Comorbidity Patterns Among Outpatient COVID-19 Cases in Turkey
Abstract
1. Introduction
2. Methodology
2.1. Patients and Data Collection
2.2. Ethics
2.3. Statistics
3. Findings
3.1. Outpatient COVID-19 Cases and Morbidity Profiles
3.2. Comorbidities Among Outpatients with COVID-19
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Coronavirus Disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 27 December 2021).
- Luo, J.; Li, M.; Sun, W.; Wang, Y.; Gu, Z.; Yang, J. Global Excess Mortality during COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1702. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, Y.V.; Zaccardi, F.; Gillies, C.L.; Razieh, C.; Yates, T.; Kloecker, D.E.; Rowlands, A.V.; Davies, M.J.; Islam, N.; Seidu, S.; et al. Patterns of multimorbidity and risk of severe SARS-CoV-2 infection: An observational study in the U.K. BMC Infect. Dis. 2021, 21, 908. [Google Scholar] [CrossRef] [PubMed]
- Amir, E.; Fatemeh, J.; Neda, P.; Ali, A. Prevalence of Underlying Diseases in Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Arch. Acad. Emerg. Med. 2020, 8, e35. [Google Scholar]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA J. Am. Med. Assoc. 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA J. Am. Med. Assoc. 2020, 323, 1775–1776. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Mattey-Mora, P.P.; Begle, C.A.; Owusu, C.K.; Chen, C.; Parker, M.A. Hospitalised versus outpatient COVID-19 patients’ background characteristics and comorbidities: A systematic review and meta-analysis. Rev. Med. Virol. 2022, 32, e2306. [Google Scholar] [CrossRef] [PubMed]
- Christian, H.N.; Bingisser, R. Mimics and chameleons of COVID-19. Swiss Med. Wkly. 2020, 150, 2–3. [Google Scholar] [CrossRef]
- Janssenswillen, G.; Depaire, B.; Swennen, M.; Jans, M.; Vanhoof, K. bupaR: Enabling reproducible business process analysis. Knowl. Based Syst. 2019, 163, 927–930. [Google Scholar] [CrossRef]
- Berti, A.; Van Zelst, S.J.; Van Der Aalst, W.M.P.; Gesellschaf, F. Process mining for python (PM4py): Bridging the gap process-and data science. CEUR Workshop Proc. 2019, 2374, 13–16. [Google Scholar]
- Voigt, P.; dem Bussche, A. The EU General Data protection Regulation (GDPR) A Practical Guide, 1st ed.; Springer: Cham, Switzerland, 2017; Volume 10, pp. 10–5555. [Google Scholar]
- Zaeem, R.N.; Barber, K.S. The Effect of the GDPR on Privacy Policies. ACM Trans. Manag. Inf. Syst. 2021, 12, 1–20. [Google Scholar] [CrossRef]
- Doe, J.; Smith, A. Analysis of Social Media Data Using IBM SPSS Statistics. J. Data Sci. Res. 2021, 5, 123–135. Available online: https://examplejournal.org/doi/full/10.1234/jdsr.v5i2.1234 (accessed on 23 January 2024).
- Wang, Z.; Deng, H.; Ou, C.; Liang, J.; Wang, Y.; Jiang, M.; Li, S. Clinical symptoms, comorbidities and complications in severe and non-severe patients with COVID-19: A systematic review and meta-analysis without cases duplication. Medicine 2020, 99, e23327. [Google Scholar] [CrossRef] [PubMed]
- Gade, N.; Nag, S.; Mishra, M.; Akkilagunta, S.; Shete, V.; Bidkar, V.; Shendre, P.; Patil, D. Incidence of COVID-19 infection and its variation with demographic and clinical profile: Lessons learned at a COVID-19 RT-PCR laboratory in Nagpur, India. Access Microbiol. 2022, 4, 330. [Google Scholar] [CrossRef] [PubMed]







| Symptoms | Number of Patients (N) | % |
|---|---|---|
| Fever | 23,146 | 48.3 |
| Fatigue/malaise | 17,490 | 36.5 |
| Cough | 15,024 | 31.4 |
| Sore throat | 13,591 | 28.4 |
| Muscle aches | 8907 | 18.6 |
| Headache | 8561 | 17.9 |
| Shortness of breath | 6325 | 13.2 |
| Runny nose | 6270 | 13.1 |
| Diarrhea | 5731 | 12.0 |
| Vomiting/nausea | 4284 | 8.9 |
| Joint pain | 1587 | 3.3 |
| Wheezing | 1458 | 3.0 |
| Lower chest wall indrawing | 179 | 0.4 |
| Chest pain | 558 | 1.2 |
| Loss of smell | 279 | 0.6 |
| Loss of taste | 778 | 1.6 |
| Abdominal pain | 1191 | 2.5 |
| With Severe COVID-19-PCR (+) | Without Severe COVID-19-PCR (−) | |||
|---|---|---|---|---|
| Characteristics | N = 40,411 | % | N = 7443 | % |
| Sex | ||||
| Male | 17,762 | 85.1 | 3097 | 14.8 |
| Female | 22,649 | 83.9 | 4346 | 16.1 |
| Age group | ||||
| 0–14 | 914 | 83.8 | 176 | 16.1 |
| 15–29 | 8659 | 81.4 | 1977 | 18.6 |
| 30–49 | 15,189 | 83.9 | 3019 | 16.0 |
| 50–69 | 9335 | 85.3 | 9335 | 14.7 |
| 70 and above | 4319 | 86.7 | 4319 | 13.3 |
| Presence of symptoms | ||||
| Yes | 6525 | 84.6 | 1266 | 15.4 |
| No | 33,867 | 83.7 | 6160 | 16.2 |
| Comorbidity | ||||
| Yes | 5066 | 82.4 | 1082 | 17.6 |
| No | 35,346 | 84.7 | 6364 | 15.3 |
| Fever | ||||
| Below 37.5 | 23,152 | 83.8 | 4463 | 16.2 |
| Above 37.5 | 12,707 | 83.4 | 2518 | 16.4 |
| Oxygen saturation | ||||
| Below 95 | 19,300 | 84.8 | 3449 | 5.2 |
| Above 95 | 19,768 | 83.1 | 3994 | 6.8 |
| Condition | N | % |
|---|---|---|
| Hypertension | 4.755 | 9.90 |
| Asthma | 3.681 | 7.70 |
| Diabetes | 193 | 0.05 |
| Chronic cardiac disease | 140 | 0.03 |
| Malignant neoplasm | 113 | 0.02 |
| Chronic pulmonary disease | 75 | 0.02 |
| Obesity | 49 | 0.01 |
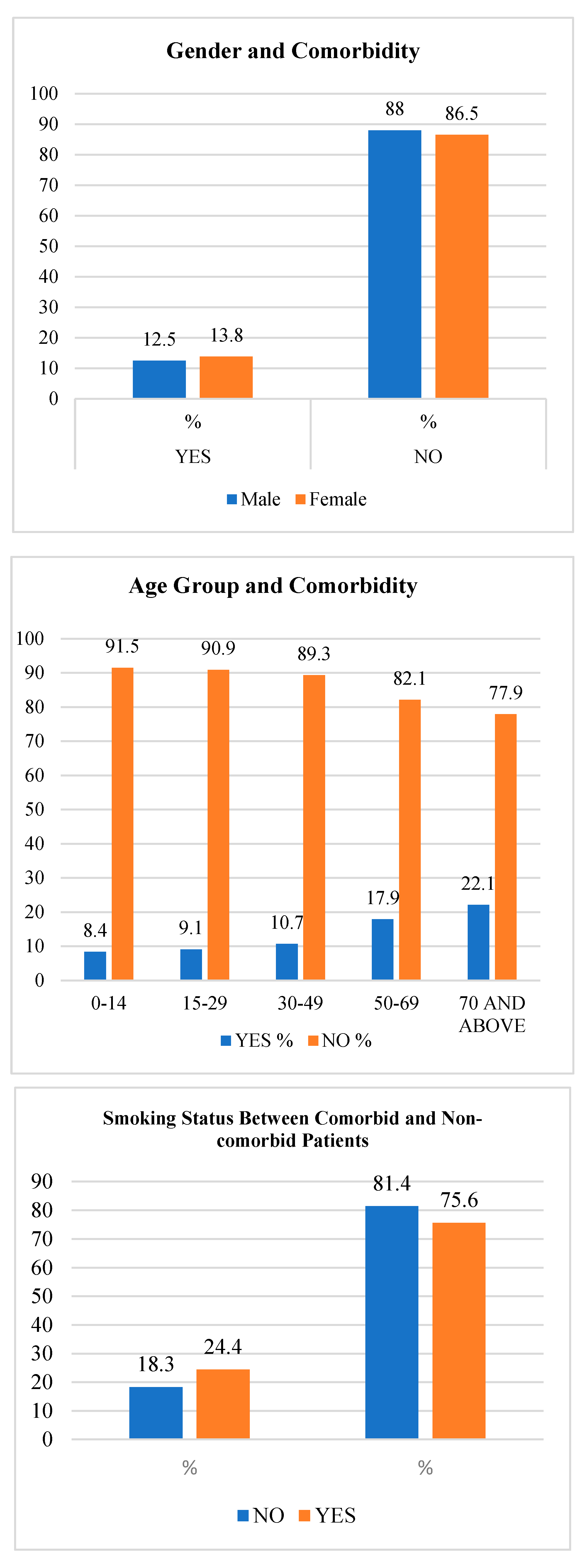
| Characteristics | Yes | No | p Value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Comorbidity by Sex | |||||
| Male | 2507 | 12.5 | 18,357 | 88.0 | p < 0.05 |
| Female | 3638 | 13.8 | 23,369 | 86.5 | |
| Comorbidity by Age Group | |||||
| 0–14 | 92 | 8.4 | 998 | 91.5 | p < 0.05 |
| 15–29 | 972 | 9.1 | 9672 | 90.9 | |
| 30–49 | 2014 | 10.7 | 16,830 | 89.3 | |
| 50–69 | 1964 | 17.9 | 8982 | 82.1 | |
| 70 and above | 1103 | 22.1 | 3879 | 77.9 | |
| Smoking | |||||
| No | 5535 | 12.6 | 38,402 | 87.4 | p < 0.05 |
| Yes | 610 | 15.6 | 3325 | 84.5 | |
| Oxygen Saturation | p > 0.05 | ||||
| Below 95 | 3028 | 13.3 | 19,726 | 86.7 | |
| Above 95 | 3116 | 13.1 | 20,658 | 86.9 | |
| Fever | p > 0.05 | ||||
| Below 37.5 | 3813 | 13.8 | 23,814 | 86.2 | |
| Above 37.5 | 2033 | 13.3 | 13,197 | 86.7 | |
| Existing Radiologic Images Related to COVID-19 | |||||
| Yes | 694 | 15.5 | 3777 | 87.4 | p < 0.05 |
| No | 5451 | 12.5 | 37,949 | 84.5 | |
| Presence of Symptoms | p < 0.05 | ||||
| Yes | 3655 | 46.9 | 4140 | 53.1 | |
| No | 2490 | 6.2 | 37,550 | 93.8 | |
| Medications | Yes | No | ||
|---|---|---|---|---|
| N | % | N | % | |
| Antiviral Therapy | 22,160 | 46.3 | 25,707 | 53.7 |
| Antibiotics | 43,360 | 90.6 | 4505 | 9.4 |
| Corticosteroid | 43,532 | 90.0 | 4333 | 9.1 |
| Variables | B | SE | p Value | Relative Risk |
|---|---|---|---|---|
| Presence of COVID-19 Symptoms | 2639 | 0.033 | 0.000 | 14.005 |
| Age | −0.115 | 0.054 | 0.032 | 0.892 |
| Sex | 0.139 | 0.032 | 0.000 | 1.149 |
| Severity of Disease | 0.365 | 0.043 | 0.000 | 1.441 |
| Antiviral Therapy | 0.530 | 0.034 | 0.000 | 1.699 |
| Smoking | 0.057 | 0.053 | 0.289 | 1.058 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Oman Medical Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akgün, H.S.; Erdoğan, T.G.; Belibağlı, M.C.; Güneş, G.; Haberal, A. Comorbidity Patterns Among Outpatient COVID-19 Cases in Turkey. J. Oman Med. Assoc. 2025, 2, 2. https://doi.org/10.3390/joma2010002
Akgün HS, Erdoğan TG, Belibağlı MC, Güneş G, Haberal A. Comorbidity Patterns Among Outpatient COVID-19 Cases in Turkey. Journal of the Oman Medical Association. 2025; 2(1):2. https://doi.org/10.3390/joma2010002
Chicago/Turabian StyleAkgün, Hediye Seval, Tuğba Gürgen Erdoğan, Mehmet Cenk Belibağlı, Gamze Güneş, and Ali Haberal. 2025. "Comorbidity Patterns Among Outpatient COVID-19 Cases in Turkey" Journal of the Oman Medical Association 2, no. 1: 2. https://doi.org/10.3390/joma2010002
APA StyleAkgün, H. S., Erdoğan, T. G., Belibağlı, M. C., Güneş, G., & Haberal, A. (2025). Comorbidity Patterns Among Outpatient COVID-19 Cases in Turkey. Journal of the Oman Medical Association, 2(1), 2. https://doi.org/10.3390/joma2010002







