Efficacy of Portable Fugitive Aerosol Mitigation Systems for Nebulizer Therapy During High-Flow Nasal Cannula and Non-Invasive Ventilation
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of Aerosol-Generating Procedures, Systems, and Mitigation Methods
2.2. Aerosol Generating (AG) Procedures
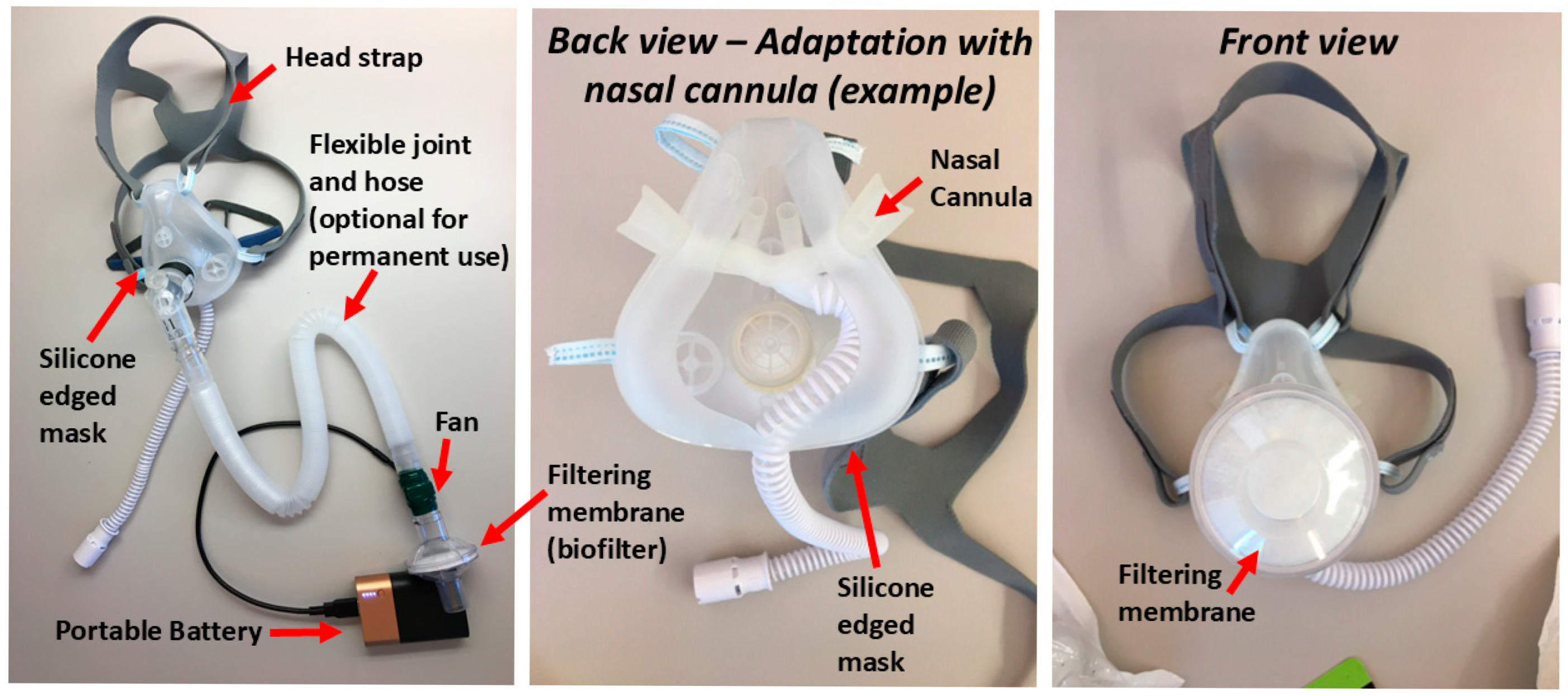
2.3. Mitigation Methods
2.4. Real and Simulated Human Subjects
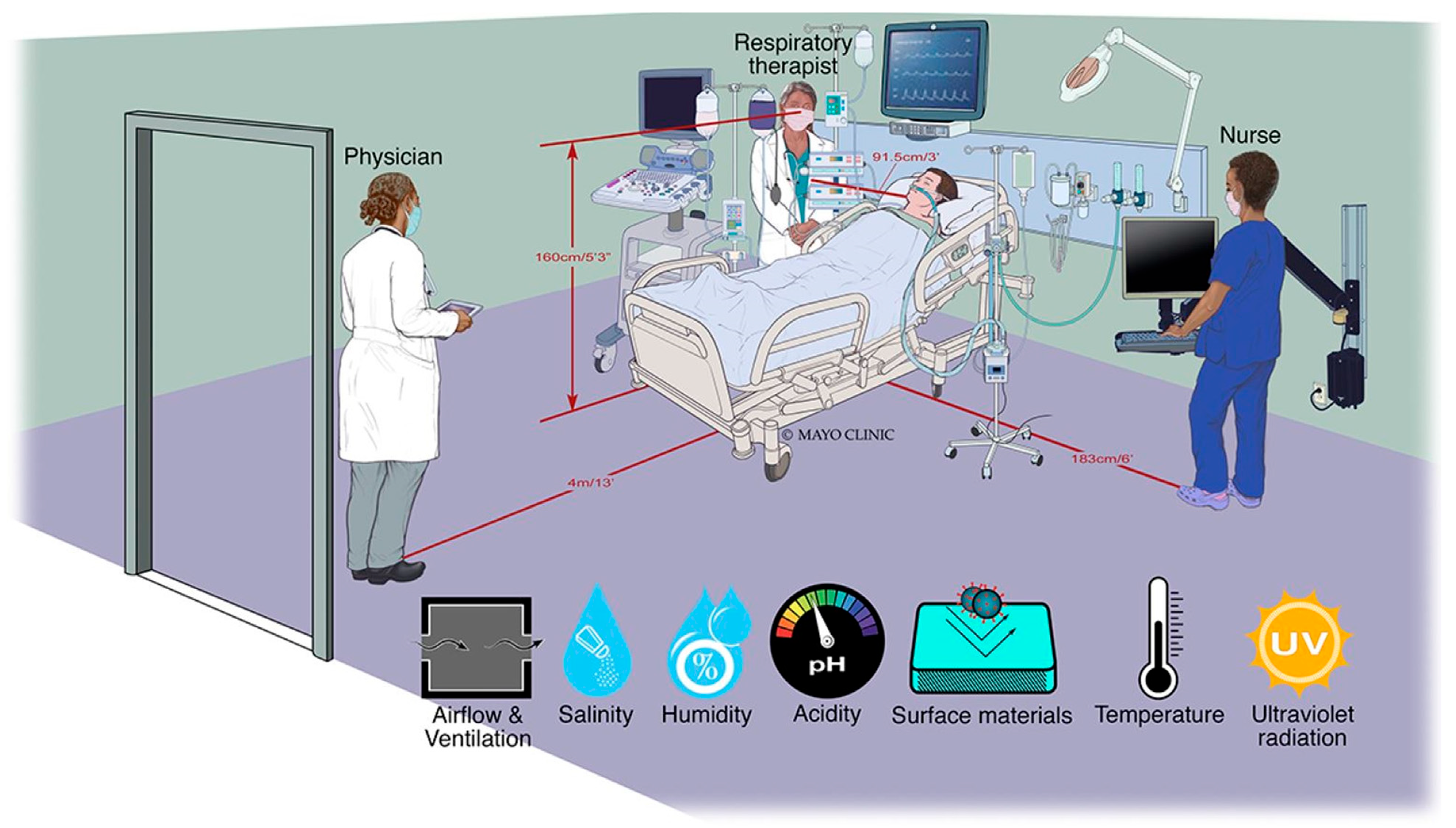
2.5. Simulated Applied Clinical Environment
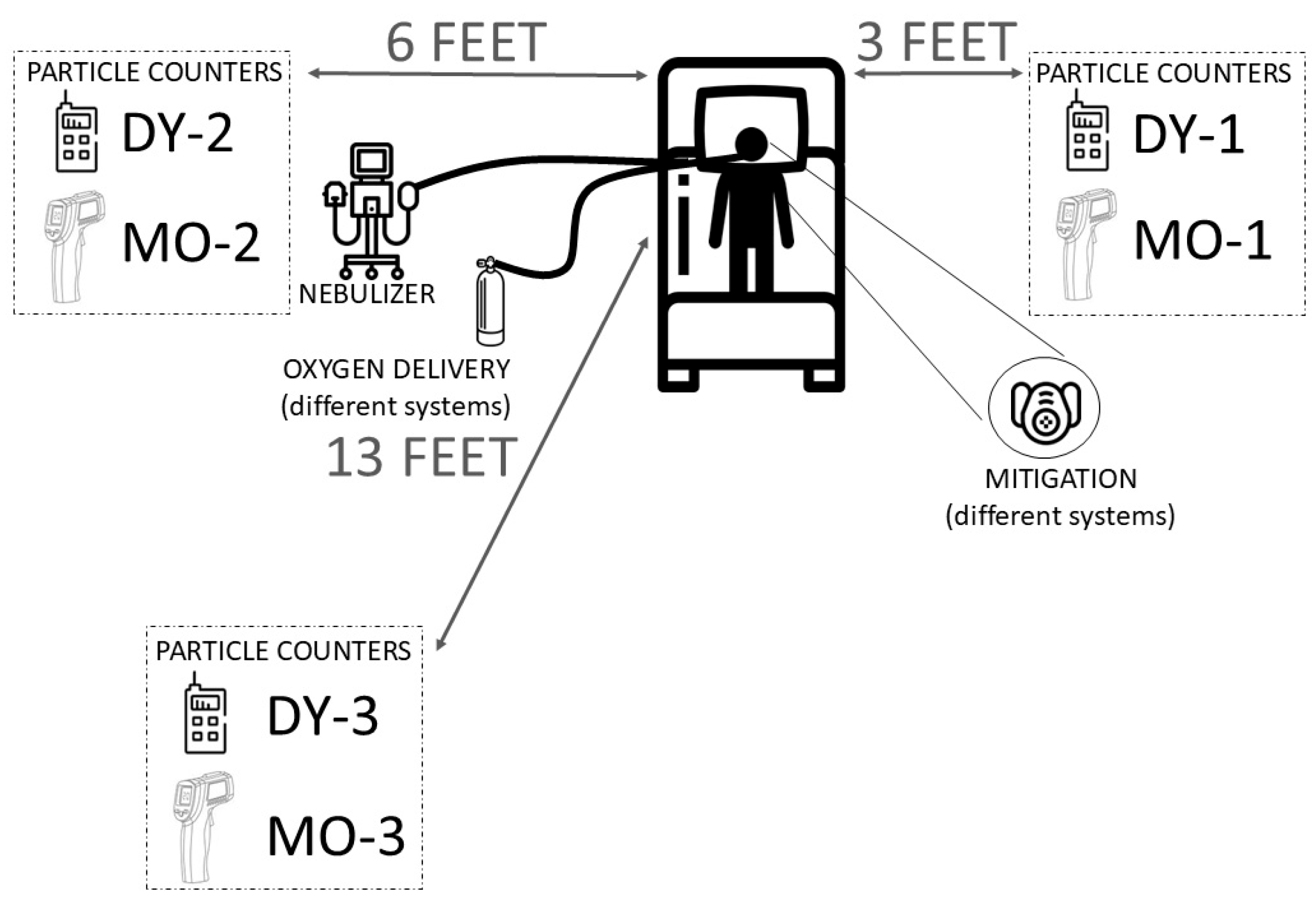
2.6. Aerosol Measurement Equipment
2.7. Measurement Protocol and Data Analysis
2.8. High-Flow Nasal Cannula Testing Procedure
2.9. Non-Invasive Ventilation Testing Procedure
2.10. Measurements of Drug Delivery Efficacy in Oronasal Silicone Mask with Active Mitigation System
- Saline solution: 5.0 mL of 0.9% sodium chloride solution (AddiPak, Hudson RCI, Temecula, CA, USA).
- Albuterol solution: 3.0 mL of 0.83 mg/mL Albuterol (Nephron Pharmaceutical Corporation, West Columbia, SC, USA).
- Vancomycin solution: 5.0 mL of 50 mg/mL Vancomycin prepared from solid Vancomycin (Klonal Laboratories, Klonal, Argentina).
- Amikacin solution: 5 mL of 100 mg/mL of Amikacin sulfate prepared from 500 mg/2 mL Amikacin sulfate (Klonal Laboratories, Klonal, Argentina).
3. Results
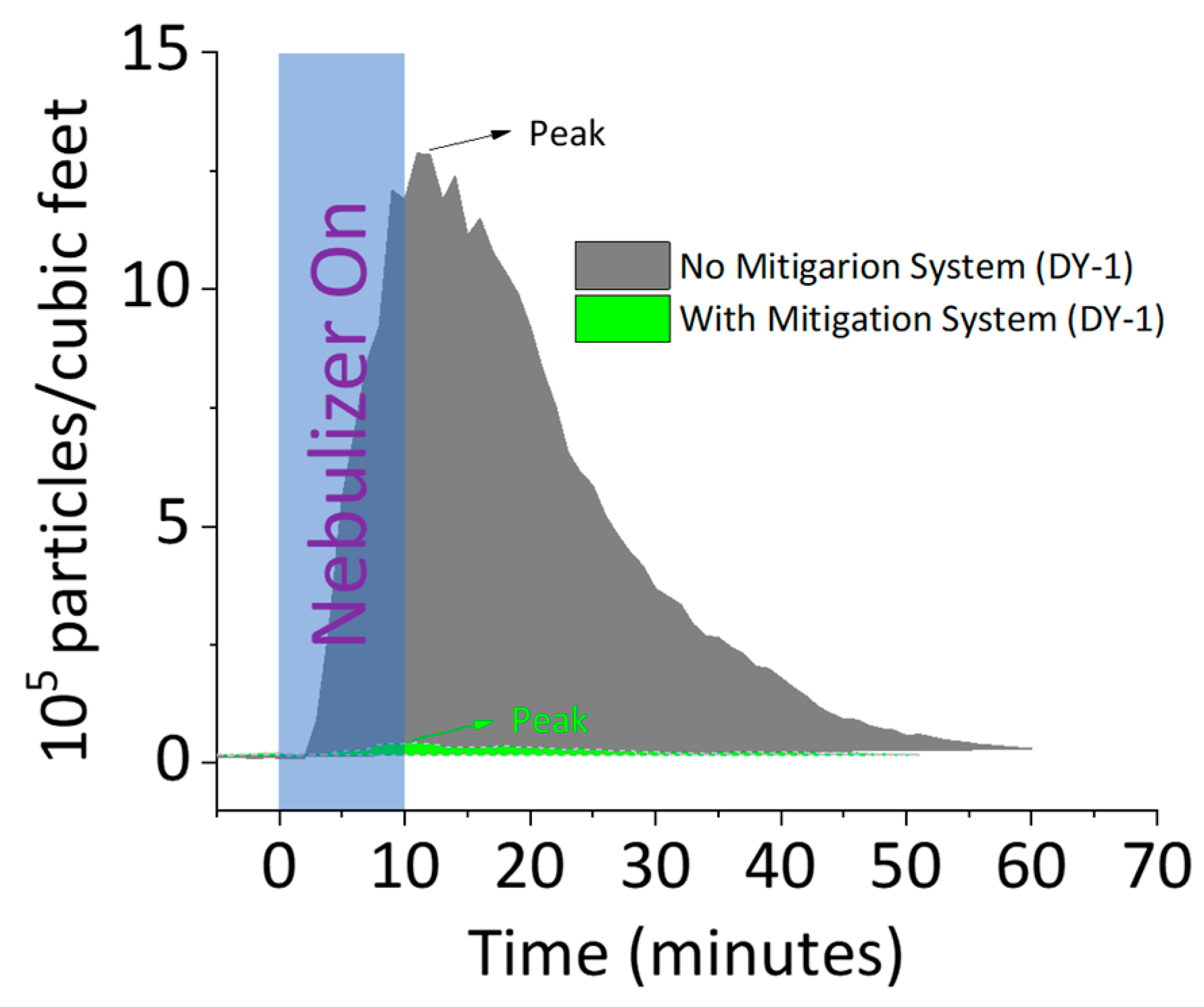
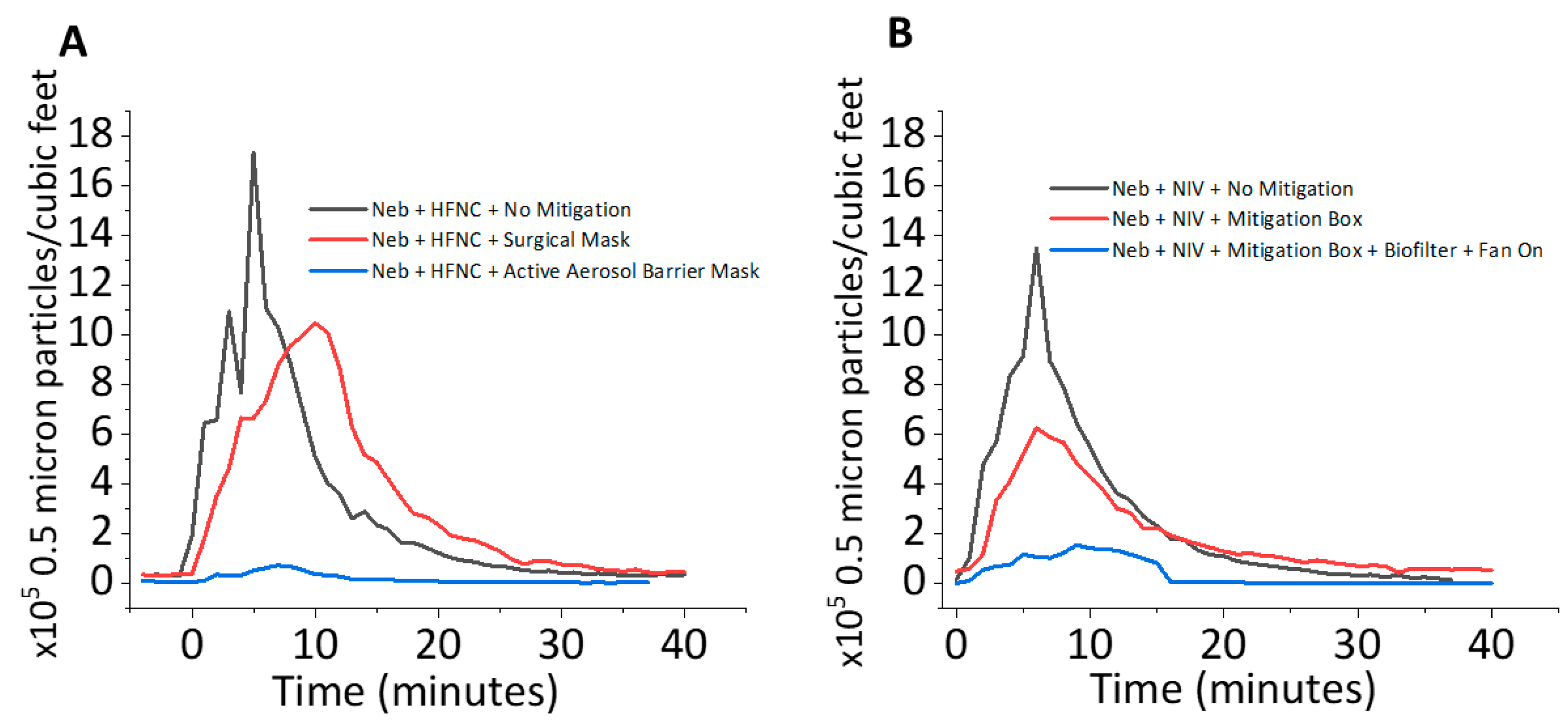
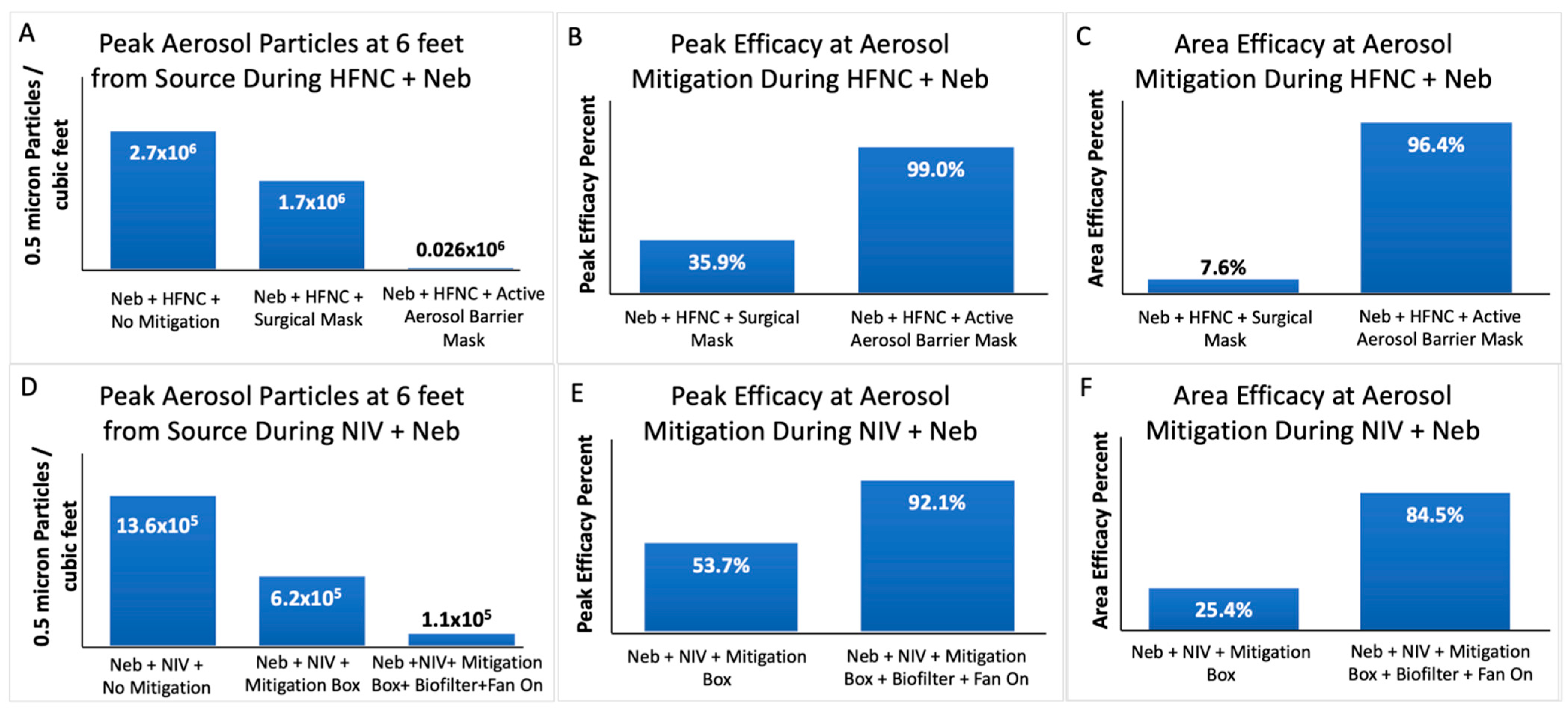
3.1. Aerosol Mitigation Efficacy
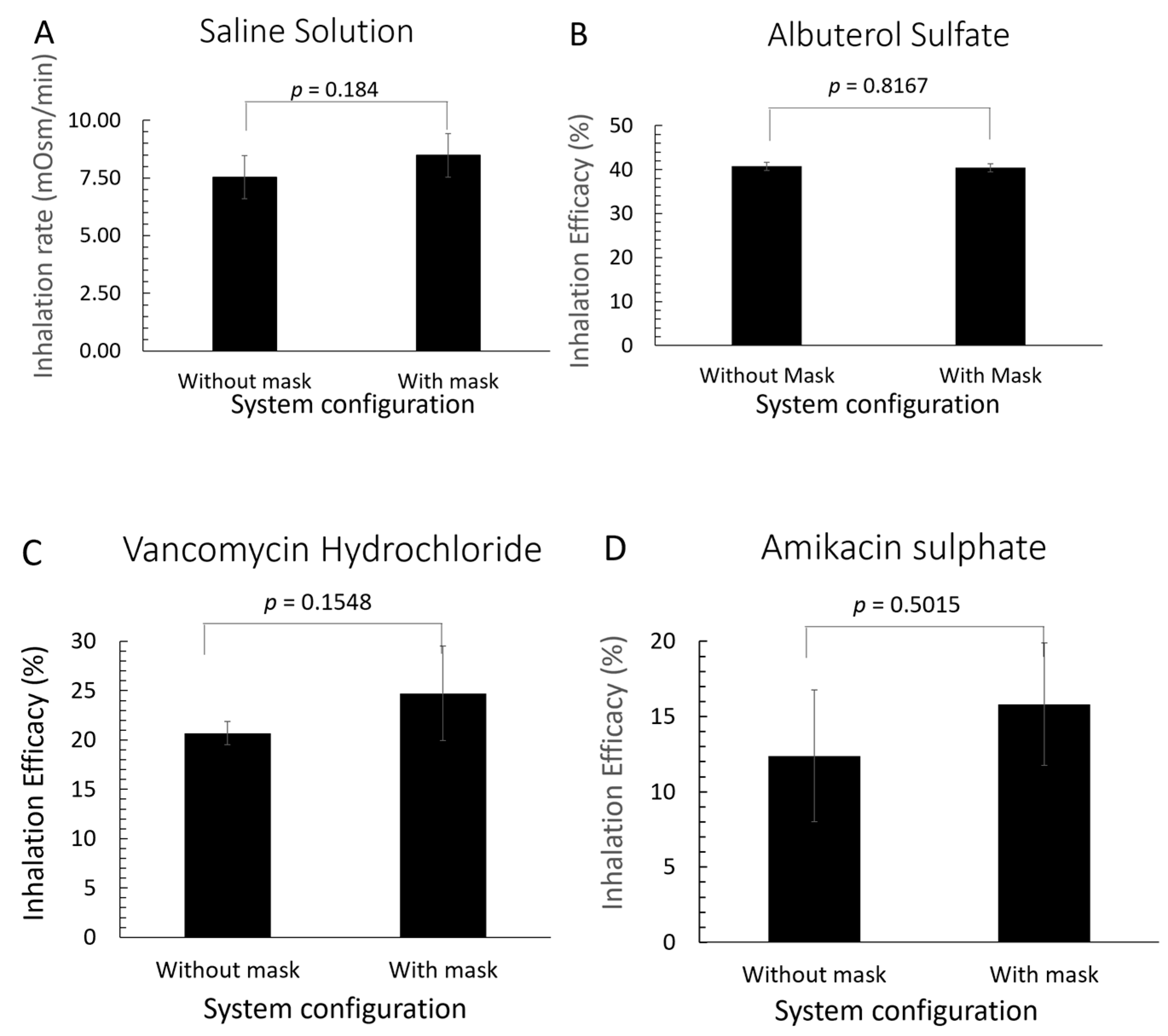
3.2. Drug Delivery Efficacy of Active Mitigation System
4. Discussion
4.1. Aerosol Mitigation Effect
4.2. Impact of Active Aerosol Mitigation on Drug Delivery Efficacy
4.3. Strengths
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGPs | aerosol-generating procedures |
| Neb | nebulization |
| µm | micrometer |
| nm | nanometer |
| cm H2O | centimeter of Water |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
| AIIR | airborne infectious isolation room |
| VMN | vibrating mesh nebulizer |
| HFNC | high-flow nasal cannula |
| IPAP | inspiratory positive airway pressure |
| EPAP | expiratory positive airway pressure |
| NIV | non-invasive ventilation |
| PPE | Personal Protective Equipment |
References
- Zayas, G.; Chiang, M.C.; Wong, E.; MacDonald, F.; Lange, C.F.; Senthilselvan, A.; King, M. Cough aerosol in healthy participants: Fundamental knowledge to optimize droplet-spread infectious respiratory disease management. BMC Pulm. Med. 2012, 12, 11. [Google Scholar] [CrossRef]
- Correia, G.; Rodrigues, L.; Gameiro da Silva, M.; Gonçalves, T. Airborne route and bad use of ventilation systems as non-negligible factors in SARS-CoV-2 transmission. Med. Hypotheses 2020, 141, 109781. [Google Scholar] [CrossRef]
- Dhand, R.; Li, J. Coughs and Sneezes: Their Role in Transmission of Respiratory Viral Infections, Including SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020, 202, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Galbadage, T.; Peterson, B.M.; Gunasekera, R.S. Does COVID-19 Spread Through Droplets Alone? Front. Public Health 2020, 8, 163. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Lo, L.J.; Waring, M.S. Review of indoor aerosol generation, transport, and control in the context of COVID-19. Int. Forum Allergy Rhinol. 2020, 10, 1173–1179. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, J.L.; Rivera, D.N.; Herrera, V.L.; Morwitzer, M.J.; Creager, H.M.; Santarpia, G.W.; Crown, K.K.; Brett-Major, D.M.; Schnaubelt, E.R.; Broadhurst, M.J.; et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci. Rep. 2020, 10, 12732. [Google Scholar] [CrossRef]
- Vuorinen, V.; Aarnio, M.; Alava, M.; Alopaeus, V.; Atanasova, N.; Auvinen, M.; Balasubramanian, N.; Bordbar, H.; Erästö, P.; Grande, R.; et al. Modelling aerosol transport and virus exposure with numerical simulations in relation to SARS-CoV-2 transmission by inhalation indoors. Saf. Sci. 2020, 130, 104866. [Google Scholar] [CrossRef] [PubMed]
- Workman, A.D.; Jafari, A.; Welling, D.B.; Varvares, M.A.; Gray, S.T.; Holbrook, E.H.; Scangas, G.A.; Xiao, R.; Carter, B.S.; Curry, W.T.; et al. Airborne Aerosol Generation During Endonasal Procedures in the Era of COVID-19: Risks and Recommendations. Otolaryngol. Head Neck Surg. 2020, 163, 465–470. [Google Scholar] [CrossRef]
- Escombe, A.R.; Ticona, E.; Chávez-Pérez, V.; Espinoza, M.; Moore, D.A.J. Improving natural ventilation in hospital waiting and consulting rooms to reduce nosocomial tuberculosis transmission risk in a low resource setting. BMC Infect. Dis. 2019, 19, 88. [Google Scholar] [CrossRef]
- Li, Y.; Leung, G.M.; Tang, J.W.; Yang, X.; Chao, C.Y.; Lin, J.Z.; Lu, J.W.; Nielsen, P.V.; Niu, J.; Qian, H.; et al. Role of ventilation in airborne transmission of infectious agents in the built environment - a multidisciplinary systematic review. Indoor Air 2007, 17, 2–18. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Khabsa, J.; Solo, K.; Khamis, A.M.; Brignardello-Petersen, R.; El-Harakeh, A.; Darzi, A.; Hajizadeh, A.; Bognanni, A.; Bak, A.; et al. Ventilation Techniques and Risk for Transmission of Coronavirus Disease, Including COVID-19: A Living Systematic Review of Multiple Streams of Evidence. Ann. Intern. Med. 2020, 173, 204–216. [Google Scholar] [CrossRef]
- Tran, K.; Cimon, K.; Severn, M.; Pessoa-Silva, C.L.; Conly, J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: A systematic review. PLoS ONE 2012, 7, e35797. [Google Scholar] [CrossRef]
- Mousavi, E.S.; Kananizadeh, N.; Martinello, R.A.; Sherman, J.D. COVID-19 Outbreak and Hospital Air Quality: A Systematic Review of Evidence on Air Filtration and Recirculation. Environ. Sci. Technol. 2021, 55, 4134–4147. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Scientific Brief: SARS-CoV-2 Transmission. Available online: https://archive.cdc.gov/www_cdc_gov/coronavirus/2019-ncov/science/science-briefs/sars-cov-2-transmission.html (accessed on 13 July 2025).
- Astuti, I.; Ysrafil. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. 2020, 14, 407–412. [Google Scholar] [CrossRef]
- Kang, S.; Yang, M.; Hong, Z.; Zhang, L.; Huang, Z.; Chen, X.; He, S.; Zhou, Z.; Zhou, Z.; Chen, Q.; et al. Crystal structure of SARS-CoV-2 nucleocapsid protein RNA binding domain reveals potential unique drug targeting sites. Acta Pharm. Sin. B 2020, 10, 1228–1238. [Google Scholar] [CrossRef]
- Reychler, G.; Vecellio, L.; Dubus, J.C. Nebulization: A potential source of SARS-CoV-2 transmission. Respir. Med. Res. 2020, 78, 100778. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.M.; Lang, A.L.; Lozano, R.; Szabo, M.; Smith, S.; Wang, J. Intensive care unit isolation hood decreases risk of aerosolization during noninvasive ventilation with COVID-19. Can. J. Anaesth. 2020, 67, 1481–1483. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Dashti, M.; Tafuro, J.; Nasiri, M.; Muntean, E.A.; Wong, N.; Kemp, T.; Hills, G.; Mallen, C.D. Methods to disinfect and decontaminate SARS-CoV-2: A systematic review of in vitro studies. Ther. Adv. Infect. Dis. 2021, 8, 2049936121998548. [Google Scholar] [CrossRef] [PubMed]
- Rosser, J.I.; Tayyar, R.; Giardina, R.; Kolonoski, P.; Kenski, D.; Shen, P.; Steinmetz, L.M.; Hung, L.Y.; Xiao, W.; Bains, K.; et al. Case-control study evaluating risk factors for SARS-CoV-2 outbreak amongst healthcare personnel at a tertiary care center. Am. J. Infect. Control 2021, 49, 1457–1463. [Google Scholar] [CrossRef]
- Chan, V.W.; Ng, H.H.; Rahman, L.; Tang, A.; Tang, K.P.; Mok, A.; Liu, J.H.P.; Ho, K.S.C.; Chan, S.M.; Wong, S.; et al. Transmission of Severe Acute Respiratory Syndrome Coronavirus 1 and Severe Acute Respiratory Syndrome Coronavirus 2 During Aerosol-Generating Procedures in Critical Care: A Systematic Review and Meta-Analysis of Observational Studies. Crit. Care Med. 2021, 49, 1159–1168. [Google Scholar] [CrossRef]
- Andrés, M.; García, M.C.; Fajardo, A.; Grau, L.; Pagespetit, L.; Plasencia, V.; Martínez, I.; Abadía, C.; Sanahuja, A.; Bella, F. Nosocomial outbreak of COVID-19 in an internal medicine ward: Probable airborne transmission. Rev. Clin. Esp. 2022, 222, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Biney, I.N.; Ari, A.; Barjaktarevic, I.Z.; Carlin, B.; Christiani, D.C.; Cochran, L.; Drummond, M.B.; Johnson, K.; Kealing, D.; Kuehl, P.J.; et al. Guidance on Mitigating the Risk of Transmitting Respiratory Infections During Nebulization by the COPD Foundation Nebulizer Consortium. Chest 2024, 165, 653–668. [Google Scholar] [CrossRef]
- Pirzada, A.R.; Aleissi, S.A.; Almeneessier, A.S.; BaHammam, A.S. Management of Aerosol during Noninvasive Ventilation for Patients with Sleep-Disordered Breathing: Important Messages during the COVID-19 Pandemic. Sleep Vigil. 2020, 4, 89–94. [Google Scholar] [CrossRef]
- Harb, H.S.; Saeed, H.; Madney, Y.M.; Abdelrahman, M.A.; Osama, H.; Esquinas, A.M.; Abdelrahim, M.E.A. Update efficacy of aerosol therapy with noninvasive ventilator approach (non-invasive ventilation and nasal high flow). J. Drug Deliv. Sci. Technol. 2020, 59, 101922. [Google Scholar] [CrossRef]
- Jackson, T.; Deibert, D.; Wyatt, G.; Durand-Moreau, Q.; Adisesh, A.; Khunti, K.; Khunti, S.; Smith, S.; Chan, X.H.S.; Ross, L.; et al. Classification of aerosol-generating procedures: A rapid systematic review. BMJ Open Respir. Res. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Barjaktarevic, I.Z.; Tashkin, D.P. The use of nebulized pharmacotherapies during the COVID-19 pandemic. Ther. Adv. Respir. Dis. 2020, 14, 1753466620954366. [Google Scholar] [CrossRef]
- Leasa, D.; Cameron, P.; Honarmand, K.; Mele, T.; Bosma, K.J.; Arntfield, R.; Basmaji, J.; Bosma, K.J.; Cameron, P.; Dashnay, I.; et al. Knowledge translation tools to guide care of non-intubated patients with acute respiratory illness during the COVID-19 Pandemic. Crit. Care 2021, 25, 22. [Google Scholar] [CrossRef]
- Bin Nafisah, S.A.; Mzahim, B.Y.; Aleid, B.S.; Sheerah, S.A.; Almatrafi, D.Q.; Ciottone, G.R.; AlAnazi, K.H.; Khan, A.A. The risk of coronavirus to healthcare providers during aerosol-generating procedures: A systematic review and meta-analysis. Ann. Thorac. Med. 2021, 16, 165–171. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Drew, D.A.; Graham, M.S.; Joshi, A.D.; Guo, C.G.; Ma, W.; Mehta, R.S.; Warner, E.T.; Sikavi, D.R.; Lo, C.H.; et al. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health 2020, 5, e475–e483. [Google Scholar] [CrossRef]
- Sze To, G.N.; Chao, C.Y. Review and comparison between the Wells-Riley and dose-response approaches to risk assessment of infectious respiratory diseases. Indoor Air 2010, 20, 2–16. [Google Scholar] [CrossRef]
- Edwards, A.J.; King, M.F.; Noakes, C.J.; Peckham, D.; López-García, M. The Wells-Riley model revisited: Randomness, heterogeneity, and transient behaviours. Risk Anal. 2024, 44, 2125–2147. [Google Scholar] [CrossRef] [PubMed]
- Riley, E.C.; Murphy, G.; Riley, R.L. Airborne spread of measles in a suburban elementary school. Am. J. Epidemiol. 1978, 107, 421–432. [Google Scholar] [CrossRef]
- Iwamura, N.; Tsutsumi, K. Predicting the Airborne Transmission of Measles: Impact of Indoor Carbon Dioxide (CO2) Levels and Mitigation Strategies. Cureus 2024, 16, e64882. [Google Scholar] [CrossRef]
- Martinot, M.; Mohseni-Zadeh, M.; Gravier, S.; Ion, C.; Eyriey, M.; Beigue, S.; Coutan, C.; Ongagna, J.C.; Henric, A.; Schieber, A.; et al. Nosocomial Coronavirus Disease 2019 during 2020–2021: Role of Architecture and Ventilation. Healthcare 2023, 12, 46. [Google Scholar] [CrossRef]
- Abi Karam, K.; Hota, P.; Mora, S.J.; Lowell, A.; McKay, K.; Xian, X.; Patel, B.; Forzani, E. Development of a new aerosol barrier mask for mitigation of spread of SARS-CoV-2 and other infectious pathogens. Respir. Med. 2021, 181, 106381. [Google Scholar] [CrossRef]
- Li, J.; Fink, J.B.; Elshafei, A.A.; Stewart, L.M.; Barbian, H.J.; Mirza, S.H.; Al-Harthi, L.; Vines, D.; Ehrmann, S. Placing a mask on COVID-19 patients during high-flow nasal cannula therapy reduces aerosol particle dispersion. ERJ Open Res. 2021, 7. [Google Scholar] [CrossRef]
- Sidler-Moix, A.L.; Di Paolo, E.R.; Dolci, U.; Berger-Gryllaki, M.; Cotting, J.; Pannatier, A. Physicochemical aspects and efficiency of albuterol nebulization: Comparison of three aerosol types in an in vitro pediatric model. Respir. Care 2015, 60, 38–46. [Google Scholar] [CrossRef]
- Simones, M.P.; Loyalka, S.K.; Duffy, C.; MacLoughlin, R.; Tatham, A.; Power, P. Measurement of the size and charge distribution of sodium chloride particles generated by an Aeroneb Pro® pharmaceutical nebulizer. Eur. J. Nanomed. 2014, 6, 29–36. [Google Scholar] [CrossRef]
- Li, J.; A, A.A.; L, J.H.; Fink, J.B.; Dhand, R. Mitigating Fugitive Aerosols During Aerosol Delivery via High-Flow Nasal Cannula Devices. Respir. Care 2022, 67, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Zou, C.; Yang, X.; Zhuang, W.; Huang, Y.; Zheng, X.; Hu, J.; Liao, L.; Yao, Y.; Sun, X.; et al. Nebulized inhalation drug delivery: Clinical applications and advancements in research. J. Mater. Chem. B 2025, 13, 821–843. [Google Scholar] [CrossRef]
- Myrianthefs, P.; Zakynthinos, G.E.; Tsolaki, V.; Makris, D. Aerosolized Antibiotics to Manage Ventilator-Associated Infections: A Comprehensive Review. Antibiotics 2023, 12, 801. [Google Scholar] [CrossRef]
- Harnois, L.J.; Alolaiwat, A.A.; Jing, G.; Fink, J.B.; Dhand, R.; Li, J. Efficacy of Various Mitigation Devices in Reducing Fugitive Emissions from Nebulizers. Respir. Care 2022, 67, 394–403. [Google Scholar] [CrossRef]
- Jayaweera, M.; Perera, H.; Gunawardana, B.; Manatunge, J. Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environ. Res. 2020, 188, 109819. [Google Scholar] [CrossRef] [PubMed]
- Gershon, R.R.M.; Pogorzelska, M.; Qureshi, K.A.; Stone, P.W.; Canton, A.N.; Samar, S.M.; Westra, L.J.; Damsky, M.R.; Sherman, M. Advances in Patient Safety Home Health Care Patients and Safety Hazards in the Home: Preliminary Findings. In Advances in Patient Safety: New Directions and Alternative Approaches (Vol. 1: Assessment); Henriksen, K., Battles, J.B., Keyes, M.A., Grady, M.L., Eds.; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2008. [Google Scholar]
- Ollier, K.; Leppänen, M.; Wu, B.; Yermakov, M.; Newman, N.C.; Reponen, T.; Grinshpun, S.A. Inhalation Exposure and Respiratory Protection of Home Healthcare Workers Administering Aerosolized Medications (Simulation Study). Aerosol Air Qual. Res. 2019, 19, 937–944. [Google Scholar] [CrossRef]
- Munjal, M.; Ahmed, S.M.; Garg, R.; Das, S.; Chatterjee, N.; Mittal, K.; Javeri, Y.; Saxena, S.; Khunteta, S. The Transport Medicine Society Consensus Guidelines for the Transport of Suspected or Confirmed COVID-19 Patients. Indian. J. Crit. Care Med. 2020, 24, 763–770. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeia and National Formulary (USP 47–NF 42). General Chapter <601> Aerosols, Nasal Sprays, Metered-Dose Inhalers, and Dry Powder Inhalers; United States Pharmacopeial Convention: Rockville, MD, USA, 2024. [Google Scholar]
- Dellweg, D.; Kerl, J.; Gena, A.W.; Alsaad, H.; Voelker, C. Exhalation Spreading During Nasal High-Flow Therapy at Different Flow Rates. Crit. Care Med. 2021, 49, e693–e700. [Google Scholar] [CrossRef] [PubMed]


| Aerosol Generating Systems | Testing Conditions | ||
|---|---|---|---|
| 1A No Mitigation | 1B Passive Mitigation: Surgical Mask | 1C Active Mitigation: Aerosol Barrier Mask = Fitted Oronasal Silicone Mask, biofilter, and fan (fan filtration) |
| 2A No Mitigation | 2B Passive Mitigation: Aerosol Mitigation Box | 2C Active Mitigation: Aerosol Mitigation Box with biofilter and fan (fan filtration) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shyamala Pandian, A.; Patel, B.; Abi Karam, K.; Lowell, A.; McKay, K.; Mora, S.J.; Hota, P.; Pyznar, G.; Batchelor, S.; Peworski, C.; et al. Efficacy of Portable Fugitive Aerosol Mitigation Systems for Nebulizer Therapy During High-Flow Nasal Cannula and Non-Invasive Ventilation. Emerg. Care Med. 2025, 2, 36. https://doi.org/10.3390/ecm2030036
Shyamala Pandian A, Patel B, Abi Karam K, Lowell A, McKay K, Mora SJ, Hota P, Pyznar G, Batchelor S, Peworski C, et al. Efficacy of Portable Fugitive Aerosol Mitigation Systems for Nebulizer Therapy During High-Flow Nasal Cannula and Non-Invasive Ventilation. Emergency Care and Medicine. 2025; 2(3):36. https://doi.org/10.3390/ecm2030036
Chicago/Turabian StyleShyamala Pandian, Adithya, Bhavesh Patel, Karam Abi Karam, Amelia Lowell, Kelly McKay, Sabrina Jimena Mora, Piyush Hota, Gabriel Pyznar, Sandra Batchelor, Charles Peworski, and et al. 2025. "Efficacy of Portable Fugitive Aerosol Mitigation Systems for Nebulizer Therapy During High-Flow Nasal Cannula and Non-Invasive Ventilation" Emergency Care and Medicine 2, no. 3: 36. https://doi.org/10.3390/ecm2030036
APA StyleShyamala Pandian, A., Patel, B., Abi Karam, K., Lowell, A., McKay, K., Mora, S. J., Hota, P., Pyznar, G., Batchelor, S., Peworski, C., Rivas, D., Sanghavi, D., Nguyen, N. A., Eltantawy, A., Li, X., Xian, X., Serhan, M., & Forzani, E. (2025). Efficacy of Portable Fugitive Aerosol Mitigation Systems for Nebulizer Therapy During High-Flow Nasal Cannula and Non-Invasive Ventilation. Emergency Care and Medicine, 2(3), 36. https://doi.org/10.3390/ecm2030036








