Carbapenem-Resistant Klebsiella pneumoniae Bacteremia: Counterbalance between the Endemic Load and the Infection Control Program in a Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Interventions
2.3. Data Collection and Outcomes
2.4. Detection of Bacteremia and Microbial Resistance
2.5. Definitions
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vading, M.; Nauclér, P.; Kalin, M.; Giske, C.G. Invasive infection caused by Klebsiella pneumoniae is a disease affecting patients with high comorbidity and associated with high long-term mortality. PLoS ONE 2018, 13, e0195258. [Google Scholar] [CrossRef] [PubMed]
- Sokhn, E.S.; Salami, A.; El Roz, A.; Salloum, L.; Bahmad, H.F.; Ghssein, G. Antimicrobial Susceptibilities and Laboratory Profiles of Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis Isolates as Agents of Urinary Tract Infection in Lebanon: Paving the Way for Better Diagnostics. Med. Sci. 2020, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.H.; Chuang, Y.C.; Chen, C.C.; Lee, M.F.; Yang, Y.C.; Tang, H.J.; Yu, W.L. Klebsiella pneumoniae Isolates from Meningitis: Epidemiology, Virulence and Antibiotic Resistance. Sci. Rep. 2017, 7, 6634. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Guo, L.; Wang, L.F.; Zhao, Q.; Shen, D.X. Prevalence and characteristics of surgical site hypervirulent Klebsiella pneumoniae isolates. J. Clin. Lab. Anal. 2020, 34, e23364. [Google Scholar] [CrossRef] [PubMed]
- Abbas, R.; Chakkour, M.; Zein El Dine, H.; Obaseki, E.F.; Obeid, S.T.; Jezzini, A.; Ghssein, G.; Ezzeddine, Z. General Overview of Klebsiella pneumonia: Epidemiology and the Role of Siderophores in Its Pathogenicity. Biology 2024, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Wilson, L.E.; Kainer, M.A.; Lynfield, R.; Greissman, S.; et al. Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N. Engl. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef] [PubMed]
- Pomakova, D.K.; Hsiao, C.-B.; Beanan, J.M.; Olson, R.; MacDonald, U.; Keynan, Y.; Russo, T.A. Clinical and phenotypic differences between classic and hypervirulent Klebsiella pneumonia: An emerging and under-recognized pathogenic variant. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Naas, T.; Poirel, L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 2011, 17, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhang, H.; Du, H. Carbapenemases in Enterobacteriaceae: Detection and Antimicrobial Therapy. Front. Microbiol. 2019, 10, 1823. [Google Scholar] [CrossRef]
- Tamma, P.D.; Goodman, K.E.; Harris, A.D.; Tekle, T.; Roberts, A.; Taiwo, A.; Simner, P.J. Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae bacteremia. Clin. Infect. Dis. 2017, 64, 257–264. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, T.; Wang, Y.; Yang, K.; Zhou, Y.; Luo, Q.; Shen, P.; Xiao, Y. Socioeconomic Burden of Bloodstream Infections Caused by Carbapenem-Resistant Enterobacteriaceae. Infect. Drug Resist. 2021, 14, 5385–5393. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Kontopidou, F.; Dedoukou, X.; Katerelos, P.; Gourgoulis, G.M.; Tsonou, P.; Maragos, A.; Gargalianos, P.; Gikas, A.; Gogos, C.; et al. Working Group for the National Action Plan to Combat Infections due to Carbapenem-Resistant, Gram-Negative Pathogens in Acute-Care Hospitals in Greece. J. Glob. Antimicrob. Resist. 2014, 2, 11–16. [Google Scholar] [PubMed]
- Karampatakis, T.; Antachopoulos, C.; Iosifidis, E.; Tsakris, A.; Roilides, E. Molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in Greece. Future Microbiol. 2016, 11, 809–823. [Google Scholar] [CrossRef]
- Tryfinopoulou, K.; Linkevicius, M.; Pappa, O.; Alm, E.; Karadimas, K.; Svartström, O.; Polemis, M.; Mellou, K.; Maragkos, A.; Brolund, A.; et al. Emergence and persistent spread of carbapenemase-producing Klebsiella pneumoniae high-risk clones in Greek hospitals, 2013 to 2022. Euro Surveill. 2023, 28, 2300571. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in Health Care Facilities. World Health Organization Website. 2017. Available online: https://www.who.int/publications/i/item/9789241550178 (accessed on 28 December 2023).
- Centers for Disease Control and Prevention. Facility Guidance for Control of Carbapenem-Resistant Enterobacteriaceae (CRE). Centers for Disease Control and Prevention Website. 2015. Available online: https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf (accessed on 28 December 2023).
- Magiorakos, A.P.; Burns, K.; Rodríguez Baño, J.; Borg, M.; Daikos, G.; Dumpis, U.; Lucet, J.C.; Moro, M.L.; Tacconelli, E.; Simonsen, G.S.; et al. Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: Guidance from the European Centre for Disease Prevention and Control. Antimicrob. Resist. Infect. Control 2017, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.W.; Kirk, A.; Lee, K.B.; Markley, J.D.; Pakyz, A.; Bearman, G.; Doll, M.E.; Stevens, M.P. The impact of formulary restriction on the relative consumption of carbapenems in intensive care units at an academic medical center. Infect. Control Hosp. Epidemiol. 2019, 40, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Kirk, A.; Pierce, J.; Doll, M.; Lee, K.; Pakyz, A.; Kim, J.; Markley, D.; De la Cruz, O.; Bearman, G.; Stevens, M.P. Effect of carbapenem restriction on prescribing trends for immunocompromised wards at an academic medical center. Am. J. Infect. Control. 2019, 47, 1035–1037. [Google Scholar] [CrossRef] [PubMed]
- Vock, I.; Tschudin-Sutter, S. Carbapenem-resistant Klebsiella pneumoniae-impact of infection-prevention and control interventions. Ann. Transl. Med. 2019, 7, S344. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, X.; Wang, J.; Tan, R.; Sun, J.; Li, L.; Huang, J.; Wu, J.; Gu, Q.; Zhao, Y.; et al. Infection-prevention and control interventions to reduce colonisation and infection of intensive care unit-acquired carbapenem-resistant Klebsiella pneumoniae: A 4-year quasi-experimental before-and-after study. Antimicrob. Resist. Infect. Control 2019, 8, 8. [Google Scholar] [CrossRef]
- Papanikolopoulou, A.; Maltezou, H.C.; Kritikou, H.; Papadopoulos, T.; Kandalepas, G.; Pentzouris, A.; Kartsonakis, I.; Chronopoulou, G.; Gargalianos-Kakolyris, P.; Pantazis, N.; et al. Six-year time-series data on multidrug-resistant bacteremia, antibiotic consumption and infection control interventions in a hospital. Microb. Drug Resist. 2022, 28, 806–818. [Google Scholar] [CrossRef]
- National Healthcare Safety Network. Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-Central Line Associated Bloodstream Infection). Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf (accessed on 28 December 2023).
- Papanikolopoulou, A.; Maltezou, H.C.; Stoupis, A.; Pangalis, A.; Kouroumpetsis, C.; Chronopoulou, G.; Kalofissoudis, Y.; Kostares, E.; Boufidou, F.; Karalexi, M.; et al. Ventilator associated pneumonia (VAP), multidrug-resistant bacteremia and infection control interventions in an intensive care unit: Analysis of six-year time-series data. Antibiotics 2022, 11, 1128. [Google Scholar] [CrossRef]
- Carrara, E.; Conti, M.; Meschiari, M.; Mussini, C. The role of antimicrobial stewardship in preventing KPC-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 2021, 76, i12–i18. [Google Scholar] [CrossRef]
- Tomczyk, S.; Zanichelli, V.; Grayson, M.L.; Twyman, A.; Abbas, M.; Pires, D.; Allegranzi, B.; Harbarth, S. Control of Carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa in Healthcare Facilities: A Systematic Review and Reanalysis of Quasi-experimental Studies. Clin. Infect. Dis. 2019, 68, 873–884. [Google Scholar] [CrossRef]
- Schwaber, M.J.; Carmeli, Y. An ongoing national intervention to contain the spread of carbapenem-resistant Enterobacteriaceae. Clin. Infect. Dis. 2014, 58, 697–703. [Google Scholar] [CrossRef]
- Hassoun-Kheir, N.; Snitser, O.; Hussein, K.; Rabino, G.; Eluk, O.; Warman, S.; Aboalhega, W.; Geffen, Y.; Mendelson, S.; Kishony, R. Concordance between epidemiological evaluation of probability of transmission and whole genome sequence relatedness among hospitalized patients acquiring Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae. Clin. Microbiol. Infect. 2021, 27, 468.e1–468.e7. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Baño, J.; Pérez-Moreno, M.A.; Peñalva, G.; Garnacho-Montero, J.; Pinto, C.; Salcedo, I.; Fernández-Urrusuno, R.; Neth, O.; Gil-Navarro, M.V.; Pérez-Milena, A.; et al. PIRASOA Programme Group. Outcomes of the PIRASOA programme, an antimicrobial stewardship programme implemented in hospitals of the Public Health System of Andalusia, Spain: An ecologic study of time-trend analysis. Clin. Microbiol. Infect. 2020, 26, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Gagliotti, C.; Cappelli, V.; Carretto, E.; Marchi, M.; Pan, A.; Ragni, P.; Sarti, M.; Suzzi, R.; Tura, G.A.; Moro, M.L.; et al. Control of carbapenemase-producing Klebsiella pneumoniae: A region-wide intervention. Euro Surveill. 2014, 19, 20943. [Google Scholar] [CrossRef][Green Version]
- Malik, B.; Bhattacharyya, S. Antibiotic drug-resistance as a complex system driven by socio-economic growth and antibiotic misuse. Sci. Rep. 2019, 9, 9788. [Google Scholar] [CrossRef]
- Kazanjian, P.H. Efforts to regulate antibiotic misuse in hospitals: A history. Infect. Control Hosp. Epidemiol. 2022, 43, 1119–1122. [Google Scholar] [CrossRef]
- Karampatakis, T.; Tsergouli, K.; Iosifidis, E.; Antachopoulos, C.; Karapanagiotou, A.; Karyoti, A.; Gritsi-Gerogianni, N.; Tsakris, A.; Roilides, E. Impact of active surveillance and infection control measures on carbapenem-resistant Gram-negative bacterial colonization and infections in intensive care. J. Hosp. Infect. 2018, 99, 396–404. [Google Scholar] [CrossRef]
- Frattari, A.; Savini, V.; Polilli, E.; Di Marco, G.; Lucisano, G.; Corridoni, S.; Spina, T.; Costantini, A.; Nicolucci, A.; Fazii, P.; et al. Control of gram-negative multi-drug resistant microorganisms in an Italian ICU: Rapid decline as a result of a multifaceted intervention, including conservative use of antibiotics. Int. J. Infect. Dis. 2019, 84, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Gordon, M.; Villarreal, E.; Frasquet, J.; Sánchez, M.Á.; Martín, M.; Castellanos, Á.; Ramirez, P. Influence of antibiotic pressure on multi-drug resistant Klebsiella pneumoniae colonisation in critically ill patients. Antimicrob. Resist. Infect. Control. 2019, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Ezzeddine, Z.; Ghssein, G. Towards new antibiotics classes targeting bacterial metallophores. Microb. Pathog. 2023, 182, 106221. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, G. Strategies to Overcome Antimicrobial Resistance (AMR) Making Use of Non-Essential Target Inhibitors: A Review. Int. J. Mol. Sci. 2019, 20, 5844. [Google Scholar] [CrossRef]
- Chibabhai, V.; Duse, A.G.; Perovic, O.; Richards, G.A. Collateral damage of the COVID-19 pandemic: Exacerbation of antimicrobial resistance and disruptions to antimicrobial stewardship programmes? S. Afr. Med. J. 2020, 110, 572–573. [Google Scholar] [CrossRef]
- Tomczyk, S.; Taylor, A.; Brown, A.; de Kraker, M.E.A.; El-Saed, A.; Alshamrani, M.; Hendriksen, R.S.; Jacob, M.; Löfmark, S.; Perovic, O.; et al. WHO AMR Surveillance and Quality Assessment Collaborating Centres Network. Impact of the COVID-19 pandemic on the surveillance, prevention and control of antimicrobial resistance: A global survey. J. Antimicrob. Chemother. 2021, 76, 3045–3058. [Google Scholar] [CrossRef]
- Perneger, T.V. What’s wrong with Bonferroni adjustments. BMJ 1998, 316, 1236–1238. [Google Scholar] [CrossRef]
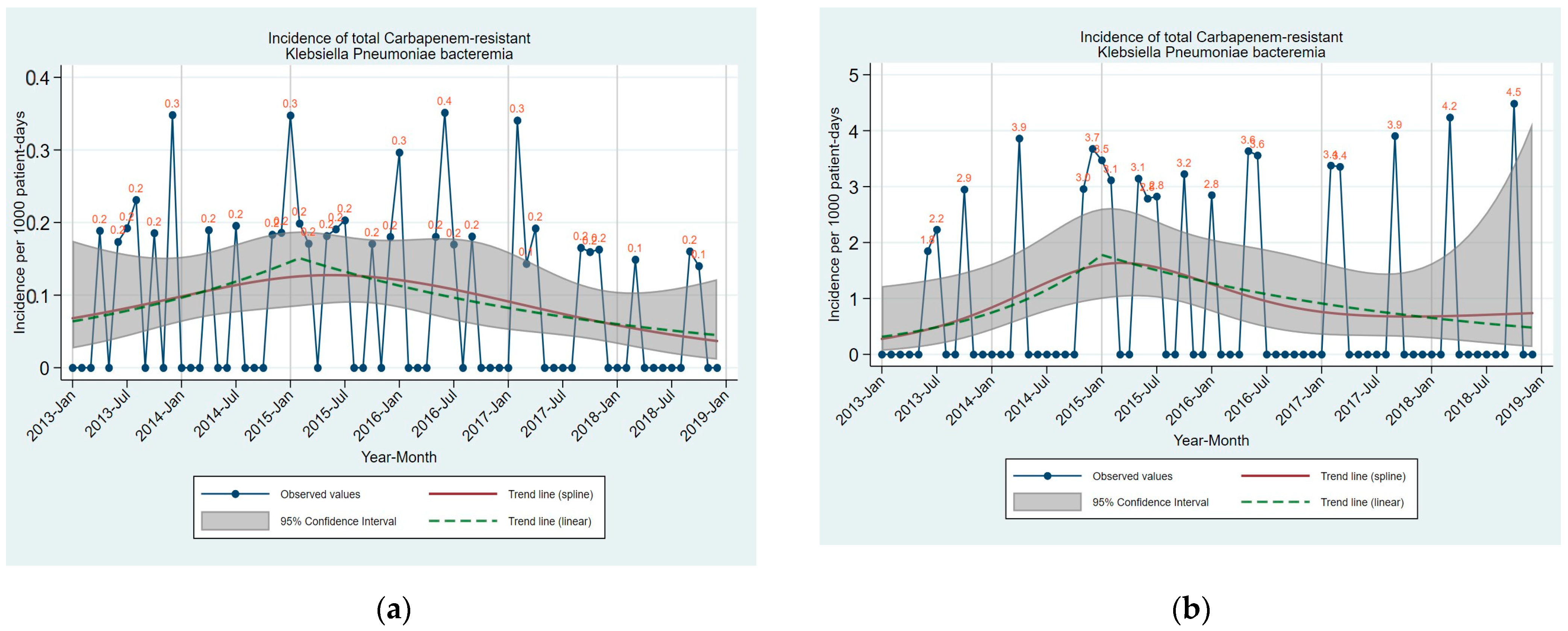
| Time Trend | |||||
|---|---|---|---|---|---|
| Incidence of Bacteremia /1000 Patient-Days | EVSP January 2013 (95% CI) | EVEP December 2018 (95% CI) | p-Value | %Relative Change/Year (95% CI) | p-Value |
| Total Hospital Clinics | |||||
| Total CRKP Bacteremia | 0.1 (0.0 to 0.2) | 0.0 (0.0 to 0.1) | 0.545 | 51.18 (−9.95 to 153.81) up to 02/2015 | 0.118 |
| −27.10 (−42.97 to −6.81) after 02/2015 | 0.012 | ||||
| Total Hospital Departments | |||||
| Total CRKP Bacteremia | 0.1 (0.0 to 0.2) | 0.0 (0.0 to 0.1) | 0.445 | −9.40 (−29.69 to 16.75) | 0.445 |
| Adults Clinic | |||||
| Total CRKP Bacteremia | 0.1 (0.0 to 0.3) | 0.1 (0.0 to 0.2) | 0.614 | 56.27 (−6.69 to 161.69) up to 02/2015 | 0.090 |
| −27.30 (−42.97 to −7.32) after 02/2015 | 0.010 | ||||
| Adults Clinic Departments | |||||
| Total CRKP Bacteremia | 0.1 (0.0 to 0.2) | 0.1 (0.0 to 0.1) | 0.495 | −8.27 (−28.40 to 17.52) | 0.495 |
| Adults ICU | |||||
| Total CRKP Bacteremia | 0.3 (0.1 to 1.2) | 0.5 (0.1 to 1.7) | 0.635 | 137.77 (8.59 to 420.63) up to 01/2015 | 0.030 |
| −28.44 (−51.84 to 6.33) after 01/2015 | 0.098 | ||||
| CRKP Bacteremia Correlation with Antibiotics | ||||||||
|---|---|---|---|---|---|---|---|---|
| Antibiotics (DDDs/100 Patient-Days) | per (n) DDD | Month 0 | Month −1 | Month −2 | Month −3 | IRR | 95% CI | p-Value |
| Total Hospital Clinics | ||||||||
| Carbapenems | 1 | ◊ | 1.54 | (1.05, 2.25) | 0.026 | |||
| Aminoglycosides | 1 | ◊ | ◊ | 2.34 | (1.09, 5.05) | 0.030 | ||
| Fluoroquinolones | 1 | ◊ | 1.35 | (0.98, 1.86) | 0.067 | |||
| Colistin | 1 | ◊ | 0.48 | (0.24, 0.96) | 0.037 | |||
| Tigecycline | 1 | ◊ | 1.48 | (0.96, 2.29) | 0.077 | |||
| Tigecycline | 1 | ◊ | 0.46 | (0.27, 0.77) | 0.003 | |||
| Fosfomycin | 0.1 | ◊ | ◊ | 1.14 | (1.01, 1.29) | 0.031 | ||
| Advanced Antibiotics | 1 | ◊ | 1.26 | (1.00, 1.58) | 0.046 | |||
| All Antibiotics | 10 | ◊ | ◊ | 2.55 | (0.96, 6.78) | 0.060 | ||
| Total Hospital Departments | ||||||||
| Monobactams | 0.1 | ◊ | ◊ | 0.30 | (0.09, 0.98) | 0.047 | ||
| Carbapenems | 1 | ◊ | 1.79 | (1.26, 2.55) | 0.001 | |||
| Colistin | 1 | ◊ | 0.17 | (0.04, 0.74) | 0.019 | |||
| Tigecycline | 1 | ◊ | 2.68 | (0.92, 7.80) | 0.071 | |||
| Tigecycline | 1 | ◊ | 0.18 | (0.05, 0.69) | 0.013 | |||
| Fosfomycin | 0.1 | ◊ | 0.75 | (0.54, 1.03) | 0.078 | |||
| Ceftolozane—tazobactam | 0.1 | ◊ | 3.92 | (1.06, 14.42) | 0.040 | |||
| Ceftolozane—tazobactam | 0.1 | ◊ | 0.11 | (0.01, 1.03) | 0.053 | |||
| Adults Clinic | ||||||||
| Monobactams | 0.1 | ◊ | 0.67 | (0.48, 0.94) | 0.021 | |||
| Carbapenems | 1 | ◊ | ◊ | 1.36 | (1.10, 1.67) | 0.004 | ||
| Aminoglycosides | 1 | ◊ | ◊ | 1.95 | (1.17, 3.25) | 0.010 | ||
| Fluoroquinolones | 1 | ◊ | 1.23 | (1.03, 1.48) | 0.023 | |||
| Colistin | 1 | ◊ | 0.67 | (0.45, 0.99) | 0.046 | |||
| Tigecycline | 1 | ◊ | 1.27 | (0.96, 1.67) | 0.091 | |||
| Tigecycline | 1 | ◊ | 0.63 | (0.46, 0.87) | 0.005 | |||
| Fosfomycin | 0.1 | ◊ | ◊ | 1.08 | (1.00, 1.16) | 0.040 | ||
| Advanced Antibiotics | 1 | ◊ | ◊ | 1.19 | (1.04, 1.36) | 0.012 | ||
| All Antibiotics | 10 | ◊ | ◊ | 2.46 | (1.19, 5.09) | 0.016 | ||
| Adults Clinic Departments | ||||||||
| Monobactams | 0.1 | ◊ | 0.46 | (0.23, 0.93) | 0.031 | |||
| Carbapenems | 1 | ◊ | 1.29 | (1.01, 1.64) | 0.044 | |||
| Aminoglycosides | 1 | ◊ | 2.20 | (1.00, 4.85) | 0.051 | |||
| Colistin | 1 | ◊ | 2.33 | (0.90, 6.04) | 0.082 | |||
| Colistin | 1 | ◊ | 0.34 | (0.13, 0.86) | 0.023 | |||
| Tigecycline | 1 | ◊ | 2.08 | (0.97, 4.46) | 0.060 | |||
| Tigecycline | 1 | ◊ | 0.32 | (0.13, 0.77) | 0.011 | |||
| Fosfomycin | 0.1 | ◊ | 0.82 | (0.66, 1.02) | 0.074 | |||
| Ceftolozane—tazobactam | 0.1 | ◊ | 2.31 | (1.02, 5.27) | 0.046 | |||
| Ceftolozane—tazobactam | 0.1 | ◊ | 0.28 | (0.08, 0.99) | 0.048 | |||
| Adults ICU | ||||||||
| Carbapenems | 10 | ◊ | 1.26 | (1.02, 1.55) | 0.033 | |||
| Aminoglycosides | 10 | ◊ | 2.89 | (1.91, 4.38) | <0.001 | |||
| Colistin | 10 | ◊ | 1.56 | (0.95, 2.58) | 0.080 | |||
| Fosfomycin | 10 | ◊ | 2.52 | (1.53, 4.14) | <0.001 | |||
| Non Advanced Antibiotics | 10 | ◊ | 1.19 | (1.02, 1.38) | 0.025 | |||
| CRKP Bacteremia Correlation with Infection Control Interventions | ||||||||
|---|---|---|---|---|---|---|---|---|
| Infection Control Interventions | Per (n) Unit | Month 0 | Month −1 | Month −2 | Month −3 | IRR | 95% CI | p-Value |
| CRKP Bacteremia | ||||||||
| Adults ICU | ||||||||
| % Isolations /Admissions | 10 | ◊ | 2.60 | (1.54, 4.39) | <0.001 | |||
| % Isolations /Admissions | 10 | ◊ | 0.35 | (0.13, 0.97) | 0.044 | |||
| L of Scrub Disinfectant sol/1000 patient-days | 10 | ◊ | 0.74 | (0.59, 0.93) | 0.008 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papanikolopoulou, A.; Vini, L.; Stoupis, A.; Kalimeri, D.; Pangalis, A.; Chronopoulou, G.; Pantazis, N.; Gargalianos-Kakolyris, P.; Kantzanou, M. Carbapenem-Resistant Klebsiella pneumoniae Bacteremia: Counterbalance between the Endemic Load and the Infection Control Program in a Hospital. Acta Microbiol. Hell. 2024, 69, 81-92. https://doi.org/10.3390/amh69020009
Papanikolopoulou A, Vini L, Stoupis A, Kalimeri D, Pangalis A, Chronopoulou G, Pantazis N, Gargalianos-Kakolyris P, Kantzanou M. Carbapenem-Resistant Klebsiella pneumoniae Bacteremia: Counterbalance between the Endemic Load and the Infection Control Program in a Hospital. Acta Microbiologica Hellenica. 2024; 69(2):81-92. https://doi.org/10.3390/amh69020009
Chicago/Turabian StylePapanikolopoulou, Amalia, Louisa Vini, Athina Stoupis, Dimitra Kalimeri, Anastasia Pangalis, Genovefa Chronopoulou, Nikos Pantazis, Panagiotis Gargalianos-Kakolyris, and Maria Kantzanou. 2024. "Carbapenem-Resistant Klebsiella pneumoniae Bacteremia: Counterbalance between the Endemic Load and the Infection Control Program in a Hospital" Acta Microbiologica Hellenica 69, no. 2: 81-92. https://doi.org/10.3390/amh69020009
APA StylePapanikolopoulou, A., Vini, L., Stoupis, A., Kalimeri, D., Pangalis, A., Chronopoulou, G., Pantazis, N., Gargalianos-Kakolyris, P., & Kantzanou, M. (2024). Carbapenem-Resistant Klebsiella pneumoniae Bacteremia: Counterbalance between the Endemic Load and the Infection Control Program in a Hospital. Acta Microbiologica Hellenica, 69(2), 81-92. https://doi.org/10.3390/amh69020009






