Elevated D-Dimer Levels in Older Medical Emergency Department Patients: Real-Life Data on Associations with Severe Acute Medical Problems and Occult Malignancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Data Collection
2.3. Occult Malignancy
2.4. D-Dimer Measurement and Classification
2.5. Analysis
3. Results
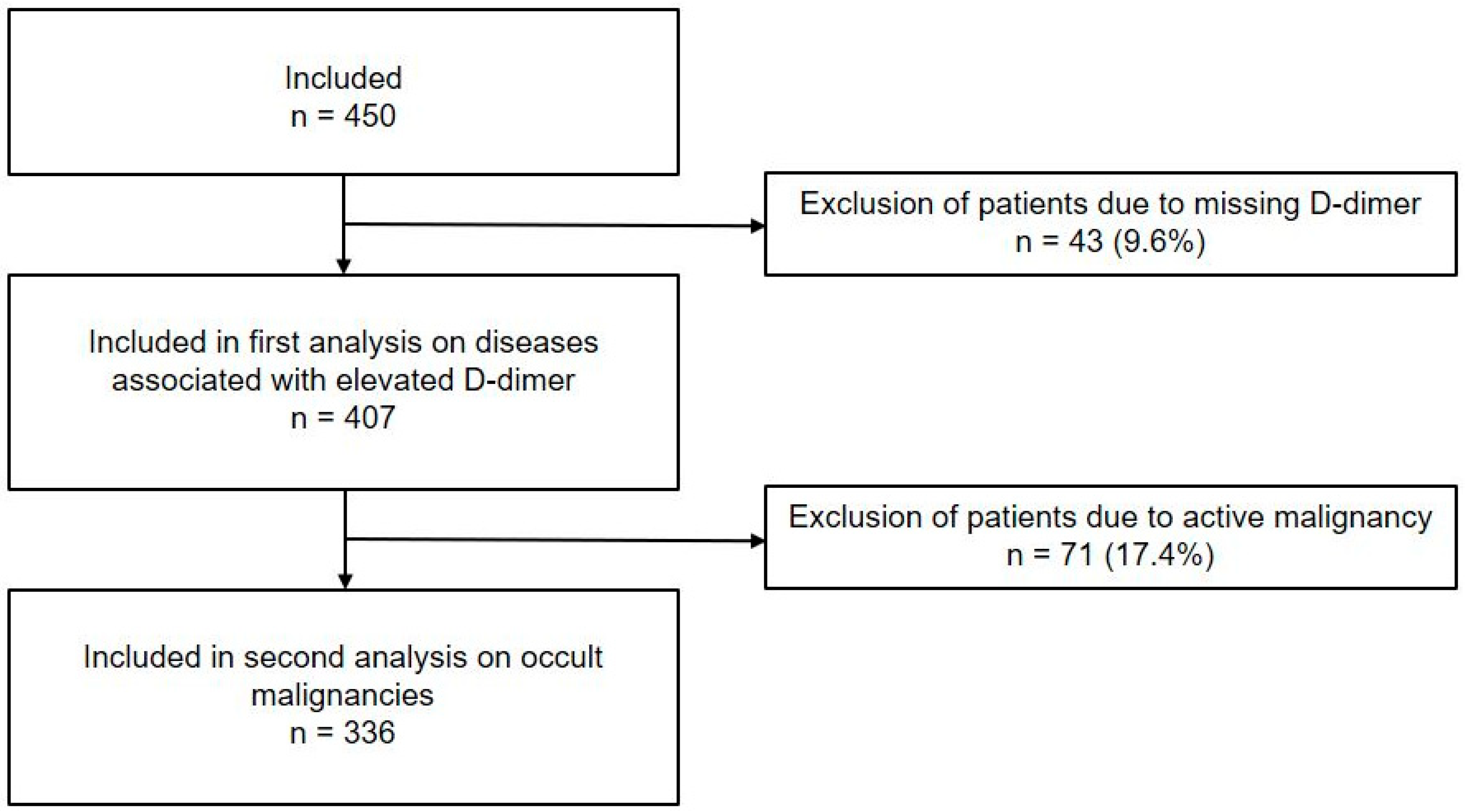
3.1. Study Population and Patient Characteristics
3.2. Distribution of D-Dimer Levels
3.3. Prevalence of Diseases Associated with Elevated D-Dimer Levels
3.4. Occult Malignancy
3.5. Association of D-Dimer Levels with Occult Malignancy
4. Discussion
4.1. Implications for Clinical Practice
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, Y.; Cao, J.; Wang, Q.; Shi, Q.; Liu, K.; Luo, Z.; Chen, X.; Chen, S.; Yu, K.; Huang, Z.; et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: A case control study. J. Intensive Care 2020, 8, 49. [Google Scholar] [CrossRef]
- Nickel, C.H.; Kellett, J.; Cooksley, T.; Lyngholm, L.E.; Chang, S.; Imfeld, S.; Bingisser, R.; Brabrand, M. The Diagnoses and Outcomes of Emergency Patients with an Elevated D-Dimer Over the Next 90 Days. Am. J. Med. 2021, 134, 260–266.e2. [Google Scholar] [CrossRef] [PubMed]
- Schutte, T.; Thijs, A.; Smulders, Y.M. Never ignore extremely elevated D-dimer levels: They are specific for serious illness. Neth. J. Med. 2016, 74, 443–448. [Google Scholar]
- Klok, F.A.; Djurabi, R.K.; Nijkeuter, M.; Eikenboom, H.C.; Leebeek, F.W.; Kramer, M.H.; Kaasjager, K.; Kamphuisen, P.W.; Büller, H.R.; Huisman, M.V. High D-dimer level is associated with increased 15-d and 3 months mortality through a more central localization of pulmonary emboli and serious comorbidity. Br. J. Haematol. 2008, 140, 218–222, Erratum in Br. J. Haematol. 2008, 141, 274. [Google Scholar] [CrossRef]
- Lippi, G.; Bonfanti, L.; Saccenti, C.; Cervellin, G. Causes of elevated D-dimer in patients admitted to a large urban emergency department. Eur. J. Intern. Med. 2014, 25, 45–48. [Google Scholar] [CrossRef]
- Lim, J.; Cardle, C.; Isles, C. Patients with markedly elevated D-dimer who do not have pulmonary embolism. Postgrad. Med. J. 2021, 97, 77–82. [Google Scholar] [CrossRef]
- Brabrand, M.; Fløjstrup, M.; Bogh, S.B.; Cooksley, T.; Nickel, C.H. Utilizing D-dimer levels for predicting survival probability in unplanned hospital admissions: Insights from a 5-year nationwide population-based register study. Eur. J. Intern. Med. 2025, 137, 149–151. [Google Scholar] [CrossRef]
- Yu, J.; Li, D.; Lei, D.; Yuan, F.; Pei, F.; Zhang, H.; Yu, A.; Wang, K.; Chen, H.; Chen, L.; et al. Tumor-Specific D-Dimer Concentration Ranges and Influencing Factors: A Cross-Sectional Study. PLoS ONE 2016, 11, e0165390. [Google Scholar] [CrossRef] [PubMed]
- Righini, M.; Van Es, J.; Den Exter, P.L.; Roy, P.M.; Verschuren, F.; Ghuysen, A.; Rutschmann, O.T.; Sanchez, O.; Jaffrelot, M.; Trinh-Duc, A.; et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: The ADJUST-PE study. JAMA 2014, 311, 1117–1124, Erratum in JAMA 2014, 311, 1694. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.D.; Tharmanathan, P.; France, B.; Din, N.U.; Cotton, S.; Fallon-Ferguson, J.; Hamilton, W.; Hendry, A.; Hendry, M.; Lewis, R.; et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br. J. Cancer 2015, 112 (Suppl. S1), S92–S107. [Google Scholar] [CrossRef]
- Rivers, E.; Nguyen, B.; Havstad, S.; Ressler, J.; Muzzin, A.; Knoblich, B.; Peterson, E.; Tomlanovich, M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 2001, 345, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Zelis, N.; Buijs, J.; de Leeuw, P.W.; van Kuijk, S.M.J.; Stassen, P.M. Study protocol for a multicentre prospective cohort study to identify predictors of adverse outcome in older medical emergency department patients (the Risk Stratification in the Emergency Department in Acutely Ill Older Patients (RISE UP) study). BMC Geriatr. 2019, 19, 65. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 5th ed.; 10th revision; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Vincent, J.L.; de Mendonça, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef]
- Douma, R.A.; le Gal, G.; Söhne, M.; Righini, M.; Kamphuisen, P.W.; Perrier, A.; Kruip, M.J.; Bounameaux, H.; Büller, H.R.; Roy, P.M. Potential of an age adjusted D-dimer cut-off value to improve the exclusion of pulmonary embolism in older patients: A retrospective analysis of three large cohorts. BMJ 2010, 340, c1475. [Google Scholar] [CrossRef]
- Zelis, N.; Buijs, J.; de Leeuw, P.W.; van Kuijk, S.M.J.; Stassen, P.M. A new simplified model for predicting 30-day mortality in older medical emergency department patients: The rise up score. Eur. J. Intern. Med. 2020, 77, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Halaby, R.; Popma, C.J.; Cohen, A.; Chi, G.; Zacarkim, M.R.; Romero, G.; Goldhaber, S.Z.; Hull, R.; Hernandez, A.; Mentz, R.; et al. D-Dimer elevation and adverse outcomes. J. Thromb. Thrombolysis 2015, 39, 55–59. [Google Scholar] [CrossRef]
- Han, D.; Hartaigh, B.ó.; Lee, J.H.; Cho, I.J.; Shim, C.Y.; Chang, H.J.; Hong, G.R.; Ha, J.W.; Chung, N. Impact of D-Dimer for Prediction of Incident Occult Cancer in Patients with Unprovoked Venous Thromboembolism. PLoS ONE 2016, 11, e0153514. [Google Scholar] [CrossRef]
- Schutgens, R.E.; Beckers, M.M.; Haas, F.J.; Biesma, D.H. The predictive value of D-dimer measurement for cancer in patients with deep vein thrombosis. Haematologica 2005, 90, 214–219. [Google Scholar]
- Lu, Y.; Zhang, L.; Zhang, Q.; Zhang, Y.; Chen, D.; Lou, J.; Jiang, J.; Ren, C. The association of D-dimer with clinicopathological features of breast cancer and its usefulness in differential diagnosis: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0221374. [Google Scholar] [CrossRef] [PubMed]
- Van Doormaal, F.F.; Terpstra, W.; Van Der Griend, R.; Prins, M.H.; Nijziel, M.R.; Van De Ree, M.A.; Büller, H.R.; Dutilh, J.C.; ten Cate-Hoek, A.; Van Den Heiligenberg, S.M.; et al. Is extensive screening for cancer in idiopathic venous thromboembolism warranted? J. Thromb. Haemost. 2011, 9, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.; Lazo-Langner, A.; Shivakumar, S.; Tagalakis, V.; Zarychanski, R.; Solymoss, S.; Routhier, N.; Douketis, J.; Danovitch, K.; Lee, A.Y.; et al. Screening for Occult Cancer in Unprovoked Venous Thromboembolism. N. Engl. J. Med. 2015, 373, 697–704. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | All Patients n = 407 |
|---|---|
| Age, median (IQR), years | 79 (73–85) |
| Male sex, n% | 215 (52.8) |
| Comorbidity | |
| Median CCI score a (IQR) | 1 (1–3) |
| Active malignancy before ED visit, n% | 71 (17.4) |
| Medication, n% | |
| Anticoagulants | 124 (30.5) |
| Antiplatelet therapy | 159 (39.1) |
| Reason for ED visit (ICD-10), n% | |
| Infectious and parasitic diseases | 113 (27.8) |
| Diseases of the digestive system | 113 (27.8) |
| Neoplasms b | 40 (9.8) |
| Diseases of the circulatory system | 34 (8.4) |
| Endocrine, nutritional, and metabolic diseases | 23 (5.7) |
| Diseases of the respiratory system | 22 (5.4) |
| Diseases of blood and blood-forming organs | 20 (4.9) |
| Diseases of the genitourinary system | 17 (4.2) |
| Miscellaneous | 25 (6.1) |
| Laboratory tests | |
| D-dimer, median (IQR), µg/L (normal <500) | 1364 (683–3314) |
| AADD cut-off value c, n% | 284 (69.8) |
| Admission, n% | 302 (74.2) |
| Mortality | |
| 90-day mortality, n% | 79 (19.4) |
| 6-month mortality, n% | 101 (24.8) |
| Diagnosis | Total | D-Dimer Levels (µg/L) | |||
|---|---|---|---|---|---|
| n = 407 | <AADD a n = 123 (30.2%) | ≥AADD-2000 n = 129 (31.7%) | ≥2000 n = 155 (38.1%) | p-Value | |
| Sepsis | 106 (26.0) | 22 (17.9) | 34 (26.4) | 50 (32.3) | 0.03 |
| Infection (excluding sepsis) | 94 (23.1) | 22 (17.9) | 36 (27.9) | 36 (23.2) | 0.18 |
| Active malignancy | 71 (17.4) | 20 (16.3) | 19 (14.7) | 32 (20.6) | 0.39 |
| VTE b | 17 (4.2) | 0 | 0 | 17 (11.0) | 0.02 |
| Acute or chronic inflammatory diseases | 15 (3.7) | 4 (3.3) | 6 (4.7) | 5 (3.2) | 0.80 |
| Ischemia | 6 (1.5) | 0 | 2 (1.6) | 4 (2.6) | 0.26 |
| Total n = 336 | |
|---|---|
| Occult malignancy, n% | 31 (9.2) |
| Metastasized at diagnosis | 11 (35.5) |
| Time to diagnosis, median (IQR), days | 5 (1–23) |
| Type of malignancy, n% | |
| Colorectal carcinoma | 5 (16.1) |
| Prostate carcinoma | 4 (12.9) |
| Acute myeloid leukemia | 3 (9.7) |
| Unknown primary | 3 (9.7) |
| Multiple myeloma | 2 (6.5) |
| Lung carcinoma | 2 (6.5) |
| Pancreatic carcinoma | 2 (6.5) |
| All other sites | 10 (32.3) |
| D-Dimer Levels (µg/L) | n = 336 | Occult Malignancy | |||
|---|---|---|---|---|---|
| Prevalence Per D-Dimer Group (%) | Yes (n%) n = 31 | No (n%) n = 305 | LR (95% CI) | ||
| 0–499 | 48 | 2.1 | 1 (3.2) | 47 (15.4) | 0.21 (0.03–1.47) |
| 500–999 | 83 | 7.2 | 6 (19.4) | 77 (25.2) | 0.77 (0.37–1.62) |
| 1000–1999 | 82 | 7.3 | 6 (19.4) | 76 (24.9) | 0.78 (0.37–1.64) |
| 2000–3999 | 55 | 12.7 | 7 (22.6) | 48 (15.7) | 1.44 (0.71–2.90) |
| 4000–7999 | 46 | 13.0 | 6 (19.4) | 40 (13.1) | 1.48 (0.68–3.21) |
| ≥8000 | 22 | 22.7 | 5 (16.1) | 17 (5.6) | 2.88 (1.14–7.26) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshout, B.; Zelis, N.; Buijs, J.; de Leeuw, P.W.; Stassen, P.M. Elevated D-Dimer Levels in Older Medical Emergency Department Patients: Real-Life Data on Associations with Severe Acute Medical Problems and Occult Malignancy. Emerg. Care Med. 2025, 2, 56. https://doi.org/10.3390/ecm2040056
Elshout B, Zelis N, Buijs J, de Leeuw PW, Stassen PM. Elevated D-Dimer Levels in Older Medical Emergency Department Patients: Real-Life Data on Associations with Severe Acute Medical Problems and Occult Malignancy. Emergency Care and Medicine. 2025; 2(4):56. https://doi.org/10.3390/ecm2040056
Chicago/Turabian StyleElshout, Beau, Noortje Zelis, Jacqueline Buijs, Peter W. de Leeuw, and Patricia M. Stassen. 2025. "Elevated D-Dimer Levels in Older Medical Emergency Department Patients: Real-Life Data on Associations with Severe Acute Medical Problems and Occult Malignancy" Emergency Care and Medicine 2, no. 4: 56. https://doi.org/10.3390/ecm2040056
APA StyleElshout, B., Zelis, N., Buijs, J., de Leeuw, P. W., & Stassen, P. M. (2025). Elevated D-Dimer Levels in Older Medical Emergency Department Patients: Real-Life Data on Associations with Severe Acute Medical Problems and Occult Malignancy. Emergency Care and Medicine, 2(4), 56. https://doi.org/10.3390/ecm2040056






