Hemodynamic Support in Cardiogenic Shock in the Cardiac Catheterization Laboratory
Abstract
1. Introduction
2. Cardiogenic Shock
2.1. Evolving Definition of Cardiogenic Shock
- Invasive hemodynamic assessment is not readily available on initial presentation. Therefore, the presence of clinical signs of hypoperfusion (i.e., cold clammy extremities, mental confusion, oliguria, and narrow pulse pressure) and/or biochemical manifestations of hypoperfusion (elevated creatinine in serum, metabolic acidosis, and elevated lactate in serum) in the context of a cardiac disorder is sufficient to suspect CS [5].
- Hypotension may not be present in all patients with CS. Some patients, especially in the initial stages with preserved compensatory mechanisms, may present without hypotension and are classified as “normotensive CS” [4]. This phenotype, more common in younger patients, is associated with a higher risk for significant hemodynamic deterioration and requires increased clinical vigilance [15].
2.2. Severity Classification
- Stage A (“at risk”): stable patients with acute cardiac conditions that put them at risk of developing CS.
- Stage B (“beginning” or pre-shock): characterized by hemodynamic instability (relative hypotension or tachycardia) without obvious signs of organ hypoperfusion such as physical signs, elevated serum creatinine, metabolic acidosis, or elevated serum lactate.
- Stage C (“classic”): classic definition of CS, with obvious signs of hypoperfusion (i.e., cold extremities, elevation of serum lactate, elevation of creatine, or low urine output requiring intervention (inotropic support).
- Stage D (“deterioration”): deteriorating hemodynamic conditions despite initial treatments or supportive interventions. Advanced interventions such as mechanical circulatory support need to be considered to stabilize the patient.
- Stage E (“extremis”): extreme shock even with circulatory support or/and cardiac arrest with ongoing cardiopulmonary resuscitation (CPR).
- -
- The distinction between SCAI shock stage B and SCAI shock stage C is critical and requires the integration of multiple clinical and laboratory exams. Patients with hypoperfusion without hypotension are at a higher risk of adverse outcomes than those patients with hypotension and normal perfusion [20]. However, this distinction may be difficult, especially in cases of mixed shock phenotypes.
- -
- Shock classification based on required therapeutic interventions: Patients needing vasoactive drugs or MCS for hypoperfusion or hemodynamic compromise are classified as stage C. If additional vasoactive drugs or MCS devices are required, the patient progresses to stage D. If perfusion cannot be restored with multiple therapies or extremely high doses of vasoactive drugs are necessary, the patient is classified as stage E [21].
- -
- Cardiac arrest (CA) modifier clarification: CA represents an “A modifier” in the SCAI classification. CA events are heterogeneous and currently, there is no specific CPR duration that qualifies a patient for the A modifier, which should refer to those at risk of an anoxic brain injury, defined as a Glasgow Coma Scale (GCS) score of less than 9 or the absence of a motor response to voice [22]. However, its heterogeneity and the following characteristics may be considered when making a decision: type of patient, place of the cardiac arrest, time to return of spontaneous circulation, duration of the cardiac arrest, rhythm of the CA, etiology, and time to defibrillation. It is difficult to establish just one severity parameter in such a complex situation.
- -
- Age might be included as a major risk for adverse outcomes that modifies risk across the SCAI shock stages [22].
- -
- Maintaining the simplicity and flexibility of the original scale is key.
- -
- The SCAI shock stage should be reassessed at intervals, the timing of which will depend on the initial severity: the improvement of the SCAI SHOCK stage by even one category is a powerful favorable prognostic indicator, and conversely, a maintaining or declining SCAI SHOCK is a potent negative marker [23].
2.3. Cause and Pathophysiological Features
2.3.1. Acute Myocardial Infarction (AMI) Related to CS
2.3.2. Heart-Failure (HF) Cardiogenic Shock
3. Therapeutic Approach to Cardiogenic Shock
3.1. Vasoactive Drugs in CS
- (a)
- Dobutamine is considered a first-line inotropic drug in CS. It is a synthetic amine with strong β1 and β2 receptor agonist properties (3:1 ratio), making it a potent inotrope with weak chronotropic effects. This allows for increased myocardial contractility with moderate effects on heart rate. Its dosing is critical when managing cardiovascular conditions [12]. At low doses, dobutamine induces mild vasodilation through β2 activation, while at high doses, it can cause vasoconstriction via α1 receptor stimulation, potentially causing an imbalance between oxygen supply and demand, especially in ischemic conditions [33]. However, dobutamine has not shown a survival benefit in clinical trials, and moreover, concerns have arisen regarding a potential detrimental effect [34].
- (b)
- Norepinephrine is also considered a first-line vasopressor in CS. It predominantly stimulates α1-adrenergic receptors, causing potent vasoconstriction and, to a lesser extent, β1 receptors provide a mild inotropic effect. This makes norepinephrine ideal for increasing systemic vascular resistance and maintaining coronary perfusion pressure without significantly raising heart rate, thus minimizing the risk of increasing myocardial oxygen demand. Its short half-life allows for precise titration. Studies, such as the Sepsis Occurrence in Acutely Ill Patients-II (SOAP-II) trial, have shown favorable outcomes in patients with CS, with a lower incidence of arrhythmias (24.1% vs. 12.4%, p < 0.001) and a trend towards reduced 28-day mortality in the cardiogenic shock subgroup (HR 0.86, 95% CI: 0.72–1.02, p = 0.07) compared to dopamine [35].
- (c)
- Epinephrine is reserved for refractory shock or mixed shock due to its powerful inotropic, chronotropic, and vasoconstrictor effects. It is an endogenous catecholamine that stimulates α1, β1, and β2 receptors. Epinephrine carries significant risks, including increased lactate production and a higher likelihood of myocardial ischemia and arrhythmias. Clinical trials, such as OptimaCC (Epinephrine Versus Norepinephrine for Cardiogenic Shock After Acute Myocardial Infarction), have reported an increased incidence of refractory CS and higher mortality with epinephrine compared to norepinephrine (HR 1.75, 95% CI: 1.06–2.88, p = 0.03), cautioning against its use in certain populations [36].
- (d)
- Dopamine was once a standard therapy for CS due to its mixed α1, β1, and dopaminergic receptor effects. Nowadays, it has fallen out of favor due to higher rates of arrhythmias and increased mortality, particularly at the higher doses needed for vasopressor effects. Low doses were believed to offer renal protection through dopaminergic receptor activation; nonetheless this theory has been debunked, and evidence now shows no benefit in renal outcomes contributing to its reduced use [37].
- (e)
- Levosimendan has emerged as a promising alternative inotropic agent in CS. It enhances myocardial contractile proteins’ sensitivity to calcium, improving contractility without increasing myocardial oxygen consumption. For this, levosimendan does not rely on increasing intracellular calcium or on the cyclic adenosine monophosphate (cAMP) mechanisms that are thought to underlie catecholamines’ and PDE inhibitors’ (PDEis) adverse effects. Levosimendan also presents vasodilatory effects, through ATP-sensitive potassium channels, reducing afterload and improving coronary perfusion. In fact, levosimendan has the fastest onset of action among cardiac inotropes, which may provide additional benefits in an acute scenario. Clinical trials, such as the RUSSLAN trial (Randomized study on Safety and effectivenesS of Levosimendan in patients with left ventricular failure due to an Acute myocardial iNfarct), demonstrated a significant reduction in mortality at 14 days compared to placebo in patients with acute heart failure (11.7% vs. 19.6%; hazard ratio 0.56, 95% CI 0.33–0.95; p = 0.031) [38]. The Levosimendan in Acute heart Failure following myocardial infarction (LEAF) trial showed a reduction in major adverse cardiac events (MACEs) at 31 days compared to dobutamine (13.1% vs. 23.3%, p = 0.049) [39]. It is important to take into account that levosimendan may not be the ideal drug in the scenario of acute CS in the catheterization lab due to its slower onset of action (30–60 min). Some authors support its role as a preconditioning agent before high-risk angioplasty procedures, similar to its use prior to coronary bypass surgeries in patients with a depressed LVEF [40]. However, randomized clinical trials are needed to support further recommendations.
- (f)
- Milrinone may be used in CS. It is a PDEi that shares inotropic and vasodilatory effects with dobutamine but differs in its mechanism of action. Milrinone prevents cAMP breakdown by inhibiting PDE3 receptors, leading to increased intracellular calcium and enhanced myocardial contractility. It thus offers a treatment advantage in heart failure patients on beta-blockers, as, unlike catecholamines, it does not depend on adrenergic receptor stimulation. Its vasodilatory effects reduce afterload. Its longer half-life warrants careful monitoring, especially in patients with renal dysfunction. The DOREMI (Dobutamine Compared with Milrinone) trial compared milrinone to dobutamine in patients with cardiogenic shock and found no significant difference in the primary composite outcome (in-hospital death from any cause, resuscitated cardiac arrest, receipt of a cardiac transplant or mechanical circulatory support, nonfatal myocardial infarction, transient ischemic attack or stroke, or initiation of renal replacement therapy.) (HR 0.90; 95% CI, 0.69–1.19; p = 0.47) [41].
- (g)
- Vasopressin is often used as a second-line vasopressor in vasodilatory shock states, such as septic shock, but it can also be useful in CS, particularly in cases of refractory hypotension or acidosis. It is an endogenous hormone, which acts on V1 receptors inducing vasoconstriction and on V2 receptors promoting water retention. Vasopressin’s effects are independent of adrenergic receptors, making it a valuable option in catecholamine-resistant shock. A study by Nguyen et al. showed that vasopressin reduced norepinephrine dose requirements in catecholamine-resistant shock (median norepinephrine equivalent dose of 0.14 mcg/kg/min vs. 0.23 mcg/kg/min, p = 0.04) [42]. Moreover, vasopressin has been associated with systemic vasoconstriction with relatively fewer effects on the pulmonary circulation, producing a desirable decrease in the pulmonary vascular resistance/systemic vascular resistance ratio [43].
- (h)
- Phenylephrine is generally avoided in cardiogenic shock, due to its potential to induce reflex bradycardia and its lack of inotropic effects. It is a pure α1 agonist, which from a pathophysiological standpoint, may be beneficial during transcatheter aortic valve replacement in patients with aortic stenosis, as it may increase blood pressure without exerting positive inotropic effects [44].
3.2. Device Therapy in CS
- (a)
- Intra-aortic balloon pumps (IABPs) are frequently used in CS, in situations such as mechanical complications of AMI and decompensated acute HF. The device consists of a latex or silicone-based balloon, using helium as an inert gas for inflation controlled by an external console. The IABP is inserted into the descending aorta, usually via the femoral artery. IABPs work by inflating during diastole to increase coronary blood flow and deflating during systole to reduce afterload, thus theoretically improving cardiac output. While they are widely available and affordable, as well as helpful in stabilizing hemodynamics, their efficacy is limited and it carries risks such as limb ischemia, infection, hemolysis, balloon rupture, and gas loss, thus translating into inconsistent survival benefits in CS. The IABP-SHOCK II (Intra-aortic Balloon Pump in Cardiogenic Shock) trial showed no significant reduction in mortality at 30 days (39.7% in the IABP group vs. 41.3% in the control group; RR 0.96, 95% CI: 0.79–1.17, p = 0.69) [45,46], leading to the 2023 European Society of Cardiology Acute Coronary Syndrome guidelines recommendation against its routine use [47]. Although IABPs have been associated with higher costs than conservative treatment, their lower upfront costs make them a more economical choice in less severe cases or when resource availability is constrained [48].
- (b)
- Micro-axial flow pumps (Impella Abiomed, Danvers, MA, USA®) are frequently used in CS to improve cardiac output. The device is an intravascular MCS pump that is inserted into the left ventricle percutaneously or surgically. It operates by drawing blood directly from the left ventricle and pumping it into the ascending aorta. It reduces both systolic and diastolic pressure within the left ventricle, decreases afterload and myocardial oxygen consumption, and enhances systemic perfusion, promoting myocardial recovery. The different models, including Impella 2.5®, CP®, 5.0® and the latest model 5.5®, offer varying levels of flow support, delivering between 2.5 and 5.5 L/min, depending on the required circulatory assistance. Additionally, Impella RP® is designed to support right ventricular function, providing up to 4.0 L/min in cases of right ventricular failure. Although the Impella is less invasive than VA-ECMO, its ability to provide blood flow is limited, making it more suitable for patients with moderate heart failure or less severe shock. In the DanGer Shock (Danish–German Cardiogenic Shock) trial, the use of Impella CP® in patients with AMI complicated by CS, combined with standard care, significantly reduced 180-day mortality compared to standard care alone (45.8% vs. 58.5%; HR 0.74, 95% CI: 0.55–0.99, p = 0.04). However, there was an increase in serious complications such as hemolysis, limb ischemia, and the need for renal replacement therapy [49]. The last released model, Impella 5.5®, has shown additional survival benefits in observational studies [50]. Impella use has been associated with a lower cost compared to ECMO, and it is additionally associated with long-term cost savings by reducing the need for long-term LVAD or a heart transplant [51].
- (c)
- Veno-arterial extracorporeal membrane oxygenation (VA-ECMO, (Getinge, Wayne, NJ, USA)) is a life-saving technique used in critical care for patients with severe CS. It works by temporarily taking over the functions of the heart and lungs. Blood is drained from the body through a large vein, passed through an oxygenator, and then pumped back into the arterial system, ensuring that oxygenated blood reaches vital organs. Despite its life-saving potential, VA-ECMO is not without risks, including complications, such as hemolysis, thromboembolism, and increased ventricular afterload caused by retrograde flow, which can potentially worsen heart failure. Recent studies have raised questions about the efficacy of VA-ECMO in improving clinical outcomes. The 2023 ECMO-CS trial found that immediate implementation of VA-ECMO did not significantly improve clinical outcomes compared to early conservative therapy in patients with CS. The composite primary endpoint (death, resuscitated cardiac arrest, or use of another mechanical support device) occurred in 63.8% of patients in the VA-ECMO group versus 71.2% in the early conservative group (HR 0.72; 95% CI 0.46–1.12; p = 0.21). Additionally, 30 days all-cause mortality remains high in both groups (50.0% in the VA-ECMO group versus 47.5% in the conservative group, p = 0.21) [52]. Similarly, the ECLS-SHOCK trial explored VA-ECMO in patients with myocardial infarction-related CS. It found no significant mortality benefit at 30 days, with 47.8% mortality in the ECMO group and 49.0% in the control group (RR, 0.98; 95% CI, 0.80–1.19; p = 0.81). In fact, patients receiving VA-ECMO experienced significantly more complications, including a higher risk of bleeding (OR 2,4 95% CI1.55–3.84) and peripheral ischemia (OR 3.53; 95% CI1.70–7.34) [9]. Moreover, a recent metanalysis of pooled data from four randomized trials confirmed that VA-ECMO did not reduce 30-day mortality in patients with infarct-related CS. The study reported similar mortality rates between the VA-ECMO and control groups (OR, 0.93; 95% CI, 0.66–1.29) but with higher related complications (bleeding or vascular complications). These findings suggest that while VA-ECMO can be a vital tool in managing cardiogenic shock, it should be used cautiously and reserved for carefully selected patients, as current evidence does not demonstrate a clear survival benefit in this population. Of note, VA-ECMO should be the preferred MCS in cases of biventricular severe dysfunction, profound CS with end-organ hypoperfusion unsuitable to be managed by an Impella alone, and refractory CA, or in cases of severe respiratory compromise (such as acute respiratory distress complicating CS). Its cost-effectiveness is generally lower than other MCS devices due to higher costs and longer hospital stays, making it more justifiable in cases of severe multi-organ failure or cardiac arrest, or as a bridge to cardiac transplantation [53].
- (d)
- Temporary ventricular overdrive pacing: although it cannot be defined as an MCS, this non-pharmacological strategy could be effective in stabilizing patients with CS secondary to a refractory electrical storm (ES) after an AMI when antiarrhythmic medications fail. It works by increasing the heart rate, which helps suppressing recurrent ventricular arrhythmias [54].
3.3. Optimal Timing
4. Hemodynamic Support During High-Risk Percutaneous Coronary Interventions
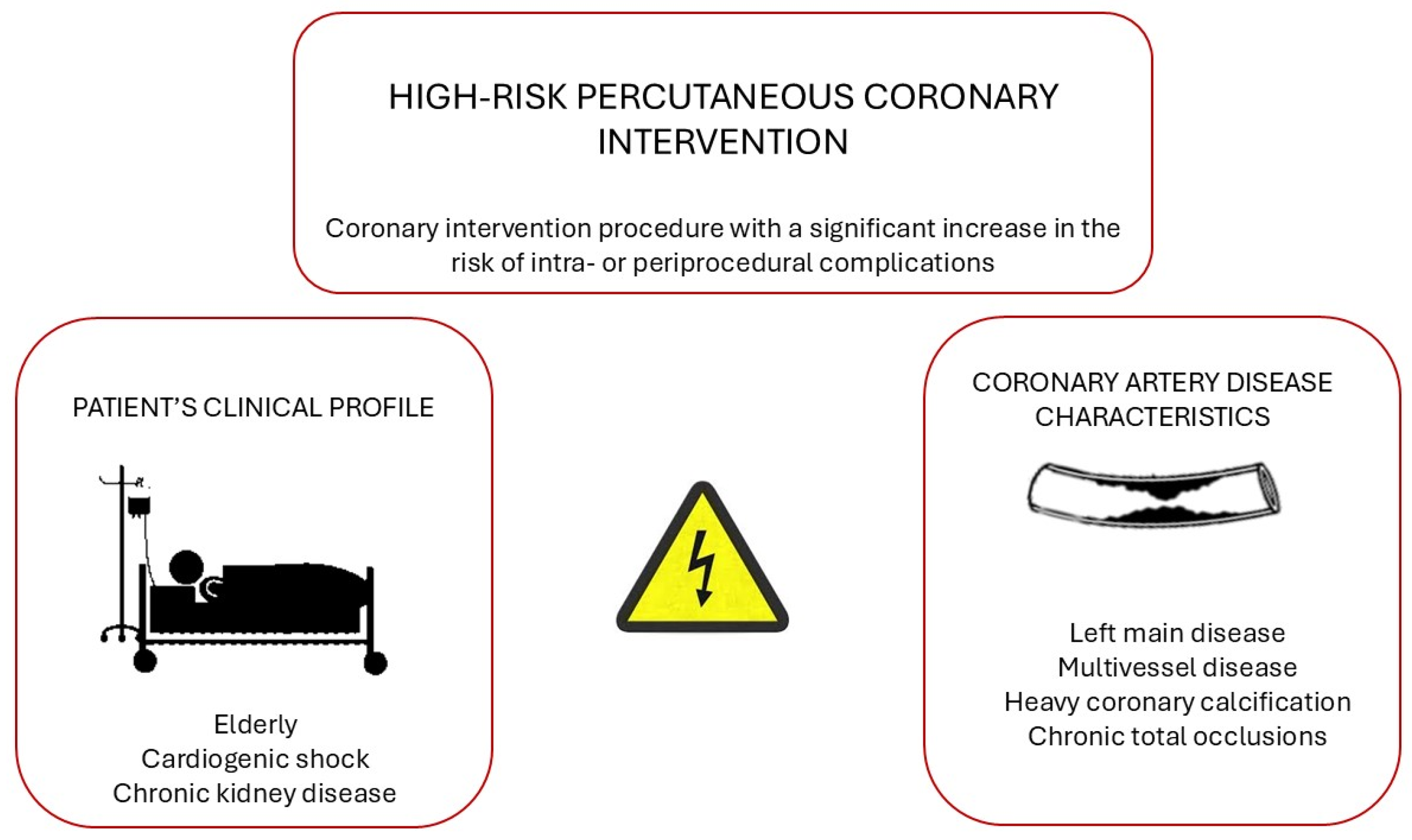
4.1. Definition of High-Risk Percutaneous Coronary Intervention (PCI)
4.2. MCS During High-Risk PCI
- (a)
- IABP
- (b)
- Microaxial flow pump (Impella, Abiomed, Danvers, MA, USA®)
4.3. MCS in Valvular Heart Disease Percutaneous Procedures
5. Future Directions
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AMI | Acute myocardial infarction |
| BiPAP | Bilevel positive airway pressure |
| BNP | Brain natriuretic peptide |
| BP | Blood pressure |
| CAD | Coronary artery disease |
| CI | Cardiac index |
| CPO | Cardiac power output |
| CPR | Cardiopulmonary resuscitation |
| CS | Cardiogenic shock |
| CVP | Central venous pressure |
| ECMO | Extracorporeal membrane oxygenation |
| GCS | Glasgow coma scale |
| GFR | Glomerular filtration rate |
| HF | Heart failure |
| IABP | Intra-aortic balloon pump |
| IVUS | Intravascular ultrasound |
| JVP | Jugular venous pressure |
| LFT | Liver function test |
| LVAD | Left ventricular assist device |
| LVEF | Left ventricular ejection fraction |
| MAP | Mean arterial pressure |
| MACEs | Major adverse cardiac events |
| MCS | Mechanical circulatory support |
| OCT | Optimal coherence tomography |
| PA | Pulmonary artery |
| PAPi | Pulmonary artery pulsatility index |
| PCI | Percutaneous coronary intervention |
| PCWP | Pulmonary capillary wedge pressure |
| PEA | Pulseless electrical activity |
| PDEi | Phosphodiesterase inhibitor |
| RAP | Right atrial pressure |
| SBP | Systolic blood pressure |
| SCAI | Society of Cardiovascular Angiography and Interventions |
| VF | Ventricular fibrillation |
| VD | Vasoactive drugs |
| VT | Ventricular tachyarrhythmia |
| cAMP | Cyclic adenosine monophosphate |
References
- Chioncel, O.; Parissis, J.; Mebazaa, A.; Thiele, H.; Desch, S.; Bauersachs, J.; Harjola, V.P.; Antohi, E.L.; Arrigo, M.; Ben Gal, T.; et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1315–1341. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; ESC Scientific Document Group; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [PubMed]
- Jentzer, J.C.; Pöss, J.; Schaubroeck, H.; Morrow, D.A.; Hollenberg, S.M.; Mebazaa, A. Advances in the management of cardiogenic shock. Crit. Care Med. 2023, 51, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, M.; Pagnesi, M.; Chioncel, O.; Mebazaa, A.; Cotter, G.; Gustafsson, F.; Tomasoni, D.; Latronico, N.; Adamo, M.; Metra, M. Medical therapy of cardiogenic shock: Contemporary use of inotropes and vasopressors. Eur. J. Heart Fail. 2024, 26, 411–431. [Google Scholar] [CrossRef]
- Alkhunaizi, F.A.; Smith, N.; Brusca, S.B.; Furfaro, D. The management of cardiogenic shock from diagnosis to devices: A narrative review. Chest Crit. Care. 2024, 2, 100071. [Google Scholar] [CrossRef]
- Martinez-Selles, M.; Hernandez-Perez, F.J.; Uribarri, A.; Villen, L.M.; Zapata, L.; Alonso, J.J.; Amat-Santos, I.J.; Ariza-Sole, A.; Barrabes, J.A.; Barrio, J.M.; et al. Cardiogenic shock code 2023. Expert document for a multidisciplinary organization that allows quality care. Rev. Esp. Cardiol. 2023, 76, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Buller, C.E.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Akin, I.; Behnes, M.; Rassaf, T.; Mahabadi, A.A.; Lehmann, R.; Eitel, I.; Graf, T.; Seidler, T.; et al. Extracorporeal life support in infarct-related cardiogenic shock. N. Engl. J. Med. 2023, 389, 1286–1297. [Google Scholar] [CrossRef]
- Møller, J.E.; Gerke, O.; DanGer Shock Investigators. Danish-German cardiogenic shock trial-DanGer shock: Trial design update. Am. Heart J. 2023, 255, 90–93. [Google Scholar] [CrossRef]
- Van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary management of cardiogenic shock: A scientific statement from the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef]
- Bloom, J.E.; Chan, W.; Kaye, D.M.; Stub, D. State of shock: Contemporary vasopressor and inotrope use in cardiogenic shock. J. Am. Heart Assoc. 2023, 12, e029787. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Rayfield, C.; Soussi, S.; Berg, D.D.; Kennedy, J.N.; Sinha, S.S.; Baran, D.A.; Brant, E.; Mebazaa, A.; Billia, F.; et al. Advances in the staging and phenotyping of cardiogenic shock: Part 1 of 2. JACC Adv. 2022, 1, 100120. [Google Scholar] [CrossRef]
- Sarma, D.; Jentzer, J.C. Cardiogenic shock: Pathogenesis, classification, and management. Crit. Care Clin. 2024, 40, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Bruno, R.R.; Jumean, M.; Price, S.; Krychtiuk, K.A.; Ramanathan, K.; Dankiewicz, J.; French, J.; Delmas, C.; Mendoza, A.-A.; et al. Management of cardiogenic shock: State-of-the-art. Intensive Care Med. 2024, 50, 1814–1829. [Google Scholar] [CrossRef]
- Krychtiuk, K.A.; Vrints, C.; Wojta, J.; Huber, K.; Speidl, W.S. Basic mechanisms in cardiogenic shock: Part 1-definition and pathophysiology. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock. Catheter. Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Naidu, S.S.; Baran, D.A.; Jentzer, J.C.; Hollenberg, S.M.; van Diepen, S.; Basir, M.B.; Grines, C.L.; Diercks, D.B.; Hall, S.; Kapur, N.K.; et al. SCAI SHOCK stage classification expert consensus update: A review and incorporation of validation studies. J. Am. Coll. Cardiol. 2022, 79, 933–946. [Google Scholar] [CrossRef]
- Jentzer, J.C.; van Diepen, S.; Barsness, G.W.; Henry, T.D.; Menon, V.; Rihal, C.S.; Naidu, S.S.; Baran, D.A. Cardiogenic Shock Classification to Predict Mortality in the Cardiac Intensive Care Unit. J. Am. Coll. Cardiol. 2019, 74, 2117–2128. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Burstein, B.; Van Diepen, S.; Murphy, J.; Holmes DRJr Bell, M.R.; Barsness, G.W.; Henry, T.D.; Menon, V.; Rihal, C.S.; Naidu, S.S.; et al. Defining shock and preshock for mortality risk stratification in cardiac intensive care unit patients. Circ. Heart Fail. 2021, 14, e007678. [Google Scholar] [CrossRef]
- Hernandez-Montfort, J.; Sinha, S.S.; Thayer, K.L.; Whitehead, E.H.; Pahuja, M.; Garan, A.R.; Mahr, C.; Haywood, J.L.; Harwani, N.M.; Schaeffer, A.; et al. Clinical outcomes associated with acute mechanical circulatory support utilization in heart failure related cardiogenic shock. Circ. Heart Fail. 2021, 14, e007924. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Schrage, B.; Holmes, D.R.; Dabboura, S.; Anavekar, N.S.; Kirchhof, P.; Barsness, G.W.; Blankenberg, S.; Bell, M.R.; Westermann, D. Influence of age and shock severity on short-term survival in patients with cardiogenic shock. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Hanson, I.D.; Tagami, T.; Mando, R.; Kara Balla, A.; Dixon, S.R.; Timmis, S.; Almany, S.; Naidu, S.S.; Baran, D.; Lemor, A.; et al. SCAI shock classification in acute myocardial infarction: Insights from the National Cardiogenic Shock Initiative. Catheter. Cardiovasc. Interv. 2020, 96, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, S.M.; Warner Stevenson, L.; Ahmad, T.; Amin, V.J.; Bozkurt, B.; Butler, J.; Davis, L.L.; Drazner, M.H.; Kirkpatrick, J.N.; Peterson, P.N.; et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: A report of the American college of cardiology solution set oversight committee. J. Am. Coll. Cardiol. 2019, 74, 1966–2011. [Google Scholar] [CrossRef]
- Presume, J.; Bello, A.R.; Gomes, D.A.; Brizido, C.; Strong, C.; Ferreira, J.; Tralhao, A. SCAI classification, the importance of its dynamic reclassification in the first 24 hours and the impact of risk modifiers in cardiogenic shock admissions. Eur. Heart J. 2023, 44 (Suppl. S2), ehad655-1148. [Google Scholar] [CrossRef]
- Díez-Villanueva, P.; Jiménez-Méndez, C.; Cepas-Guillén, P.; Arenas-Loriente, A.; Fernández-Herrero, I.; García-Pardo, H.; Díez-Delhoyo, F. Current Management of Non-ST-Segment Elevation Acute Coronary Syndrome. Biomedicines 2024, 12, 1736. [Google Scholar] [CrossRef]
- Mehta, A.; Vavilin, I.; Nguyen, A.H.; Batchelor, W.B.; Blumer, V.; Cilia, L.; Dewanjee, A.; Desai, M.; Desai, S.S.; Flanagan, M.C.; et al. Contemporary approach to cardiogenic shock care: A state-of-the-art review. Front. Cardiovasc. Med. 2024, 11, 1354158. [Google Scholar] [CrossRef]
- Samsky, M.D.; Morrow, D.A.; Proudfoot, A.G.; Hochman, J.S.; Thiele, H.; Rao, S.V. Cardiogenic shock after acute myocardial infarction: A review. JAMA 2021, 326, 1840–1850. [Google Scholar] [CrossRef]
- Zeymer, U.; Alushi, B.; Noc, M.; Mamas, M.A.; Montalescot, G.; Fuernau, G.; Huber, K.; Poess, J.; de Waha-Thiele, S.; Schneider, S.; et al. Influence of culprit lesion intervention on outcomes in infarct-related Cardiogenic Shock with cardiac arrest. J. Am. Coll. Cardiol. 2023, 81, 1165–1176. [Google Scholar] [CrossRef]
- Narang, N.; Blumer, V.; Jumean, M.F.; Kar, B.; Kumbhani, D.J.; Bozkurt, B.; Uriel, N.; Guglin, M.; Kapur, N.K. Management of heart failure-related cardiogenic shock: Practical guidance for clinicians. JACC Heart Fail. 2023, 11, 845–851. [Google Scholar] [CrossRef]
- Osman, M.; Syed, M.; Patibandla, S.; Sulaiman, S.; Kheiri, B.; Shah, M.K.; Bianco, C.; Balla, S.; Patel, B. Fifteen-year trends in incidence of cardiogenic shock hospitalization and in-hospital mortality in the United States. J. Am. Heart Assoc. 2021, 10, e021061. [Google Scholar] [CrossRef] [PubMed]
- Barnett, C.F.; Brusca, S.B.; Hanff, T.C.; Blumer, V.; Kalif, A.; Kanwar, M. Management of cardiogenic shock unrelated to acute myocardial infarction. Can. J. Cardiol. 2023, 39, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.R.; Wolff, G.; Kelm, M.; Jung, C. Pharmacological treatment of cardiogenic shock—A state of the art review. Pharmacol. Ther. 2022, 240, 108230. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.A.; Timmins, A.C.; Yau, E.H.; Palazzo, M.; Hinds, C.J.; Watson, D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N. Engl. J. Med. 1994, 330, 1717–1722. [Google Scholar] [CrossRef]
- De Backer, D.; Biston, P.; Devriendt, J.; Madl, C.; Chochrad, D.; Aldecoa, C.; Brasseur, A.; Defrance, P.; Gottignies, P.; Vincent, J.L. Comparison of dopamine and norepinephrine in the treatment of shock. N. Engl. J. Med. 2010, 362, 779–789. [Google Scholar] [CrossRef]
- Levy, B.; Clere-Jehl, R.; Legras, A.; Morichau-Beauchant, T.; Leone, M.; Frederique, G.; Quenot, J.P.; Kimmoun, A.; Cariou, A.; Lassus, J.; et al. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J. Am. Coll. Cardiol. 2018, 72, 173–182. [Google Scholar] [CrossRef]
- Mebazaa, A.; Motiejunaite, J.; Gayat, E.; Crespo-Leiro, M.G.; Lund, L.H.; Maggioni, A.P.; Chioncel, O.; Akiyama, E.; Harjola, V.P.; Seferovic, P.; et al. Long-term safety of intravenous cardiovascular agents in acute heart failure: Results from the European Society of Cardiology Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2018, 20, 430–440. [Google Scholar] [CrossRef]
- Moiseyev, V.S.; Poder, P.; Andrejevs, N.; Ruda, M.Y.; Golikov, A.P.; Lazebnik, L.B.; Kobalava, Z.D.; Lehtonen, L.A.; Laine, T.; Nieminen, M.S.; et al. Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction: A randomized, placebo-controlled, double-blind study (RUSSLAN). Eur Heart J. 2002, 23, 1422–1432. [Google Scholar] [CrossRef]
- Follath, F.; Cleland, J.G.; Just, H.; Papp, J.G.Y.; Scholz, H.; Peuhkurinen, K.; Harjola, V.P.; Mitrovic, V.; Abdalla, M.; Sandell, E.P.; et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): A randomized double-blind trial. J. Am. Coll. Cardiol. 2002, 39, 1888–1898. [Google Scholar] [CrossRef]
- Ayala, R.; Gewher, D.M.; Godoi, A.; Velasquez, C.; Fernandez, M.; Carvalho, P.E.P.; Goebel, N. Preoperative levosimendan in patients with severe left ventricular dysfunction undergoing isolated coronary artery bypass grafting: A meta-analysis of randomized controlled trials. J. Cardiothorac. Vasc. Anesth. 2024, 38, 649–659. [Google Scholar] [CrossRef]
- Mathew, R.; Di Santo, P.; Jung, R.G.; Marbach, J.A.; Hutson, J.; Simard, T.; Ramirez, F.D.; Harnett, D.T.; Merdad, A.; Almufleh, A.; et al. Milrinone as compared with dobutamine in the treatment of cardiogenic shock. N. Engl. J. Med. 2021, 385, 516–525. [Google Scholar] [CrossRef]
- Nguyen, M.; Berthoud, V.; Rizk, A.; Bouhemad, B.; Guinot, P.G. Real life use of vasopressin in patients with cardiogenic shock: A retrospective cohort analysis. Crit. Care 2023, 27, 291. [Google Scholar] [CrossRef]
- Sarkar, J.; Golden, P.J.; Kajiura, L.N.; Murata, L.A.; Uyehara, C.F. Vasopressin decreases pulmonary-to-systemic vascular resistance ratio in a porcine model of severe hemorrhagic shock. Shock 2015, 43, 475–482. [Google Scholar] [CrossRef]
- Goertz, A.W.; Lindner, K.H.; Schültz, W.; Schirmer, U.; Beyer, M.; Georgieff, M. Influence of Phenylephrine Bolus Administration on Left Ventricular Filling Dynamics in Patients with Coronary Artery Disease and Patients with Valvular Aortic Stenosis. Anesthesiology 1994, 81, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, L.E.; Lane, B.H.; Shaw, C.R.; Gorder, K.; Grisoli, A.; Lavallee, M.; Gobble, O.; Vidosh, J.; Deimling, D.; Ahmad, S.; et al. The intra-aortic balloon pump: A focused review of physiology, transport logistics, mechanics, and complications. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 101337. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Neumann, F.J.; Ferenc, M.; Olbrich, H.G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.; Faulkner, M.; Zeymer, U.; Ouarrak, T.; Eitel, I.; Desch, S.; Hasenfuß, G.; Thiele, H. Economic implications of intra-aortic balloon support for myocardial infarction with cardiogenic shock: An analysis from the IABP-SHOCK II-trial. Clin. Res. Cardiol. 2015, 104, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Møller, J.E.; Engstrøm, T.; Jensen, L.O.; Eiskjær, H.; Mangner, N.; Polzin, A.; Schulze, P.C.; Skurk, C.; Nordbeck, P.; Clemmensen, P.; et al. Microaxial flow pump or standard care in infarct-related cardiogenic shock. N. Engl. J. Med. 2024, 390, 1382–1393. [Google Scholar] [CrossRef]
- Fried, J.; Farr, M.; Kanwar, M.; Uriel, N.; Hernandez-Montfort, J.; Blumer, V.; Li, S.; Sinha, S.S.; Garan, A.R.; Li, B.; et al. Clinical outcomes among cardiogenic shock patients supported with high-capacity Impella axial flow pumps: A report from the Cardiogenic Shock Working Group. J. Heart Lung Transplant. 2024, 43, 1478–1488. [Google Scholar] [CrossRef]
- Stretch, R.; Sauer, C.M.; Yuh, D.D.; Bonde, P. National trends in the utilization of short-term mechanical circulatory support: Incidence, outcomes, and cost analysis. J. Am. Coll. Cardiol. 2014, 64, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Ostadal, P.; Rokyta, R.; Karasek, J.; Kruger, A.; Vondrakova, D.; Janotka, M.; Naar, J.; Smalcova, J.; Hubatova, M.; Hromadka, M.; et al. Extracorporeal membrane oxygenation in the therapy of cardiogenic shock: Results of the ECMO-CS randomized clinical trial. Circulation 2023, 147, 454–464. [Google Scholar] [CrossRef]
- Jäämaa-Holmberg, S.; Salmela, B.; Suojaranta, R.; Lemström, K.B.; Lommi, J. Cost-utility of venoarterial extracorporeal membrane oxygenation in cardiogenic shock and cardiac arrest. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 333–341. [Google Scholar] [CrossRef]
- Meter, M.; Borovac, J.A. A Refractory Electrical Storm after Acute Myocardial Infarction: The Role of Temporary Ventricular Overdrive Pacing as a Bridge to ICD Implantation. Pathophysiology 2024, 31, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, A.; Campinas, A.; Brochado, B.; Braga, M.; Sá-Couto, D.; Santos, M.; Ribeiro, D.; Brandão, M.; Silva, M.P.; de Morais, G.P.; et al. Twelve-year trends in unprotected left main coronary artery occlusion: Insights from a real-world multicentre study. ESC Heart Fail. 2024, 11, 1981–1994. [Google Scholar] [CrossRef]
- Bass, T.A. High-Risk Percutaneous Coronary Interventions in Modern Day Clinical Practice: Current Concepts and Challenges. Circ. Cardiovasc. Interv. 2015, 8, e003405. [Google Scholar] [CrossRef]
- Bottardi, A.; Prado, G.F.; Lunardi, M.; Fezzi, S.; Pesarini, G.; Tavella, D.; Scarsini, R.; Ribichini, F. Clinical Updates in Coronary Artery Disease: A Comprehensive Review. J. Clin. Med. 2024, 13, 4600. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, K.H.; Song, Y.B.; Lee, J.Y.; Lee, S.J.; Lee, S.Y.; Kim, S.M.; Yun, K.H.; Cho, J.Y.; Kim, C.J.; et al. Intravascular Imaging-Guided or Angiography-Guided Complex PCI. N. Engl. J. Med. 2023, 388, 1668–1679. [Google Scholar] [CrossRef]
- Di Muro, F.M.; Bellino, M.; Esposito, L.; Attisano, T.; Meucci, F.; Mattesini, A.; Galasso, G.; Vecchione, C.; Di Mario, C. Role of Mechanical Circulatory Support in Complex High-Risk and Indicated Percutaneous Coronary Intervention: Current Indications, Device Options, and Potential Complications. J. Clin. Med. 2024, 13, 4931. [Google Scholar] [CrossRef]
- Lauga, A.; Perel, C.; D’Ortencio, A.O. Balón de contrapulsación intraaórtico. Insuf. Card. 2008, 3, 184–195. [Google Scholar]
- Perera, D.; Stables, R.; Clayton, T.; De Silva, K.; Lumley, M.; Clack, L.; Thomas, M.; Redwood, S. BCIS-1 Investigators. Long-term mortality data from the balloon pump-assisted coronary intervention study (BCIS-1): A randomized, controlled trial of elective balloon counterpulsation during high-risk percutaneous coronary intervention. Circulation 2013, 127, 207–212. [Google Scholar] [CrossRef]
- Sazzad, F.; Luo, H.D.; Chang, G.; Wu, D.; Ong, Z.X.; Kofidis, T.; Kang, G.S. Is preoperative IABP insertion significantly reducing postoperative complication in augmented high-risk coronary artery bypass grafting patients? J. Cardiothorac. Surg. 2024, 19, 363. [Google Scholar] [CrossRef]
- Saito, S.; Okubo, S.; Matsuoka, T.; Hirota, S.; Yokoyama, S.; Kanazawa, Y.; Takei, Y.; Tezuka, M.; Tsuchiya, G.; Konishi, T.; et al. Impella—Current issues and future expectations for the percutaneous, microaxial flow left ventricular assist device. J. Cardiol. 2024, 83, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Pieri, M.; Sorrentino, T.; Oppizzi, M.; Melisurgo, G.; Lembo, R.; Colombo, A.; Zangrillo, A.; Pappalardo, F. The role of different mechanical circulatory support devices and their timing of implantation on myocardial damage and mid-term recovery in acute myocardial infarction related cardiogenic shock. J. Interv. Cardiol. 2018, 31, 717–724. [Google Scholar] [CrossRef]
- Schrage, B.; Ibrahim, K.; Loehn, T.; Werner, N.; Sinning, J.M.; Pappalardo, F.; Pieri, M.; Skurk, C.; Lauten, A.; Landmesser, U.; et al. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation 2019, 139, 1249–1258. [Google Scholar] [CrossRef]
- Lauten, A.; Engström, A.E.; Jung, C.; Empen, K.; Erne, P.; Cook, S.; Windecker, S.; Bergmann, M.W.; Klingenberg, R.; Lüscher, T.F.; et al. Percutaneous left-ventricular support with the Impella-2.5-assist device in acute cardiogenic shock: Results of the Impella-EUROSHOCK-registry. Circ. Heart Fail. 2013, 6, 23–30. [Google Scholar] [CrossRef]
- Rihal, C.S.; Naidu, S.S.; Givertz, M.M.; Szeto, W.Y.; Burke, J.A.; Kapur, N.K.; Kern, M.; Garratt, K.N.; Goldstein, J.A.; Dimas, V.; et al. Society for Cardiovascular Angiography and Interventions (SCAI), Heart Failure Society of America (HFSA), Society for Thoracic Surgeons (STS), American Heart Association (AHA), American College of Cardiology (ACC). 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care. J. Card. Fail. 2015, 21, 499–518. [Google Scholar]
- Kovacic, J.C.; Kini, A.; Banerjee, S.; Dangas, G.; Massaro, J.; Mehran, R.; Popma, J.; O’Neill, W.W.; Sharma, S.K. Patients with 3-vessel coronary artery disease and impaired ventricular function undergoing PCI with Impella 2.5 hemodynamic support have improved 90-day outcomes compared to intra-aortic balloon pump: A sub-study of the PROTECT II trial. J. Interv. Cardiol. 2015, 28, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Becher, T.; Eder, F.; Baumann, S.; Loßnitzer, D.; Pollmann, B.; Behnes, M.; Borggrefe, M.; Akin, I. Unprotected versus protected high-risk percutaneous coronary intervention with the Impella 2.5 in patients with multivessel disease and severely reduced left ventricular function. Medicine 2018, 97, e12665. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Anderson, M.; Burkhoff, D.; Grines, C.L.; Kapur, N.K.; Lansky, A.J.; Mannino, S.; McCabe, J.M.; Alaswad, K.; Daggubati, R.; et al. Improved outcomes in patients with severely depressed LVEF undergoing percutaneous coronary intervention with contemporary practices. Am. Heart J. 2022, 248, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Chitturi, K.R.; Zhang, C.; Abusnina, W.; Sawant, V.; Banerjee, A.; Ahmed, S.; Merdler, I.; Haberman, D.; Chaturvedi, A.; Lupu, L.; et al. High-risk percutaneous coronary intervention with or without mechanical circulatory support: Will. Impella show superiority in the PROTECT IV randomized trial? Cardiovasc. Revasc. Med. 2024, 14, 00580–00583. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e21–e129. [Google Scholar] [PubMed]
- Shah, A.P.; Retzer, E.M.; Nathan, S.; Paul, J.D.; Friant, J.; Dill, K.E.; Thomas, J.L. Clinical and economic effectiveness of percutaneous ventricular assist devices for high-risk patients undergoing percutaneous coronary intervention. J. Invasive Cardiol. 2015, 27, 148–154. [Google Scholar]
- Ryan, M.; Ezad, S.M.; Webb, I.; O’Kane, P.D.; Dodd, M.; Evans, R.; Laidlaw, L.; Khan, S.Q.; Weerackody, R.; Bagnall, A.; et al. Percutaneous Left Ventricular Unloading During High-Risk Coronary Intervention: Rationale and Design of the CHIP-BCIS3 Randomized Controlled Trial. Circ. Cardiovasc. Interv. 2024, 17, e013367. [Google Scholar] [CrossRef]
- Van den Buijs, D.M.F.; Wilgenhof, A.; Knaapen, P.; Zivelonghi, C.; Meijers, T.; Vermeersch, P.; Arslan, F.; Verouden, N.; Nap, A.; Sjauw, K.; et al. Prophylactic Impella CP versus VA-ECMO in Patients Undergoing Complex High-Risk Indicated PCI. J. Interv. Cardiol. 2022, 8167011. [Google Scholar] [CrossRef]
- Leon, S.A.; Rosen, J.L.; Ahmad, D.; Austin, M.A.; Vishnevsky, A.; Rajapreyar, I.N.; Ruggiero, N.J.; Rame, J.E.; Entwistle, J.W.; Massey, H.T.; et al. Microaxial circulatory support for percutaneous coronary intervention: A systematic review and meta-analysis. Artif. Organs. 2023, 47, 934–942. [Google Scholar] [CrossRef]
- Scotti, A.; Leone, P.P.; Sturla, M.; Curio, J.; Spring, A.M.; Ressa, G.; Ludwig, S.; Sugiura, T.; Assafin, M.; Granada, J.F.; et al. Prophylactic intra-aortic balloon pump in transfemoral transcatheter aortic valve implantation. EuroIntervention 2023, 19, e188. [Google Scholar] [CrossRef]
- Geppert, A.; Mashayekhi, K.; Huber, K. The use of mechanical circulatory support in elective high-risk percutaneous coronary interventions: A literature-based review. Eur. Heart J. Open 2024, 4, oeae007. [Google Scholar] [CrossRef]
- Almajed, M.R.; Mahmood, S.; Obri, M.; Nona, P.; Gonzalez, P.E.; Chiang, M.; Wang, D.D.; Frisoli, T.; Lee, J.; Basir, M.; et al. Application of Impella Mechanical Circulatory Support Devices in Transcatheter Aortic Valve Replacement and Balloon Aortic Valvuloplasty: A Single-Center Experience. Cardiovasc. Revasc Med. 2023, 53, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Alkhalil, A.; Hajjar, R.; Ibrahim, H.; Ruiz, C.E. Mechanical Circulatory Support in Transcatheter Aortic Valve Implantation in the United States (from the National Inpatient Sample). Am. J. Cardiol. 2019, 124, 1615–1620. [Google Scholar] [CrossRef]
- Yeo, I.; Wong, S.C.; Mack, C.A.; Ko, W.; Kim, L.K.; Feldman, D.N.; Reisman, M.; Mick, S.L.; Iannacone, E.M.; Shah, T.; et al. Feasibility and Safety of Impella-Assisted High-Risk PCI Before TAVR in Patients With Severe Aortic Stenosis. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 101061. [Google Scholar] [CrossRef] [PubMed]

| Physical Examination | Laboratory Tests | Hemodynamics | |
|---|---|---|---|
| SCAI A (at risk) Patient who is not currently experiencing signs or symptoms of CS. | Normal JVP Lung sound Warm and well-perfused | Normal exams | Normotensive (SBP ≥ 100 mmHg or normal for patient) Hemodynamic: CI ≥ 2.5 L/min CVP < 10 mmHg PCWP ≤ 15 mmHg PA saturation ≥ 65% |
| SCAI B (beginning) Relative hypotension or tachycardia without hypoperfusion. | Elevated JVP Rales in lung fields Warm and well-perfused | Normal lactate Minimal renal function impairment Elevated BNP | SBP < 90 mmHg OR MAP < 60 mmHg OR > 30 mmHg drop from baseline Heart rate ≥ 100 bpm Hemodynamic: CI ≥ 2.2 L/min PA saturation ≥ 65% |
| SCAI C (classic) Hypoperfusion that requires intervention beyond volume resuscitation. | May include any of the following: Looks unwell, panicked, ashen, mottled, or dusky Cold, clammy Volume overload Extensive rales Killip–Kimball classification 3 or 4 Mechanical ventilatory support Cold, clammy Acute alteration in mental status Urine output < 30 mL/h | May include any of: Lactate ≥ 2 mmL/L Creatinine doubling OR > 50% drop in GFR Increased LFTs Elevated BNP | May include any of the following: >30 mmHg SPB drop from baseline AND drugs/device used to maintain BP above target CI < 2.2 L/min PCWP > 15 mmHg RAP/PCWP ≥ 0.8 PAPi < 1.85 CPO ≤ 0.6 W |
| SCAI D (deteriorating) Similar to category C but getting worse. | Any of stage C | Any of stage C AND Deteriorating | Any of stage C AND requiring multiple vasopressors OR addition of MCS devices to maintain perfusion |
| SCAI E (extremis) Cardiac arrest with ongoing CPR and/or ECMO, being supported by multiple interventions. | Near pulselessness Cardiac collapse Mechanical ventilation Defibrillator used | “Trying to die” CPR (A-modifier) PH ≤ 7.2 Lactate ≥ 5 mmoL/L | No SBP without resuscitation PEA or refractory VT/VF Hypotension despite maximal support |
| Drug | Mechanism of Action | Dose | Advantages | Disadvantages |
|---|---|---|---|---|
| Dobutamine | β1 and β2 agonist, mild α1 effects at higher doses. | 2–20 mcg/kg/min | Potent inotrope improving cardiac output. Mild vasodilation at low doses. | Increases myocardial oxygen consumption. Risk of arrhythmias and hypotension at high doses. |
| Norepinephrine | Predominantly α1 agonist with mild β1 inotropic effects. | 0.05–1.0 mcg/kg/min | Potent vasoconstriction, increases SVR, and maintains coronary perfusion pressure without excessive HR increase. | Limited chronotropic effect. May cause ischemia. Increases afterload. |
| Epinephrine | Non-selective α1, β1, and β2 agonist. | 0.01–0.5 mcg/kg/min | Potent inotropic and chronotropic effects. Useful in refractory shock. | Can induce stress cardiomyopathy. Increases lactate production. |
| Dopamine | α1, β1, and dopaminergic agonist. | 2–20 mcg/kg/min | Useful for hypotension with concurrent low CO. | Dose-dependent effects. Higher risk of arrhythmias. No proven renal protection. |
| Levosimendan | Calcium sensitizer and ATP-sensitive K + channel opener. | 0.05–0.2 mcg/kg/min | Increases contractility without increasing myocardial oxygen demand. | Limited data on long-term survival benefit. Hypotension risk due to vasodilation. |
| Milrinone | Phosphodiesterase-3 inhibitor, increases cAMP and intracellular calcium. | 0.375–0.75 mcg/kg/min | Inotropic effects even in patients on beta-blockers. | Long half-life requires cautious use in renal failure. Hypotension risk due to vasodilation. |
| Vasopressin | V1 receptor agonist and V2. | 0.03 units/min | Efficacy in acidosis and refractory hypotension. | Risk of ischemia and peripheral vasoconstriction. Risk of hyponatremia. |
| Phenylephrine | Pure α1 agonist. | 0.5–10 mcg/min | Increases SVR without direct impact on heart rate. | Reflex bradycardia. Not suitable for patients with low-output states. |
| Device | Mechanism of Action | Advantages | Complications | Estimated Cost (€-euros) |
|---|---|---|---|---|
| IABP (Intra-Aortic Balloon Pump) | A balloon inflates in the descending aorta during diastole to improve coronary perfusion and deflates in systole to reduce afterload. | Improves coronary perfusion. Relatively low cost. Widely available. | Limited hemodynamic benefits. Risk of peripheral ischemia. Less effective in severe ventricular dysfunction. | 800–1500 € |
| Impella 2.5 (Abiomed, Danvers, MA, USA)® | Axial flow pump that provides up to 2.5 L/min blood flow, inserted percutaneously via femoral artery. | Easy percutaneous insertion. Provides adequate support for moderate left ventricular dysfunction. | Bleeding. Hemolysis. Risk of vascular injury during insertion. | 18,000–23,000 € |
| Impella CP (Abiomed, Danvers, MA, USA)® | Axial flow pump delivering 3.5–4.3 L/min flow, used for more severe cases with additional monitoring features (Smart Assist). | Increased flow capacity. Enhanced hemodynamic monitoring with Smart Assist system. Suited for high-risk interventions. | Similar to Impella 2.5® but with additional risks due to higher flow, such as device migration and hemolysis. | 20,000–25,000 € |
| Impella RP(Abiomed, Danvers, MA, USA)® | Provides 4 L/min of blood flow, specifically designed for right ventricular support. | Specialized for right ventricular failure. Effective unloading of the right ventricle. | Specific for RV use. Risk of vascular injury, bleeding, and hemolysis. | 22,000–25,000 € |
| VA-ECMO (Getinge, Wayne, NJ, USA) | Cardiopulmonary support device that oxygenates blood outside the body and returns it to arterial circulation. | Simultaneous circulatory and respiratory support. Useful in severe cardiogenic shock. | Increased ventricular afterload. Risk of renal failure. Vascular complications. | 45,000–75,000 € per case, including monitoring and consumables costs. |
| Temporary Ventricular Overdrive Pacing (VOP) | Increases heart rate to suppress arrhythmias, effective in stabilizing electrical storms. | Quick stabilization of arrhythmias. Useful as a bridge to permanent ICD implantation. | Requires constant monitoring. Possible discomfort from pacing leads. | 600–1300 € |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Méndez, C.; Lara-Palomo, A.; Pérez-Asensio, A.; Martín-Alfaro, L.; Urgiles, M.; Vázquez-García, R.; Gheorghe, L. Hemodynamic Support in Cardiogenic Shock in the Cardiac Catheterization Laboratory. Emerg. Care Med. 2025, 2, 39. https://doi.org/10.3390/ecm2030039
Jiménez-Méndez C, Lara-Palomo A, Pérez-Asensio A, Martín-Alfaro L, Urgiles M, Vázquez-García R, Gheorghe L. Hemodynamic Support in Cardiogenic Shock in the Cardiac Catheterization Laboratory. Emergency Care and Medicine. 2025; 2(3):39. https://doi.org/10.3390/ecm2030039
Chicago/Turabian StyleJiménez-Méndez, Cesar, Ana Lara-Palomo, Ana Pérez-Asensio, Luis Martín-Alfaro, Mauricio Urgiles, Rafael Vázquez-García, and Livia Gheorghe. 2025. "Hemodynamic Support in Cardiogenic Shock in the Cardiac Catheterization Laboratory" Emergency Care and Medicine 2, no. 3: 39. https://doi.org/10.3390/ecm2030039
APA StyleJiménez-Méndez, C., Lara-Palomo, A., Pérez-Asensio, A., Martín-Alfaro, L., Urgiles, M., Vázquez-García, R., & Gheorghe, L. (2025). Hemodynamic Support in Cardiogenic Shock in the Cardiac Catheterization Laboratory. Emergency Care and Medicine, 2(3), 39. https://doi.org/10.3390/ecm2030039








