Ciraparantag Does Not Remove Anticoagulant Activities In Vitro, but DOAC-Stop™ May Mitigate Ciraparantag-Associated Interferences in Coagulation Testing
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Human Plasma with and Without Anticoagulants
2.2. Removal of Anticoagulant-Associated Activities from Human Plasma
2.3. Routine and Specialised Coagulation Testing of Human Plasma
2.4. Statistical Analysis
3. Results
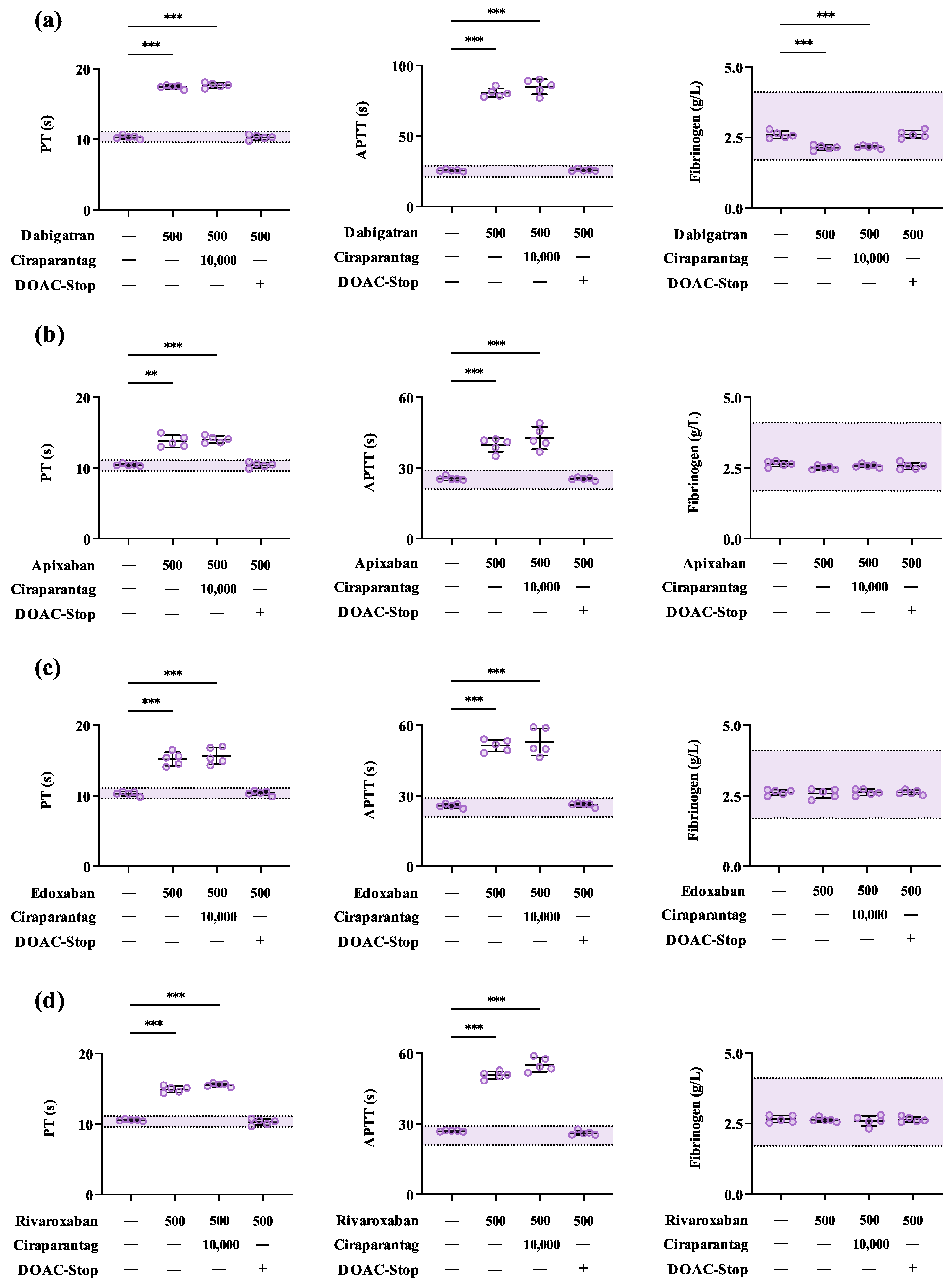
3.1. Ciraparantag Has Minimal Impact on Routine Coagulation Parameters In Vitro
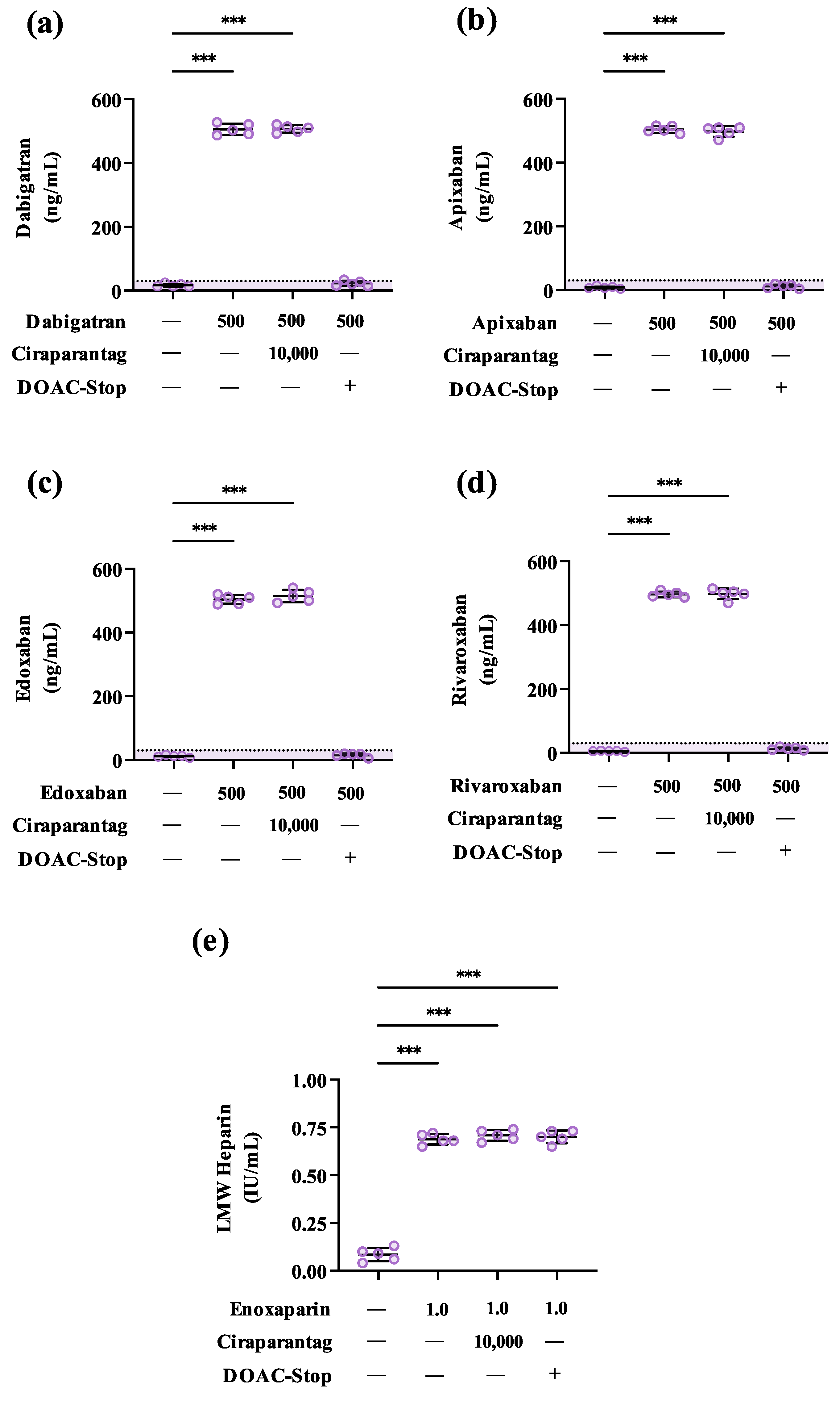
3.2. Ciraparantag Does Not Remove Anticoagulant-Associated Activities in Routine and Specialised Coagulation Assays
3.3. DOAC-Stop™ May Mitigate Ciraparantag-Associated Interferences in Coagulation Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Wheelock, K.M.; Ross, J.S.; Murugiah, K.; Lin, Z.; Krumholz, H.M.; Khera, R. Clinician Trends in Prescribing Direct Oral Anticoagulants for US Medicare Beneficiaries. JAMA Netw. Open 2021, 4, e2137288. [Google Scholar] [CrossRef] [PubMed]
- Iyer, G.S.; Tesfaye, H.; Khan, N.F.; Zakoul, H.; Bykov, K. Trends in the Use of Oral Anticoagulants for Adults With Venous Thromboembolism in the US, 2010–2020. JAMA Netw. Open 2023, 6, e234059. [Google Scholar] [CrossRef] [PubMed]
- Moser, K.A.; Smock, K.J. Direct Oral Anticoagulant (DOAC) Interference in Hemostasis Assays. Hematol. Am. Soc. Hematol. Educ. Program 2021, 2021, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Buckley, G.T.; Murphy, B.; Fleming, N.; Crowley, M.P.; Harte, J.V. Removing Direct Oral Factor Xa Inhibitor Interferences from Routine and Specialised Coagulation Assays Using a Raw Activated Charcoal Product. Clin. Chim. Acta 2023, 550, 117565. [Google Scholar] [CrossRef]
- Buckley, G.T.; Bowen, A.; Crowley, M.P.; Harte, J.V. Precision-Weighing of Raw Activated Charcoal Using a 3D-Printed Micromeasure for the Removal of Direct Oral Anticoagulant-Associated Interferences in Coagulation Testing. Int. J. Lab. Hematol. 2024, 46, 405–407. [Google Scholar] [CrossRef]
- Frackiewicz, A.; Kalaska, B.; Miklosz, J.; Mogielnicki, A. The Methods for Removal of Direct Oral Anticoagulants and Heparins to Improve the Monitoring of Hemostasis: A Narrative Literature Review. Thromb. J. 2023, 21, 58. [Google Scholar] [CrossRef]
- Baker, S.A.; Jin, J.; Pfaffroth, C.; Vu, T.; Zehnder, J.L. DOAC-Stop in Lupus Anticoagulant Testing: Direct Oral Anticoagulant Interference Removed in Most Samples. Res. Pract. Thromb. Haemost. 2021, 5, 314–325. [Google Scholar] [CrossRef]
- Devreese, K.M.J.; de Groot, P.G.; de Laat, B.; Erkan, D.; Favaloro, E.J.; Mackie, I.; Martinuzzo, M.; Ortel, T.L.; Pengo, V.; Rand, J.H.; et al. Guidance from the Scientific and Standardization Committee for Lupus Anticoagulant/Antiphospholipid Antibodies of the International Society on Thrombosis and Haemostasis: Update of the Guidelines for Lupus Anticoagulant Detection and Interpretation. J. Thromb. Haemost. 2020, 18, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Cohen, H.; Devreese, K.M.J. Lupus Anticoagulant Detection in Anticoagulated Patients. Guidance from the Scientific and Standardization Committee for Lupus Anticoagulant/Antiphospholipid Antibodies of the International Society on Thrombosis and Haemostasis. J. Thromb. Haemost. 2020, 18, 1569–1575. [Google Scholar] [CrossRef]
- Exner, T.; Ahuja, M.; Ellwood, L. Effect of an Activated Charcoal Product (DOAC StopTM) Intended for Extracting DOACs on Various Other APTT-Prolonging Anticoagulants. Clin. Chem. Lab. Med. 2019, 57, 690–696. [Google Scholar] [CrossRef]
- Favaloro, E.J.; (Adcock) Funk, D.M.; Lippi, G. Pre-Analytical Variables in Coagulation Testing Associated with Diagnostic Errors in Hemostasis. Lab. Med. 2012, 43, 1–10. [Google Scholar] [CrossRef]
- Lindhoff-Last, E.; Ansell, J. Reversal of Direct Oral Anticoagulants: Meeting a Need & Filling a Gap. Med. Res. Arch. 2023, 11, 1–17. [Google Scholar] [CrossRef]
- Ansell, J.; Laulicht, B.E.; Bakhru, S.H.; Burnett, A.; Jiang, X.; Chen, L.; Baker, C.; Villano, S.; Steiner, S. Ciraparantag, an Anticoagulant Reversal Drug: Mechanism of Action, Pharmacokinetics, and Reversal of Anticoagulants. Blood 2021, 137, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Ansell, J.E.; Bakhru, S.H.; Laulicht, B.E.; Steiner, S.S.; Grosso, M.; Brown, K.; Dishy, V.; Noveck, R.J.; Costin, J.C. Use of PER977 to Reverse the Anticoagulant Effect of Edoxaban. N. Engl. J. Med. 2014, 371, 2141–2142. [Google Scholar] [CrossRef] [PubMed]
- Ansell, J.E.; Laulicht, B.E.; Bakhru, S.H.; Hoffman, M.; Steiner, S.S.; Costin, J.C. Ciraparantag Safely and Completely Reverses the Anticoagulant Effects of Low Molecular Weight Heparin. Thromb. Res. 2016, 146, 113–118. [Google Scholar] [CrossRef]
- Ansell, J.; Bakhru, S.; Laulicht, B.E.; Tracey, G.; Villano, S.; Freedman, D. Ciraparantag Reverses the Anticoagulant Activity of Apixaban and Rivaroxaban in Healthy Elderly Subjects. Eur. Heart J. 2022, 43, 985–992. [Google Scholar] [CrossRef]
- Laulicht, B.; Bakhru, S.; Bakhru, S.; Lee, C.; Baker, C.; Jiang, X.; Mathiowitz, E.; Costin, J.; Steiner, S. Small Molecule Antidote for Anticoagulants. Circulation 2012, 126, A11395. [Google Scholar]
- Ansell, J.E.; Bakhru, S.H.; Laulicht, B.E.; Steiner, S.S.; Grosso, M.A.; Brown, K.; Dishy, V.; Lanz, H.J.; Mercuri, M.F.; Noveck, R.J.; et al. Single-Dose Ciraparantag Safely and Completely Reverses Anticoagulant Effects of Edoxaban. Thromb. Haemost. 2017, 117, 238–245. [Google Scholar] [CrossRef]
- Exner, T.; Dangol, M.; Favaloro, E.J. Simplified Method for Removing Direct Oral Anticoagulant Interference in Mechanical Coagulation Test Systems—A Proof of Concept. J. Clin. Med. 2024, 13, 1042. [Google Scholar] [CrossRef]
- Hirsh, J.; Warkentin, T.E.; Shaughnessy, S.G.; Anand, S.S.; Halperin, J.L.; Raschke, R.; Granger, C.; Ohman, E.M.; Dalen, J.E. Heparin and Low-Molecular-Weight Heparin: Mechanisms of Action, Pharmacokinetics, Dosing, Monitoring, Efficacy, and Safety. Chest 2001, 119, 64S–94S. [Google Scholar] [CrossRef]
- Boneu, B.; de Moerloose, P. How and When to Monitor a Patient Treated with Low Molecular Weight Heparin. Semin. Thromb. Hemost. 2001, 27, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Z.; Mu, G.; Wang, Z.; Zhou, S.; Xie, Q.; Ma, L.; Wang, Z.; Hu, K.; Gong, Y.; et al. Diagnostic Performance of Coagulation Indices for Direct Oral Anticoagulant Concentration. Thromb. Res. 2020, 195, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Leentjens, J.; Middeldorp, S.; Jung, C. A Short Review of Ciraparantag in Perspective of the Currently Available Anticoagulant Reversal Agents. Drug Discov. Today 2022, 27, 103332. [Google Scholar] [CrossRef] [PubMed]
- Hillarp, A.; Baghaei, F.; Blixter, I.F.; Gustafsson, K.M.; Stigendal, L.; Sten-Linder, M.; Strandberg, K.; Lindahl, T.L. Effects of the Oral, Direct Factor Xa Inhibitor Rivaroxaban on Commonly Used Coagulation Assays. J. Thromb. Haemost. 2011, 9, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Lindahl, T.L.; Baghaei, F.; Blixter, I.F.; Gustafsson, K.M.; Stigendal, L.; Sten-Linder, M.; Strandberg, K.; Hillarp, A.; Expert Group on Coagulation of the External Quality Assurance in Laboratory Medicine in Sweden. Effects of the Oral, Direct Thrombin Inhibitor Dabigatran on Five Common Coagulation Assays. Thromb. Haemost. 2011, 105, 371–378. [Google Scholar] [CrossRef]
- Hillarp, A.; Gustafsson, K.M.; Faxälv, L.; Strandberg, K.; Baghaei, F.; Fagerberg Blixter, I.; Berndtsson, M.; Lindahl, T.L. Effects of the Oral, Direct Factor Xa Inhibitor Apixaban on Routine Coagulation Assays and Anti-FXa Assays. J. Thromb. Haemost. 2014, 12, 1545–1553. [Google Scholar] [CrossRef]
- Hillarp, A.; Strandberg, K.; Baghaei, F.; Fagerberg Blixter, I.; Gustafsson, K.M.; Lindahl, T.L. Effects of the Oral, Direct Factor Xa Inhibitor Edoxaban on Routine Coagulation Assays, Lupus Anticoagulant and Anti-Xa Assays. Scand. J. Clin. Lab. Investig. 2018, 78, 575–583. [Google Scholar] [CrossRef]
| Parameter | PT (s) | APTT (s) | Fibrinogen (g/L) |
|---|---|---|---|
| Reference Range | 9.5–11.1 | 21.0–29.0 | 1.7–4.1 |
| N † | 5 | 5 | 5 |
| Baseline | 10.5 (0.97) | 26.2 (0.60) | 2.44 (0.15) |
| +100 ng/mL Ciraparantag | 10.6 (0.51) | 25.6 (0.80) | 2.51 (0.13) |
| +250 ng/mL Ciraparantag | 10.9 (0.58) | 24.8 (0.89) | 2.42 (0.08) |
| +500 ng/mL Ciraparantag | 10.5 (0.47) | 25.5 (0.30) | 2.54 (0.12) |
| +1000 ng/mL Ciraparantag | 11.0 (0.56) | 26.1 (1.22) | 2.58 (0.12) |
| +10,000 ng/mL Ciraparantag | 11.7 (0.87) | 30.9 (1.43) *** | 2.36 (0.12) |
| Parameter | PT (s) | APTT (s) | Fibrinogen (g/L) | |
|---|---|---|---|---|
| Reference Range | 9.5–11.1 | 21.0–29.0 | 1.7–4.1 | |
| N † | DOAC-Stop™ | 5 | 5 | 5 |
| Baseline | − | 10.4 (0.44) | 26.4 (0.84) | 2.49 (0.08) |
| + 10,000 ng/mL Ciraparantag | − | 10.4 (0.41) | 30.4 (0.91) ** | 2.41 (0.09) |
| + 10,000 ng/mL Ciraparantag | + | 10.6 (0.32) | 26.9 (1.00) | 2.58 (0.09) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harte, J.V.; Buckley, G.T. Ciraparantag Does Not Remove Anticoagulant Activities In Vitro, but DOAC-Stop™ May Mitigate Ciraparantag-Associated Interferences in Coagulation Testing. LabMed 2024, 1, 33-42. https://doi.org/10.3390/labmed1010006
Harte JV, Buckley GT. Ciraparantag Does Not Remove Anticoagulant Activities In Vitro, but DOAC-Stop™ May Mitigate Ciraparantag-Associated Interferences in Coagulation Testing. LabMed. 2024; 1(1):33-42. https://doi.org/10.3390/labmed1010006
Chicago/Turabian StyleHarte, James V., and Gavin T. Buckley. 2024. "Ciraparantag Does Not Remove Anticoagulant Activities In Vitro, but DOAC-Stop™ May Mitigate Ciraparantag-Associated Interferences in Coagulation Testing" LabMed 1, no. 1: 33-42. https://doi.org/10.3390/labmed1010006
APA StyleHarte, J. V., & Buckley, G. T. (2024). Ciraparantag Does Not Remove Anticoagulant Activities In Vitro, but DOAC-Stop™ May Mitigate Ciraparantag-Associated Interferences in Coagulation Testing. LabMed, 1(1), 33-42. https://doi.org/10.3390/labmed1010006







