton, J.M.; Kochar, D.K.; Alonso, P.L.; Portillo, H.A. del Key Gaps in the Knowledge of Plasmodium Vivax, a Neglected Human Malaria Parasite. Lancet Infect. Dis. 2009, 9, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Collins, W.E.; Jeffery, G.M. Plasmodium Malariae: Parasite and Disease. Clin. Microbiol. Rev. 2007, 20, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Crompton, P.D.; Moebius, J.; Portugal, S.; Waisberg, M.; Hart, G.; Garver, L.S.; Miller, L.H.; Barillas-Mury, C.; Pierce, S.K. Malaria Immunity in Man and Mosquito: Insights into Unsolved Mysteries of a Deadly Infectious Disease. Annu. Rev. Immunol. 2014, 32, 157–187. [Google Scholar] [CrossRef]
- CDC-Centers for Disease Control and Prevention CDC—Malaria—About Malaria—Biology. Available online: https://www.cdc.gov/malaria/about/biology/index.html (accessed on 10 February 2024).
- World Health Organization. Guidelines for the Treatment of Malaria, 3rd ed.; World Health Organization: Geneva, Switzerland, 2015; ISBN 978-92-4-154912-7. [Google Scholar]
- Paaijmans, K.P.; Read, A.F.; Thomas, M.B. Understanding the Link between Malaria Risk and Climate. Proc. Natl. Acad. Sci. USA 2009, 106, 13844–13849. [Google Scholar] [CrossRef]
- Chandra, G.; Mukherjee, D. Chapter 35—Effect of Climate Change on Mosquito Population and Changing Pattern of Some Diseases Transmitted by Them. In Advances in Animal Experimentation and Modeling; Sobti, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 455–460. ISBN 978-0-323-90583-1. [Google Scholar]
- RTS,S Clinical Trials Partnership. Efficacy and Safety of the RTS,S/AS01 Malaria Vaccine during 18 Months after Vaccination: A Phase 3 Randomized, Controlled Trial in Children and Young Infants at 11 African Sites. PLoS Med. 2014, 11, e1001685. [Google Scholar] [CrossRef]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The Effect of Malaria Control on Plasmodium Falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207–211. [Google Scholar] [CrossRef]
- Anoopkumar, A.N.; Aneesh, E.M. A Critical Assessment of Mosquito Control and the Influence of Climate Change on Mosquito-Borne Disease Epidemics. Environ. Dev. Sustain. 2022, 24, 8900–8929. [Google Scholar] [CrossRef]
- Kester, K.E.; Cummings, J.F.; Ofori-Anyinam, O.; Ockenhouse, C.F.; Krzych, U.; Moris, P.; Schwenk, R.; Nielsen, R.A.; Debebe, Z.; Pinelis, E.; et al. Randomized, Double-Blind, Phase 2a Trial of Falciparum Malaria Vaccines RTS,S/AS01B and RTS,S/AS02A in Malaria-Naive Adults: Safety, Efficacy, and Immunologic Associates of Protection. J. Infect. Dis. 2009, 200, 337–346. [Google Scholar] [CrossRef]
- Adepoju, P. RTS,S Malaria Vaccine Pilots in Three African Countries. Lancet 2019, 393, 1685. [Google Scholar] [CrossRef] [PubMed]
- Neafsey, D.; Juraska, M.; Bedford, T.; Benkeser, D.; Valim, C.; Griggs, A.; Lievens, M.; Abdulla, S.; Adjei, S.; Agbenyega, T.; et al. Genetic Diversity and Protective Efficacy of the RTS,S/AS01 Malaria Vaccine. N. Engl. J. Med. 2015, 373, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Olotu, A.; Lusingu, J.; Leach, A.; Lievens, M.; Vekemans, J.; Msham, S.; Lang, T.; Gould, J.; Dubois, M.; Jongert, E.; et al. Efficacy of RTS,S/AS01E Malaria Vaccine and Exploratory Analysis on Anti-Circumsporozoite Antibody Titres and Protection in Children Aged 5–17 Months in Kenya and Tanzania: A Randomised Controlled Trial. Lancet Infect. Dis. 2011, 11, 102–109. [Google Scholar] [CrossRef]
- Olotu, A.; Fegan, G.; Wambua, J.K.; Nyangweso, G.; Leach, A.; Lievens, M.; Kaslow, D.; Njuguna, P.; Marsh, K.; Bejon, P. Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children. N. Engl. J. Med. 2016, 374, 2519–2529. [Google Scholar] [CrossRef] [PubMed]
- Olotu, A.; Moris, P.; Mwacharo, J.; Vekemans, J.; Kimani, D.; Janssens, M.; Kai, O.; Jongert, E.; Lievens, M.; Leach, A.; et al. Circumsporozoite-Specific T Cell Responses in Children Vaccinated with RTS,S/AS01E and Protection against P Falciparum Clinical Malaria. PLoS ONE 2011, 6, e25786. [Google Scholar] [CrossRef]
- Agnandji, S.; Asante, K.P.; Lyimo, J.; Vekemans, J.; Soulanoudjingar, S.; Owusu, R.; Shomari, M.; Leach, A.; Fernandes, J.; Dosoo, D.; et al. Evaluation of the Safety and Immunogenicity of the RTS,S/AS01E Malaria Candidate Vaccine When Integrated in the Expanded Program of Immunization. J. Infect. Dis. 2010, 202, 1076–1087. [Google Scholar] [CrossRef]
- Datoo, M.S.; Natama, M.H.; Somé, A.; Traoré, O.; Rouamba, T.; Bellamy, D.; Yameogo, P.; Valia, D.; Tegneri, M.; Ouedraogo, F.; et al. Efficacy of a Low-Dose Candidate Malaria Vaccine, R21 in Adjuvant Matrix-M, with Seasonal Administration to Children in Burkina Faso: A Randomised Controlled Trial. Lancet 2021, 397, 1809–1818. [Google Scholar] [CrossRef]
- Datoo, M.S.; Dicko, A.; Tinto, H.; Ouédraogo, J.-B.; Hamaluba, M.; Olotu, A.; Beaumont, E.; Lopez, F.R.; Natama, H.M.; Weston, S.; et al. Safety and Efficacy of Malaria Vaccine Candidate R21/Matrix-M in African Children: A Multicentre, Double-Blind, Randomised, Phase 3 Trial. Lancet 2024, 403, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Seder, R.A.; Chang, L.-J.; Enama, M.E.; Zephir, K.L.; Sarwar, U.N.; Gordon, I.J.; Holman, L.A.; James, E.R.; Billingsley, P.F.; Gunasekera, A.; et al. Protection against Malaria by Intravenous Immunization with a Nonreplicating Sporozoite Vaccine. Science 2013, 341, 1359–1365. [Google Scholar] [CrossRef]
- Jongo, S.A.; Shekalaghe, S.A.; Church, L.W.P.; Ruben, A.J.; Schindler, T.; Zenklusen, I.; Rutishauser, T.; Rothen, J.; Tumbo, A.; Mkindi, C.; et al. Safety, Immunogenicity, and Protective Efficacy against Controlled Human Malaria Infection of Plasmodium Falciparum Sporozoite Vaccine in Tanzanian Adults. Am. J. Trop. Med. Hyg. 2018, 99, 338–349. [Google Scholar] [CrossRef]
- Olotu, A.; Urbano, V.; Hamad, A.; Eka, M.; Chemba, M.; Nyakarungu, E.; Raso, J.; Eburi, E.; Mandumbi, D.O.; Hergott, D.; et al. Advancing Global Health through Development and Clinical Trials Partnerships: A Randomized, Placebo-Controlled, Double-Blind Assessment of Safety, Tolerability, and Immunogenicity of PfSPZ Vaccine for Malaria in Healthy Equatoguinean Men. Am. J. Trop. Med. Hyg. 2017, 98, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Sissoko, M.S.; Healy, S.A.; Katile, A.; Omaswa, F.; Zaidi, I.; Gabriel, E.E.; Kamate, B.; Samake, Y.; Guindo, M.A.; Dolo, A.; et al. Safety and Efficacy of PfSPZ Vaccine against Plasmodium Falciparum via Direct Venous Inoculation in Healthy Malaria-Exposed Adults in Mali: A Randomised, Double-Blind Phase 1 Trial. Lancet Infect. Dis. 2017, 17, 498–509. [Google Scholar] [CrossRef]
- Oneko, M.; Steinhardt, L.C.; Yego, R.; Wiegand, R.E.; Swanson, P.A.; Kc, N.; Akach, D.; Sang, T.; Gutman, J.R.; Nzuu, E.L.; et al. Safety, Immunogenicity and Efficacy of PfSPZ Vaccine against Malaria in Infants in Western Kenya: A Double-Blind, Randomized, Placebo-Controlled Phase 2 Trial. Nat. Med. 2021, 27, 1636–1645. [Google Scholar] [CrossRef]
- Thera, M.A.; Doumbo, O.K.; Coulibaly, D.; Laurens, M.B.; Ouattara, A.; Kone, A.K.; Guindo, A.B.; Traore, K.; Traore, I.; Kouriba, B.; et al. A Field Trial to Assess a Blood-Stage Malaria Vaccine. N. Engl. J. Med. 2011, 365, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Draper, S.J.; Sack, B.K.; King, C.R.; Nielsen, C.M.; Rayner, J.C.; Higgins, M.K.; Long, C.A.; Seder, R.A. Malaria Vaccines: Recent Advances and New Horizons. Cell Host Microbe 2018, 24, 43–56. [Google Scholar] [CrossRef]
- Arama, C.; Troye-Blomberg, M. The Path of Malaria Vaccine Development: Challenges and Perspectives. J. Intern. Med. 2014, 275, 456–466. [Google Scholar] [CrossRef]
- Bennett, J.W.; Yadava, A.; Tosh, D.; Sattabongkot, J.; Komisar, J.; Ware, L.A.; McCarthy, W.F.; Cowden, J.J.; Regules, J.; Spring, M.D.; et al. Phase 1/2a Trial of Plasmodium Vivax Malaria Vaccine Candidate VMP001/AS01B in Malaria-Naive Adults: Safety, Immunogenicity, and Efficacy. PLoS Negl. Trop. Dis. 2016, 10, e0004423. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.H.; Gangnon, R.; Silveira, G.A.; Patz, J.A. Deforestation and Malaria in Mâncio Lima County, Brazil. Emerg. Infect. Dis. 2010, 16, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Guerra, C.A.; Snow, R.W.; Hay, S.I. A Global Assessment of Closed Forests, Deforestation and Malaria Risk. Ann. Trop. Med. Parasitol. 2006, 100, 189–204. [Google Scholar] [CrossRef]
- Moyes, C.L.; Shearer, F.M.; Huang, Z.; Wiebe, A.; Gibson, H.S.; Nijman, V.; Mohd-Azlan, J.; Brodie, J.F.; Malaivijitnond, S.; Linkie, M.; et al. Predicting the Geographical Distributions of the Macaque Hosts and Mosquito Vectors of Plasmodium Knowlesi Malaria in Forested and Non-Forested Areas. Parasites Vectors 2016, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.L.; Henry, J.M.; Citron, D.T.; Ssebuliba, D.M.; Nsumba, J.N.; Sánchez, C.H.M.; Brady, O.J.; Guerra, C.A.; García, G.A.; Carter, A.R.; et al. Spatial Dynamics of Malaria Transmission. PLoS Comput. Biol. 2023, 19, e1010684. [Google Scholar] [CrossRef]
- Benelli, G.; Duggan, M.F. Management of Arthropod Vector Data—Social and Ecological Dynamics Facing the One Health Perspective. Acta Trop. 2018, 182, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Taffese, H.S.; Hemming-Schroeder, E.; Koepfli, C.; Tesfaye, G.; Lee, M.; Kazura, J.; Yan, G.-Y.; Zhou, G.-F. Malaria Epidemiology and Interventions in Ethiopia from 2001 to 2016. Infect. Dis. Poverty 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Singh, M.; Wasnik, K.; Pareek, D.; Gupta, P.S.; Mukherjee, S.; Paik, P. Polymeric Nanoparticle Based Diagnosis and Nanomedicine for Treatment and Development of Vaccines for Cerebral Malaria: A Review on Recent Advancement. ACS Appl. Bio Mater. 2021, 4, 7342–7365. [Google Scholar] [CrossRef]
- Burkhard, P.; Lanar, D.E. Malaria Vaccine Based on Self-Assembling Protein Nanoparticles. Expert Rev. Vaccines 2015, 14, 1525–1527. [Google Scholar] [CrossRef]
- Wu, Y.; Narum, D.L.; Fleury, S.; Jennings, G.; Yadava, A. Particle-Based Platforms for Malaria Vaccines. Vaccine 2015, 33, 7518–7524. [Google Scholar] [CrossRef]
- Dong, S.; Dong, Y.; Simões, M.L.; Dimopoulos, G. Mosquito Transgenesis for Malaria Control. Trends Parasitol. 2022, 38, 54–66. [Google Scholar] [CrossRef]
- Knols, B.G.J.; Bossin, H.C.; Mukabana, W.R.; Robinson, A.S. Transgenic Mosquitoes and the Fight against Malaria: Managing Technology Push in a Turbulent GMO World. Am. J. Trop. Med. Hyg. 2007, 77, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Christophides, G.K. Transgenic Mosquitoes and Malaria Transmission. Cell. Microbiol. 2005, 7, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Tripet, F.; Aboagye-Antwi, F.; Hurd, H. Ecological Immunology of Mosquito–Malaria Interactions. Trends Parasitol. 2008, 24, 219–227. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines on Ethical Issues in Public Health Surveillance. In WHO Guidelines on Ethical Issues in Public Health Surveillance; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- MacLennan, C.A.; Saul, A. Vaccines against Poverty. Proc. Natl. Acad. Sci. USA 2014, 111, 12307–12312. [Google Scholar] [CrossRef]
- Ricci, F. Social Implications of Malaria and Their Relationships with Poverty. Mediterr. J. Hematol. Infect. Dis. 2012, 4, e2012048. [Google Scholar] [CrossRef] [PubMed]
- Chima, R.I.; Goodman, C.A.; Mills, A. The Economic Impact of Malaria in Africa: A Critical Review of the Evidence. Health Policy 2003, 63, 17–36. [Google Scholar] [CrossRef]
- Khuu, D.; Eberhard, M.L.; Bristow, B.N.; Javanbakht, M.; Ash, L.R.; Shafir, S.C.; Sorvillo, F.J. Economic Impact of Malaria-Related Hospitalizations in the United States, 2000–2014. J. Infect. Public Health 2019, 12, 424–433. [Google Scholar] [CrossRef]
- Penny, M.A.; Verity, R.; Bever, C.A.; Sauboin, C.; Galactionova, K.; Flasche, S.; White, M.T.; Wenger, E.A.; Velde, N.V.d.; Pemberton-Ross, P.; et al. Public Health Impact and Cost-Effectiveness of the RTS,S/AS01 Malaria Vaccine: A Systematic Comparison of Predictions from Four Mathematical Models. Lancet 2016, 387, 367–375. [Google Scholar] [CrossRef]
- Tediosi, F.; Maire, N.; Penny, M.; Studer, A.; Smith, T.A. Simulation of the Cost-Effectiveness of Malaria Vaccines. Malar. J. 2009, 8, 127. [Google Scholar] [CrossRef]
- Galactionova, K.; Tediosi, F.; Camponovo, F.; Smith, T.A.; Gething, P.W.; Penny, M.A. Country Specific Predictions of the Cost-Effectiveness of Malaria Vaccine RTS,S/AS01 in Endemic Africa. Vaccine 2017, 35, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, K.; Hammond, A.M.; Galizi, R.; Kranjc, N.; Burt, A.; Beaghton, A.K.; Nolan, T.; Crisanti, A. A CRISPR–Cas9 Gene Drive Targeting Doublesex Causes Complete Population Suppression in Caged Anopheles Gambiae Mosquitoes. Nat. Biotechnol. 2018, 36, 1062–1066. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, B.; Jiang, Y.; Zhao, Y.; Li, Z.; Gao, H.; Ling, Y.; Wei, J.; Li, S.; Lu, M.; et al. Efficient Editing of Malaria Parasite Genome Using the CRISPR/Cas9 System. mBio 2014, 5, e01414-e14. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Fidock, D.A. CRISPR-Mediated Genome Editing of Plasmodium Falciparum Malaria Parasites. Genome Med. 2014, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Garrood, W.T.; Kranjc, N.; Petri, K.; Kim, D.Y.; Guo, J.A.; Hammond, A.M.; Morianou, I.; Pattanayak, V.; Joung, J.K.; Crisanti, A.; et al. Analysis of Off-Target Effects in CRISPR-Based Gene Drives in the Human Malaria Mosquito. Proc. Natl. Acad. Sci. USA 2021, 118, e2004838117. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.B.; Smithyman, R.; O’Neill, S.L.; Moreira, L.A. How to Engage Communities on a Large Scale? Lessons from World Mosquito Program in Rio de Janeiro, Brazil. Gates Open Res. 2021, 4, 109. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; Moreira, L.A. Can Wolbachia Be Used to Control Malaria? Mem. Inst. Oswaldo Cruz 2011, 106, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.M.; Barillas-Mury, C. Infection of Anopheline Mosquitoes with Wolbachia: Implications for Malaria Control. PLoS Pathog. 2018, 14, e1007333. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.L.; Rivero, A.; Rasgon, J.L. Wolbachia Can Enhance Plasmodium Infection in Mosquitoes: Implications for Malaria Control? PLoS Pathog. 2014, 10, e1004182. [Google Scholar] [CrossRef]
- Dimala, C.A.; Kika, B.T.; Kadia, B.M.; Blencowe, H. Current Challenges and Proposed Solutions to the Effective Implementation of the RTS, S/AS01 Malaria Vaccine Program in Sub-Saharan Africa: A Systematic Review. PLoS ONE 2018, 13, e0209744. [Google Scholar] [CrossRef]
- van der Graaf, R.; Macklin, R.; Rid, A.; Bhan, A.; Gefenas, E.; Greco, D.; Haerry, D.; Hurst, S.; London, A.J.; Saracci, R.; et al. Integrating Public Health Programs and Research after the Malaria Vaccine Implementation Program (MVIP): Recommendations for next Steps. Vaccine 2020, 38, 6975–6978. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, K. Japan’s Innovation for Global Health—GHIT’s Catalytic Role. Parasitol. Int. 2021, 80, 102232. [Google Scholar] [CrossRef] [PubMed]
| Feature | P. falciparum | P. vivax | P. ovale | P. malariae | P. knowlesi |
|---|---|---|---|---|---|
| Geographical Distribution | Primarily in Africa; also in parts of South America, Southeast Asia. | Asia, Latin America, and some parts of Africa. | West Africa primarily; sporadically elsewhere. | Worldwide, but less common. | Southeast Asia, particularly in Malaysian Borneo, Peninsular Malaysia, Thailand, Myanmar, the Philippines, and Vietnam. |
| Incubation Period in Humans | Shortest, 9–14 days. | 12–17 days; can be longer due to relapses. | Similar to P. vivax; relapses common. | Longest, 18–40 days. | 9 to 12 days; can range from 7 to 21 days. |
| Liver Stage | No hypnozoites; single acute phase. | Hypnozoites present; causes relapses. | Hypnozoites present; causes relapses. | No hypnozoites; single acute phase. | No hypnozoites. |
| Blood Stage | High parasitemia levels; severe symptoms. | Lower parasitemia; symptoms less severe than P. falciparum. | Similar to P. vivax but less frequent. | Lowest parasitemia; chronic infection possible. | Replicates every 24 h, leading to a rapid increase in parasitemia. |
| Clinical Manifestations | Severe malaria, cerebral malaria, anemia, respiratory distress. | Relapsing malaria, splenomegaly, anemia. | Milder symptoms; relapsing pattern similar to P. vivax. | Quartan malaria; nephrotic syndrome. | Fever, chills, headache, myalgia, and gastrointestinal symptoms. Severe cases: acute respiratory distress, renal failure and jaundice. |
| Vector | Anopheles spp. mosquitoes. | Anopheles spp. mosquitoes. | Anopheles spp. mosquitoes. | Anopheles spp. mosquitoes. | Anopheles latens , Anopheles balabacensis, and Anopheles hackeri. |
| Diagnostic Challenges | Rapid diagnostic tests and microscopy are effective. | Requires skilled microscopy for differentiation; dormant liver stages undetectable. | Requires skilled microscopy; often underdiagnosed. | Band-form trophozoites distinctive; can be confused with P. falciparum. | Morphological similarities to Plasmodium malariae under microscopy. |
| Treatment | Artemisinin-based combination therapies (ACTs). | Chloroquine (where resistance is not present); primaquine for hypnozoites. | Similar to P. vivax. | Chloroquine; primaquine not required. | Artemisinin-based combination therapy (ACT). |
| Vaccine Candidate | Properties | Clinical Trial Status | Reference |
|---|---|---|---|
| RTS,S/AS01 (Mosquirix™) |
|
| [8] |
| R21/Matrix-M |
|
| [36] |
| PfSPZ Vaccine |
|
| [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutanto, H. Combating Malaria with Vaccines: Insights from the One Health Framework. Acta Microbiol. Hell. 2024, 69, 153-166. https://doi.org/10.3390/amh69030015
Sutanto H. Combating Malaria with Vaccines: Insights from the One Health Framework. Acta Microbiologica Hellenica. 2024; 69(3):153-166. https://doi.org/10.3390/amh69030015
Chicago/Turabian StyleSutanto, Henry. 2024. "Combating Malaria with Vaccines: Insights from the One Health Framework" Acta Microbiologica Hellenica 69, no. 3: 153-166. https://doi.org/10.3390/amh69030015
APA StyleSutanto, H. (2024). Combating Malaria with Vaccines: Insights from the One Health Framework. Acta Microbiologica Hellenica, 69(3), 153-166. https://doi.org/10.3390/amh69030015






