Improving Turnaround Time in Pediatric Clinical Microbiology Results: Implementation of the Kaizen Method in a Chilean Hospital Laboratory
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AST | Antimicrobial susceptibility |
| COVID-19 | Coronavirus disease 2019 |
| HRRIO | Hospital Roberto del Río |
| IQR | Interquartile range |
| IT | Information technology |
| LIS | Laboratory information system |
| MALDI-TOF | Matrix-assisted laser desorption/ionization–time of flight mass spectrometry |
| POC | Point of Care |
| PCR | Polymerase Chain Reaction |
| PDCA | Plan-Do-Check-Act cycle |
| TAT | Turn-around time |
| V2C | Vitek 2 Compact® automated identification and susceptibility testing system |
References
- Plebani, M. Quality in laboratory medicine: An unfinished journey. J. Lab. Precis. Med. 2017, 2, 63. [Google Scholar] [CrossRef]
- Trigueiro, G.; Oliveira, C.; Rodrigues, A.; Seabra, S.; Pinto, R.; Bala, Y.; Gutiérrez Granado, M.; Vallejo, S.; Gonzalez, V.; Cardoso, C. Conversion of a classical microbiology laboratory to a total automation laboratory enhanced by the application of lean principles. Microbiol. Spectr. 2024, 12, e02153-23. [Google Scholar] [CrossRef] [PubMed]
- Tapia, C.; Vega, C.; Rojas, C. Implementación del laboratorio clínico moderno. Rev. Médica Clínica Las Condes 2015, 26, 794–801. [Google Scholar] [CrossRef][Green Version]
- Antonios, K.; Croxatto, A.; Culbreath, K. Current State of Laboratory Automation in Clinical Microbiology Laboratory. Clin. Chem. 2022, 68, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R. Laboratory turnaround time. Clin. Biochem. Rev. 2007, 28, 179–194. [Google Scholar]
- Kotter, J. Choosing Strategies for Change. 2013. Available online: https://projects.iq.harvard.edu/files/sdpfellowship/files/day3_2_choosing_strategies_for_change.pdf (accessed on 20 September 2025).
- Alvarado, K.; Pumisasho, V. Continuous improvement practices with Kaizen approach in companies of the metropolitan district of Quito: An exploratory study. Intang. Cap. 2017, 13, 901. [Google Scholar] [CrossRef]
- Declerck, B.; Swaak, M.; Martin, M.; Kesteloot, K. Activity-based costing analysis of laboratory testing in clinical chemistry. Clin. Chem. Lab. Med. 2021, 59, 1369–1375. [Google Scholar] [CrossRef]
- Da Silva, A.; Emmendoerfer, M. Innovation labs in South American governments: Congruencies and peculiarities. Braz. Adm. Rev. 2023, 20, e220173. [Google Scholar] [CrossRef]
- Samara, M.N.; Harry, K.D. Leveraging Kaizen with process mining in healthcare settings: A conceptual framework for data-driven continuous improvement. Healthcare 2025, 13, 941. [Google Scholar] [CrossRef]
- Senok, A.; Dabal, L.A.; Alfaresi, M.; Habous, M.; Celiloglu, H.; Bashiri, S.; Almaazmi, N.; Ahmed, H.; Mohmed, A.A.; Bahaaldin, O.; et al. Clinical impact of the BioFire Blood Culture Identification 2 Panel in adult patients with bloodstream infection: A multicenter observational study in the United Arab Emirates. Diagnostics 2023, 13, 2433. [Google Scholar] [CrossRef]
- Rezaei, M.; Razavi Bazaz, S.; Zhand, S.; Sayyadi, N.; Jin, D.; Stewart, M.P.; Ebrahimi Warkiani, M. Point-of-care diagnostics in the age of COVID-19. Diagnostics 2020, 11, 9. [Google Scholar] [CrossRef]
- Peri, A.M.; Ling, W.; Furuya-Kanamori, L.; Harris, P.N.A.; Paterson, D.L. Performance of BioFire Blood Culture Identification 2 Panel (BCID2) for the detection of bloodstream pathogens and their associated resistance markers: A systematic review and meta-analysis of diagnostic test accuracy studies. BMC Infect. Dis. 2022, 22, 794. [Google Scholar] [CrossRef]
- Reszetnik, G.; Hammond, K.; Mahshid, S.; AbdElFatah, T.; Nguyen, D.; Corsini, R.; Caya, C.; Papenburg, J.; Cheng, M.P.; Yansouni, C.P. Next-generation rapid phenotypic antimicrobial susceptibility testing. Nat. Commun. 2024, 15, 9719. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.Y.; Chen, C.L.; Chen, W.C.; Kuo, Y.C.; Liang, S.J.; Tu, C.Y.; Lin, Y.C.; Hsueh, P.R. Reduced mortality with antimicrobial stewardship guided by BioFire FilmArray Blood Culture Identification 2 panel in critically ill patients with bloodstream infection: A retrospective propensity score-matched study. Int. J. Antimicrob. Agents 2024, 64, 107300. [Google Scholar] [CrossRef]
- Cintrón, M.; Clark, B.; Miranda, E.; Delgado, M.; Babady, N.E. Development and evaluation of a direct disk diffusion, rapid antimicrobial susceptibility testing method from blood cultures positive for Gram-negative bacilli using rapid molecular testing and microbiology laboratory automation. Microbiol. Spectr. 2025, 13, e0240124. [Google Scholar] [CrossRef]
- Totty, H.; Ullery, M.; Spontak, J.; Viray, J.; Adamik, M.; Katzin, B.; Dunne, W.M.; Deol, P. A controlled comparison of the BacT/ALERT® 3D and VIRTUO™ microbial detection systems. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1795–1800. [Google Scholar] [CrossRef]
- Yarbrough, M.L.; Wallace, M.A.; Burnham, C.D. Comparison of Microorganism Detection and Time to Positivity in Pediatric and Standard Media from Three Major Commercial Continuously Monitored Blood Culture Systems. J. Clin. Microbiol. 2021, 59, e0042921. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.F.; Ruppé, E.; Barbier, F.; Tabah, A.; Bassetti, M. Bloodstream infections in critically ill patients: An expert statement. Intensive Care Med. 2020, 46, 266–284. [Google Scholar] [CrossRef]
- Bailey, A.L.; Ledeboer, N.; Burnham, C.D. Clinical Microbiology Is Growing up: The Total Laboratory Automation Revolution. Clin. Chem. 2019, 65, 634–643. [Google Scholar] [CrossRef]
- Chan, W.S.; Ho, C.W.; Chan, T.C.; Hung, J.; To, M.Y.; Leung, S.M.; Lai, K.C.; Wong, C.Y.; Leung, C.P.; Au, C.H.; et al. Clinical Evaluation of the BIOFIRE SPOTFIRE Respiratory Panel. Viruses 2024, 16, 600. [Google Scholar] [CrossRef] [PubMed]
- Graff, K.E.; Palmer, C.; Anarestani, T.; Velasquez, D.; Hamilton, S.; Pretty, K.; Parker, S.; Dominguez, S.R. Clinical Impact of the Expanded BioFire Blood Culture Identification 2 Panel in a U.S. Children’s Hospital. Microbiol. Spectr. 2021, 9, e0042921. [Google Scholar] [CrossRef]
- Peri, A.M.; Chatfield, M.D.; Ling, W.; Furuya-Kanamori, L.; Harris, P.N.A.; Paterson, D.L. Rapid Diagnostic Tests and Antimicrobial Stewardship Programs for the Management of Bloodstream Infection: What Is Their Relative Contribution to Improving Clinical Outcomes? A Systematic Review and Network Meta-analysis. Clin. Infect. Dis. 2024, 16, 502–515. [Google Scholar] [CrossRef]
- Antiochia, R. Paper-Based Biosensors: Frontiers in Point-of-Care Detection of COVID-19 Disease. Biosensors 2021, 11, 110. [Google Scholar] [CrossRef]
- Truong, W.R.; Hidayat, L.; Bolaris, M.A.; Nguyen, L.; Yamaki, J. The antibiogram: Key considerations for its development and utilization. JAC-Antimicrob. Resist. 2021, 3, dlab060. [Google Scholar] [CrossRef] [PubMed]
- Klinker, K.P.; Hidayat, L.K.; DeRyke, C.A.; DePestel, D.D.; Motyl, M.; Bauer, K.A. Antimicrobial stewardship and antibiograms: Importance of moving beyond traditional antibiograms. Ther. Adv. Infect. Dis. 2021, 8, 20499361211011373. [Google Scholar] [CrossRef]
- Chokkalla, A.K.; Recio, B.D.; Devaraj, S. Best Practices for Effective Management of Point of Care Testing. eJIFCC 2023, 34, 245–249. [Google Scholar] [PubMed]
- Khalifa, M.; Khalid, P. Improving laboratory results turnaround time by reducing pre analytical phase. Stud. Health Technol. Inform. 2014, 202, 71–74. [Google Scholar] [PubMed]
- Sancho, D.; Rezusta, A.; Acero, R. Integrating Lean Six Sigma into Microbiology Laboratories: Insights from a Literature Review. Healthcare 2025, 13, 917. [Google Scholar] [CrossRef]
- White, B.A.; Baron, J.M.; Dighe, A.S.; Camargo, C.A., Jr.; Brown, D.F. Applying Lean methodologies reduces ED laboratory turnaround times. Am. J. Emerg. Med. 2015, 33, 1572–1576. [Google Scholar] [CrossRef]
- Cherie, N.; Berta, D.M.; Tamir, M.; Yiheyis, Z.; Angelo, A.A.; Mekuanint Tarekegn, A.; Chane, E.; Nigus, M.; Teketelew, B.B. Improving laboratory turnaround times in clinical settings: A systematic review of the impact of lean methodology application. PLoS ONE 2024, 19, e0312033. [Google Scholar] [CrossRef]
- Vyas, S.; Patel, D.; Bandali, A.; Giordano, P.; Roland, R.; Kessler, J. Impact of BioFire® Blood Culture Identification (BCID) panels on antibiotic management of bacteremia due to select organisms. Diagn. Microbiol. Infect. Dis. 2024, 110, 116384. [Google Scholar] [CrossRef] [PubMed]
- Donnars, A.; Mahieu, R.; Declerck, C.; Chenouard, R.; Lemarié, C.; Pailhoriès, H.; Requin, J.; Kempf, M.; Eveillard, M. BIOFIRE® Blood Culture IDentification 2 (BCID2) panel for early adaptation of antimicrobial therapy in adult patients with bloodstream infections: A real-life experience. Diagn. Microbiol. Infect. Dis. 2023, 105, 115858. [Google Scholar] [CrossRef] [PubMed]
- Graff, K.E.; Galvez, N.; Lamb, M.M.; Cortes, R.; Sanchez Villeda, E.A.; Calvimontes, D.M.; Melgar, M.A.; Olson, D.; Asturias, E.J.; Dominguez, S.R. Evaluating the Clinical Impact of the BCID2 Panel and Antimicrobial Stewardship in Pediatric Bloodstream Infections: A Pragmatic Trial in Guatemala. Pediatr. Infect. Dis. J. 2025, 44, 1051–1058. [Google Scholar] [CrossRef] [PubMed]

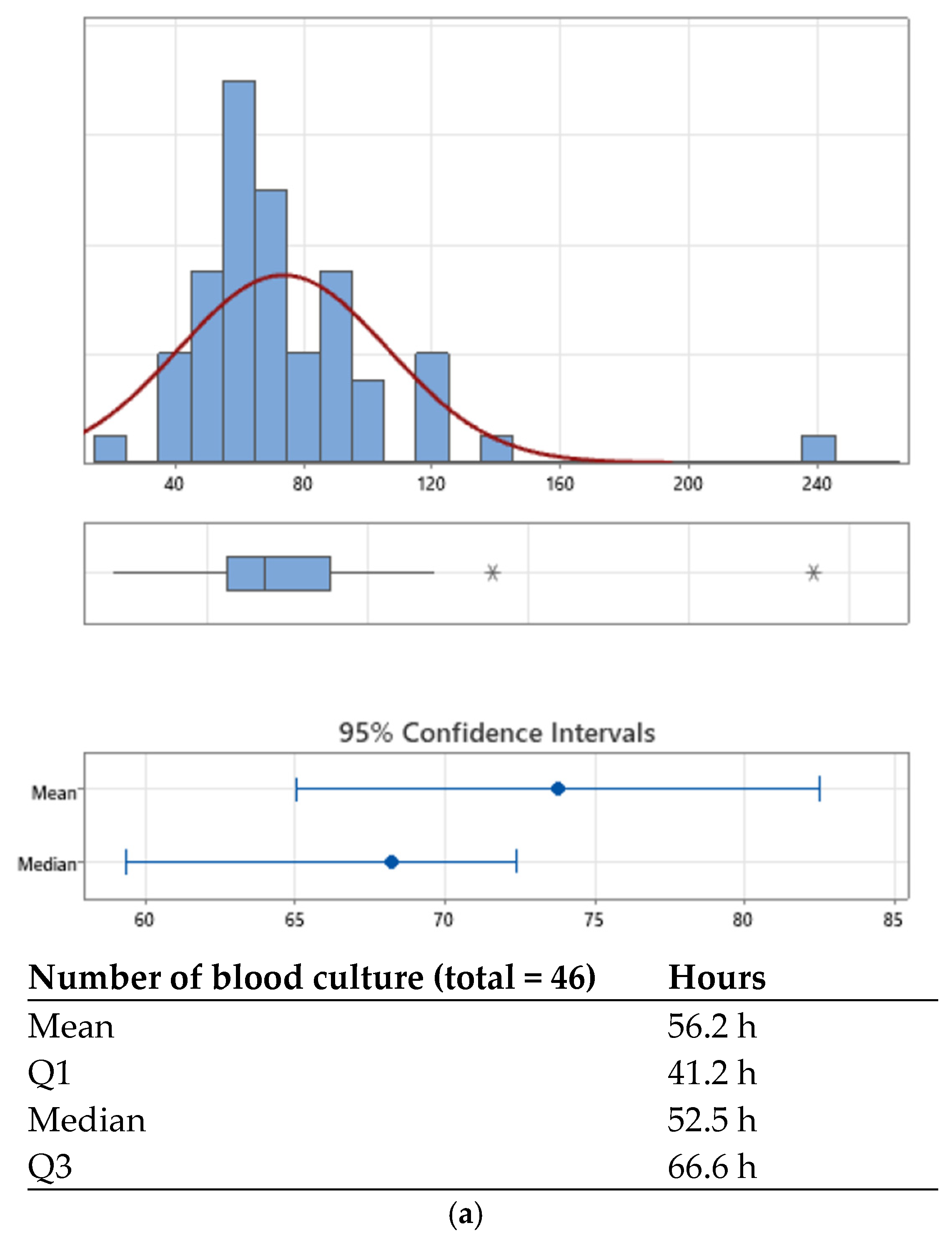

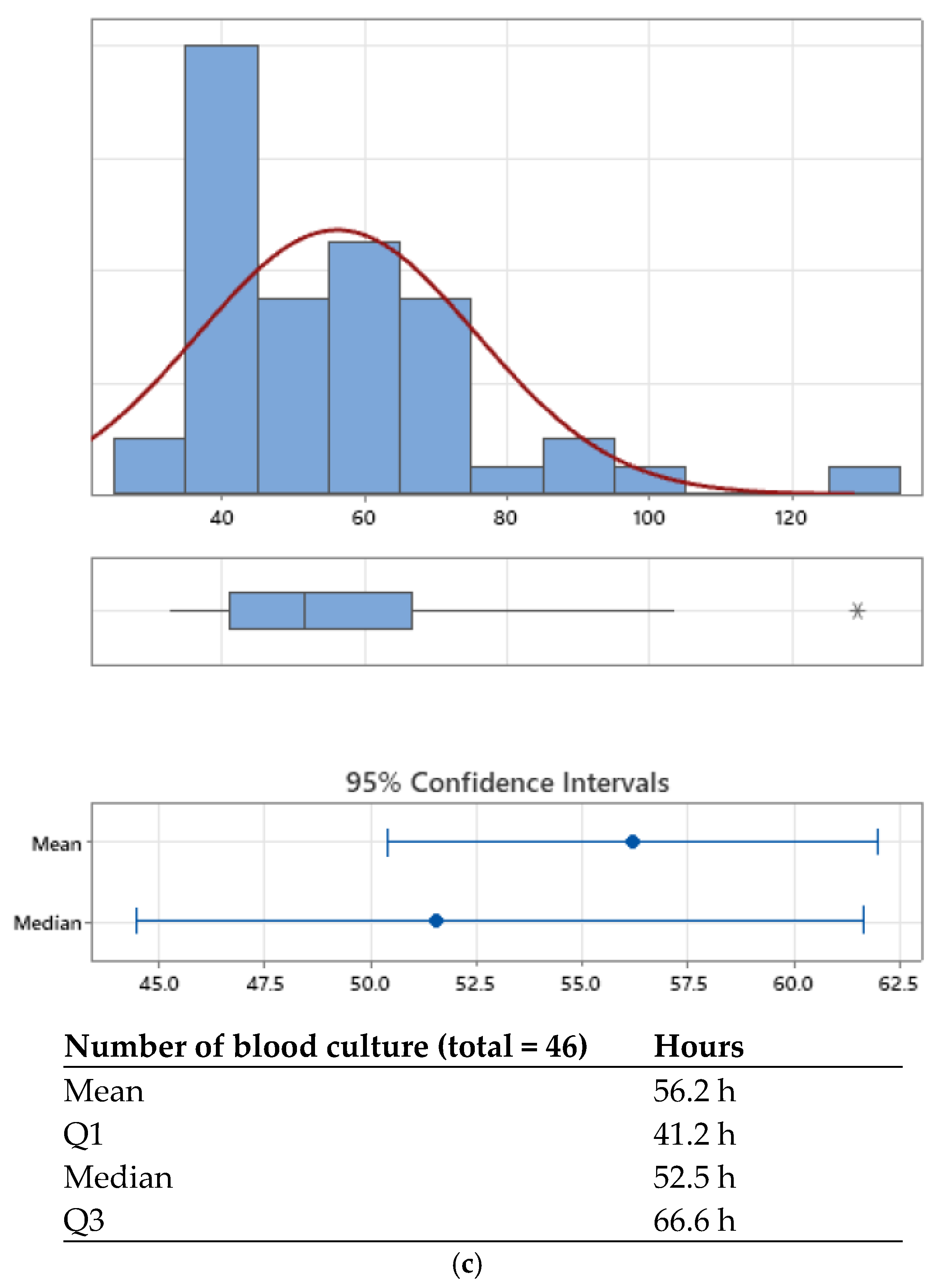

| Kaizen Principle | Definition | Practical Application in the Pediatric Microbiology Laboratory |
|---|---|---|
| Continuous Improvement (Iteration) | Progressive implementation of sustained, incremental changes aimed at increasing efficiency. | Short-, medium-, and long-term improvements in result delivery were planned, based on operational observations. |
| Cross-functional Team Involvement | Active engagement of all staff levels in the improvement process. | Laboratory technical staff collaborated with bioMérieux facilitators to define outcomes. |
| Collaborative Work | Formation of multidisciplinary teams to identify improvement opportunities. | Kaizen event (workshop). The participants developed solutions tailored to the local realities of the hospital. |
| On-site Observation (Gemba) | Direct analysis of processes at the point of execution to identify critical issues. | Operational workflows were observed in real time, highlighting the need to optimize sample collection and processing steps. |
| Elimination of Waste (Muda) | Identification and removal of non–value-adding activities. | Process redesigns were proposed to reduce idle times, eliminate redundant tasks, and enhance human resource utilization. |
| Standardization | Formalization of successful improvements through clear, reproducible protocols. | Implementation of a standardized 24/7 workflow was discussed to ensure consistent performance across all shifts. |
| Improvement Tools | Use of methods such as Plan–Do–Check–Act (PDCA), root cause analysis, and 5S to guide change implementation (sort, set in order, shine, standardize, sustain). | Kaizen tools were applied during bioMérieux-facilitated event to support structured decision-making. |
| Data-Driven and Measurable Outcomes | Evaluation of impact through quantifiable indicators before and after intervention. | Focus was placed on reducing validation and result delivery times by increasing daily validations and tracking performance metrics. |
| Organizational Improvement Culture | Promotion of an institutional mindset focused on continuous improvement identification. | A collaborative approach was established, integrating the team in the design of sustainable and context-sensitive solutions. |
| Innovation and Technological Support | Gradual incorporation of digital tools to support continuous improvement. | Optimization of REAL V1.5® (*) and VITEK® 2 Compact software (bioMerieux, Marcy l’Etoile, France) was recommended, along with IT integration for real-time monitoring. |
| Day | Manual Activity Time (h) | Equipment/Incubation Time (h) | Expected Time Savings (h) | % Expected Time Savings |
|---|---|---|---|---|
| 1 | 8.0 | 16.0 | 4.3 | 54% |
| 2 | 6.0 | 18.0 | 0.4 | 7% |
| 3 | 11.7 | 12.3 | 0.004 | 0% |
| 4 y 5 | 0.3 | 0.3 | 0.2 | 50% |
| Total time saved | 4.8 |
| # | Details Strategy |
|---|---|
| 1 | Elimination of approximately 5 h from the total processing time per positive blood culture bottle (see Table 2). |
| 2 | Increased productivity through the addition of a dedicated workstation at the laboratory’s sample admission area. |
| 3 | Technological improvements in the real® to enable integration with the central LIS, improve sample tracking (e.g., anatomical site, urinary sediment analysis), and enhance dashboard compatibility with the real® environment. |
| 4 | Full automation of the validation process for negative blood culture vials. |
| 5 | Implementation of culture plate boxes by time range of incubation, allowing continuous evaluation, MALDI-TOF identification, and as setup throughout the shift, ending once-daily batch processing. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benadof, D.; Zamorano, A.; Aguirre, J.; Veas, A.; Araneda, E.; Saint-Pierre, G. Improving Turnaround Time in Pediatric Clinical Microbiology Results: Implementation of the Kaizen Method in a Chilean Hospital Laboratory. LabMed 2025, 2, 20. https://doi.org/10.3390/labmed2040020
Benadof D, Zamorano A, Aguirre J, Veas A, Araneda E, Saint-Pierre G. Improving Turnaround Time in Pediatric Clinical Microbiology Results: Implementation of the Kaizen Method in a Chilean Hospital Laboratory. LabMed. 2025; 2(4):20. https://doi.org/10.3390/labmed2040020
Chicago/Turabian StyleBenadof, Dona, Agustin Zamorano, Judith Aguirre, Abigail Veas, Esteban Araneda, and Gustavo Saint-Pierre. 2025. "Improving Turnaround Time in Pediatric Clinical Microbiology Results: Implementation of the Kaizen Method in a Chilean Hospital Laboratory" LabMed 2, no. 4: 20. https://doi.org/10.3390/labmed2040020
APA StyleBenadof, D., Zamorano, A., Aguirre, J., Veas, A., Araneda, E., & Saint-Pierre, G. (2025). Improving Turnaround Time in Pediatric Clinical Microbiology Results: Implementation of the Kaizen Method in a Chilean Hospital Laboratory. LabMed, 2(4), 20. https://doi.org/10.3390/labmed2040020






