Surgical Decision-Making for the Treatment of Acute Diverticulitis: A Single-Center Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Hartmann Resection vs. Resection with Primary Anastomosis
3.2. Sigmoidectomy vs. Peritoneal Lavage
3.3. Hartmann Resection vs. Peritoneal Lavage
3.4. Primary Anastomosis vs. Peritoneal Lavage
3.5. Peritoneal Lavage Analysis—Patients with and Without Complications
3.6. Hartmann’s Resection—Patients with and Without Complications
3.7. Sigmoidectomy with Primary Anastomosis—Patients with and Without Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACPGBI | Association of Coloproctology of Great Britain and Ireland |
| ASA | American Society of Anesthesiologists |
| ASCRS | American Society of Colon and Rectal Surgeons |
| BMI | Body Mass Index |
| CRP | C-reactive protein |
| EAE | European Association for Endoscopic Surgery |
| HP | Hartmann’s procedure |
| LPL | Laparoscopic peritoneal lavage |
| MOF | Multi-organ failure |
| PA | Primary anastomosis |
| WSES | World Society of Emergency Surgery |
| WBC | White blood cell |
References
- Strate, L.L.; Morris, A.M. Epidemiology, Pathophysiology, and Treatment of Diverticulitis. Gastroenterology 2019, 156, 5. [Google Scholar] [CrossRef]
- Delvaux, M. Diverticular disease of the colon in Europe: Epidemiology, impact on citizen health and prevention. In Alimentary Pharmacology and Therapeutics, Supplement; Wiley-Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2003; Volume 18. [Google Scholar] [CrossRef]
- Bridoux, V.; Regimbeau, J.M.; Ouaissi, M.; Mathonnet, M.; Mauvais, F.; Houivet, E.; Schwarz, L.; Mege, D.; Sielezneff, I.; Sabbagh, C.; et al. Hartmann’s Procedure or Primary Anastomosis for Generalized Peritonitis due to Perforated Diverticulitis: A Prospective Multicenter Randomized Trial (DIVERTI). J. Am. Coll. Surg. 2017, 225, 798–805. [Google Scholar] [CrossRef]
- Sartelli, M.; Weber, D.G.; Kluger, Y.; Ansaloni, L.; Coccolini, F.; Abu-Zidan, F.; Augustin, G.; Ben-Ishay, O.; Biffl, W.L.; Bouliaris, K.; et al. 2020 update of the WSES guidelines for the management of acute colonic diverticulitis in the emergency setting. World J. Emerg. Surg. 2020, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.K.; Yaqub, S.; Wallon, C.; Blecic, L.; Forsmo, H.M.; Folkesson, J.; Buchwald, P.; Körner, H.; Dahl, F.A.; Øresland, T.; et al. Laparoscopic lavage vs. primary resection for acute perforated diverticulitis: The SCANDIV randomized clinical trial. JAMA—J. Am. Med. Assoc. 2015, 314, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Swank, H.A.; Vermeulen, J.; Lange, J.F.; Mulder, I.M.; van der Hoeven, J.A.B.; Stassen, L.P.S.; Crolla, R.M.P.; Sosef, M.N.; Nienhuijs, S.W.; Bosker, R.J.I.; et al. The LADIES trial: Laparoscopic peritoneal lavage or resection for purulent peritonitis and Hartmann’s procedure or resection with primary anastomosis for purulent or faecal peritonitis in perforated diverticulitis (NTR2037). BMC Surg. 2010, 10, 29. [Google Scholar] [CrossRef]
- Kohl, A.; Rosenberg, J.; Bock, D.; Bisgaard, T.; Skullman, S.; Thornell, A.; Gehrman, J.; Angenete, E.; Haglind, E. Two-year results of the randomized clinical trial DILALA comparing laparoscopic lavage with resection as treatment for perforated diverticulitis. Br. J. Surg. 2018, 105, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- NCT01019239. LapLAND Laparoscopic Lavage for Acute Non-Faeculant Diverticulitis. Published online 2009. Available online: https://clinicaltrials.gov/show/NCT01019239 (accessed on 11 July 2024).
- Gervaz, P.; Ambrosetti, P. Critical appraisal of laparoscopic lavage for Hinchey III diverticulitis. World J. Gastrointest. Surg. 2016, 8, 371–375. [Google Scholar] [CrossRef]
- Hall, J.; Hardiman, K.; Lee, S.; Lightner, A.; Stocchi, L.; Paquette, I.M.; Steele, S.R.; Feingold, D.L. Prepared on behalf of the Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Treatment of Left-Sided Colonic Diverticulitis. Dis. Colon Rectum 2020, 63, 728–747. [Google Scholar] [CrossRef]
- O’Leary, D.P.; Lynch, N.; Clancy, C.; Winter, D.C.; Myers, E. International, expert-based, consensus statement regarding the management of acute diverticulitis. JAMA Surg. 2015, 150, 899–904. [Google Scholar] [CrossRef]
- Miller, A.S.; Boyce, K.; Box, B.; Clarke, M.D.; Duff, S.E.; Foley, N.M.; Guy, R.J.; Massey, L.H.; Ramsay, G.; Slade, D.A.J.; et al. The Association of Coloproctology of Great Britain and Ireland consensus guidelines in emergency colorectal surgery. Color. Dis. 2021, 23, 476–547. [Google Scholar] [CrossRef]
- Schultz, J.K.; Azhar, N.; Binda, G.A.; Barbara, G.; Biondo, S.; Boermeester, M.A.; Chabok, A.; Consten, E.C.J.; van Dijk, S.T.; Johanssen, A.; et al. European Society of Coloproctology: Guidelines for the management of diverticular disease of the colon. Color. Dis. 2020, 22, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Gachabayov, M.; Oberkofler, C.E.; Tuech, J.J.; Hahnloser, D.; Bergamaschi, R. Resection with primary anastomosis vs. nonrestorative resection for perforated diverticulitis with peritonitis: A systematic review and meta-analysis. Color. Dis. 2018, 20, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Vennix, S.; Musters, G.D.; Mulder, I.M.; Swank, H.A.; Belgers, E.H.; van Geloven, A.A.; Govaert, M.J.; van Grevenstein, W.M.; van Geloven, A.A.; Gerhards, M.F.; et al. Laparoscopic peritoneal lavage or sigmoidectomy for perforated diverticulitis with purulent peritonitis: A multicentre, parallel-group, randomised, open-label trial. Lancet 2015, 386, 1269–1277. [Google Scholar] [CrossRef]
- Lambrichts, D.P.V.; Vennix, S.; Musters, G.D.; Mulder, I.M.; Swank, H.A.; Hoofwijk, A.G.M.; Belgers, E.H.J.; Stockmann, H.B.A.C.; Eijsbouts, Q.A.J.; Gerhards, M.F.; et al. Hartmann’s procedure versus sigmoidectomy with primary anastomosis for perforated diverticulitis with purulent or faecal peritonitis (LADIES): A multicentre, parallel-group, randomised, open-label, superiority trial. Lancet Gastroenterol. Hepatol. 2019, 4, 599–610. [Google Scholar] [CrossRef]
- Toro, A.; Mannino, M.; Reale, G.; Cappello, G.; Di Carlo, I. Primary anastomosis vs. Hartmann procedure in acute complicated diverticulitis. Evolution over the last twenty years. Chirurgia 2012, 107, 598–604. [Google Scholar] [PubMed]
- Uematsu, D.; Akiyama, G.; Magishi, A.; Sano, T.; Niitsu, H.; Narita, M.; Komatsu, H. Laparoscopic Hartmann’s procedure for fecal peritonitis resulting from perforation of the left-sided colon in elderly and severely ill patients. Tech. Coloproctol. 2012, 16, 243–246. [Google Scholar] [CrossRef]
- Costantini, R. Hartmann’s procedure for complicated diverticulitis: A critical reappraisal. Open Anesth. J. 2019, 13, 121–131. [Google Scholar] [CrossRef]
- Shaban, F.; Carney, K.; McGarry, K.; Holtham, S. Perforated diverticulitis: To anastomose or not to anastomose? A systematic review and meta-analysis. Int. J. Surg. 2018, 58, 11–21. [Google Scholar] [CrossRef]
- Ahmadi, N.; Howden, W.B.; Ahmadi, N.; Byrne, C.M.; Young, C.J. Increasing primary anastomosis rate over time for the operative management of acute diverticulitis. ANZ J. Surg. 2019, 89, 1080–1084. [Google Scholar] [CrossRef]
- Lee, J.M.; Bai PChang, J.; el Hechi, M.; Kongkaewpaisan, N.; Bonde, A.; Mendoza, A.; Saillant, N.; Fagenholz, P.; Velmahos, G.; Kaafarani, H. Hartmann’s Procedure vs. Primary Anastomosis with Diverting Loop Ileostomy for Acute Diverticulitis: Nationwide Analysis of 2,729 Emergency Surgery Patients. J. Am. Coll. Surg. 2019, 229, 48–55. [Google Scholar] [CrossRef]
- Cauley, C.E.; Patel, R.; Bordeianou, L. Use of primary anastomosis with diverting ileostomy in patients with acute diverticulitis requiring urgent operative intervention. Dis. Colon Rectum 2018, 61, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Quinn, S.; Akram, W.; Hao, S.; Honaker, M.D. Emergency Surgery for Diverticulitis: Relationship of Outcomes to Patient Age and Surgical Procedure. J. Am. Med. Dir. Assoc. 2022, 23, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Acuna, S.A.; Dossa, F.; Baxter, N.N. The end of the Hartmann’s era for perforated diverticulitis. Lancet Gastroenterol. Hepatol. 2019, 4, 573–575. [Google Scholar] [CrossRef]
- Cirocchi, R.; Sapienza, P.; Anania, G.; Binda, G.A.; Avenia, S.; di Saverio, S.; Tebala, G.D.; Zago, M.; Donini, A.; Mingoli, A.; et al. State-of-the-art surgery for sigmoid diverticulitis. Langenbecks Arch. Surg. 2022, 407, 1–14. [Google Scholar] [CrossRef]
- Tsujinaka, S.; Konishi, F.; Kawamura, Y.J.; Saito, M.; Tajima, N.; Tanaka, O.; Lefor, A.T. Visceral obesity predicts surgical outcomes after laparoscopic colectomy for sigmoid colon cancer. Dis. Colon Rectum 2008, 51, 1757–1767. [Google Scholar] [CrossRef]
- Ri, M.; Aikou, S.; Seto, Y. Obesity as a surgical risk factor. Ann. Gastroenterol. Surg. 2018, 2, 13–21. [Google Scholar] [CrossRef]
- Matsubara, N.; Miyata, H.; Gotoh, M.; Tomita, N.; Baba, H.; Kimura, W.; Nakagoe, T.; Simada, M.; Kitagawa, Y.; Sugihara, K.; et al. Mortality after common rectal surgery in Japan: A study on low anterior resection from a newly established nationwide large-scale clinical database. Dis. Colon Rectum 2014, 57, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Ri, M.; Miyata, H.; Aikou, S.; Seto, Y.; Akazawa, K.; Takeuchi, M.; Matsui, Y.; Konno, H.; Gotoh, M.; Mori, M.; et al. Effects of body mass index (BMI) on surgical outcomes: A nationwide survey using a Japanese web-based database. Surg. Today 2015, 45, 1271–1279. [Google Scholar] [CrossRef]
- Govaert, J.A.; Lijftogt, N.; Van Dijk, W.A.; Tseng, L.N.L.; Liem, R.S.L.; Tollenaar, R.A.E.M.; Fiocco, M.; Wouters, M.W.J.M.; On behalf of the Dutch Value Based Healthcare Study Group. Colorectal cancer surgery for obese patients: Financial and clinical outcomes of a Dutch population-based registry. J. Surg. Oncol. 2016, 113, 489–495. [Google Scholar] [CrossRef]
- Thelwall, S.; Harrington, P.; Sheridan, E.; Lamagni, T. Impact of obesity on the risk of wound infection following surgery: Results from a nationwide prospective multicentre cohort study in England. Clin. Microbiol. Infect. 2015, 21, 1008.E1–1008.E8. [Google Scholar] [CrossRef] [PubMed]
- Anaya, D.A.; Dellinger, E.P. The obese surgical patient: A susceptible host for infection. Surg. Infect. 2006, 7, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Greilsamer, T.; Abet, E.; Meurette, G.; Comy, M.; Hamy, A.; Lehur, P.-A.; Venara, A.; Duchalais, E. Is the Failure of Laparoscopic Peritoneal Lavage Predictable in Hinchey III Diverticulitis Management? Dis. Colon Rectum 2017, 60, 965–970. [Google Scholar] [CrossRef]
- Radé, F.; Bretagnol, F.; Auguste, M.; di Guisto, C.; Huten, N.; de Calan, L. Determinants of outcome following laparoscopic peritoneal lavage for perforated diverticulitis. Br. J. Surg. 2014, 101, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
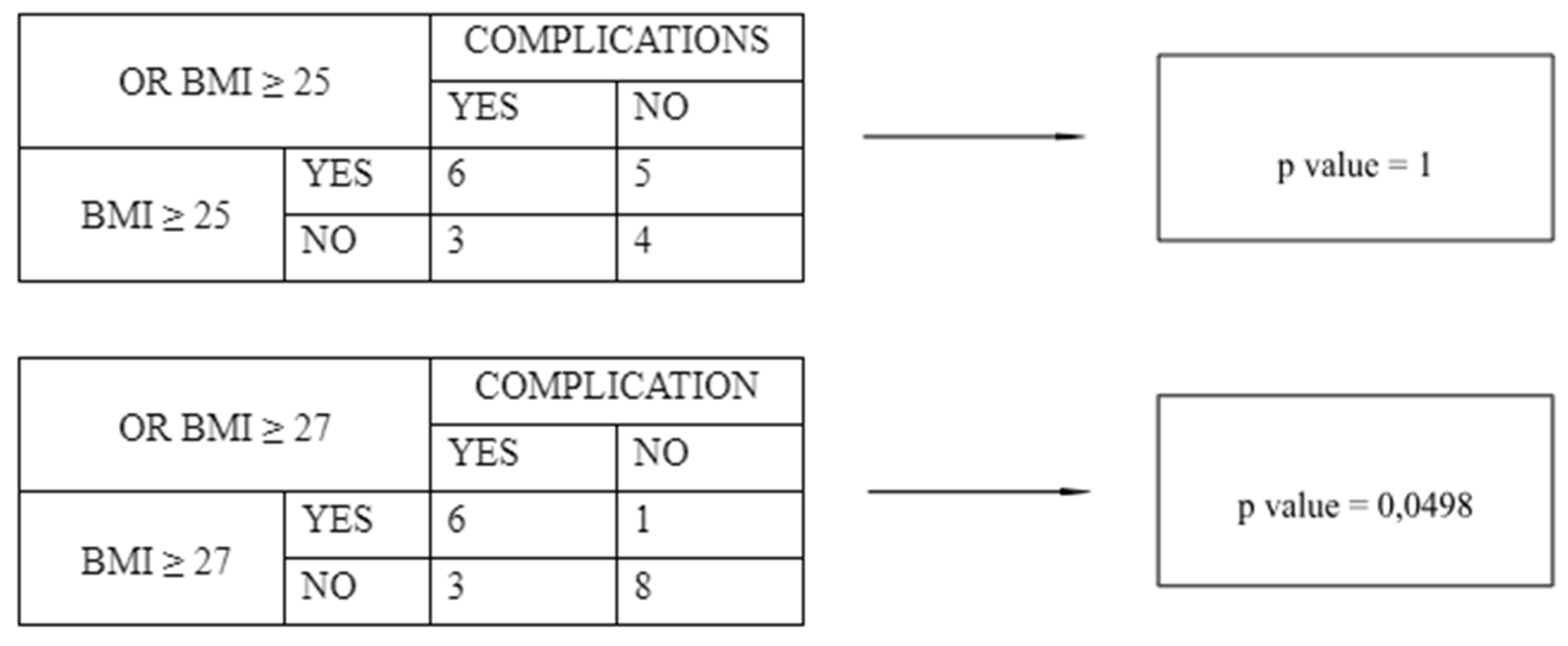

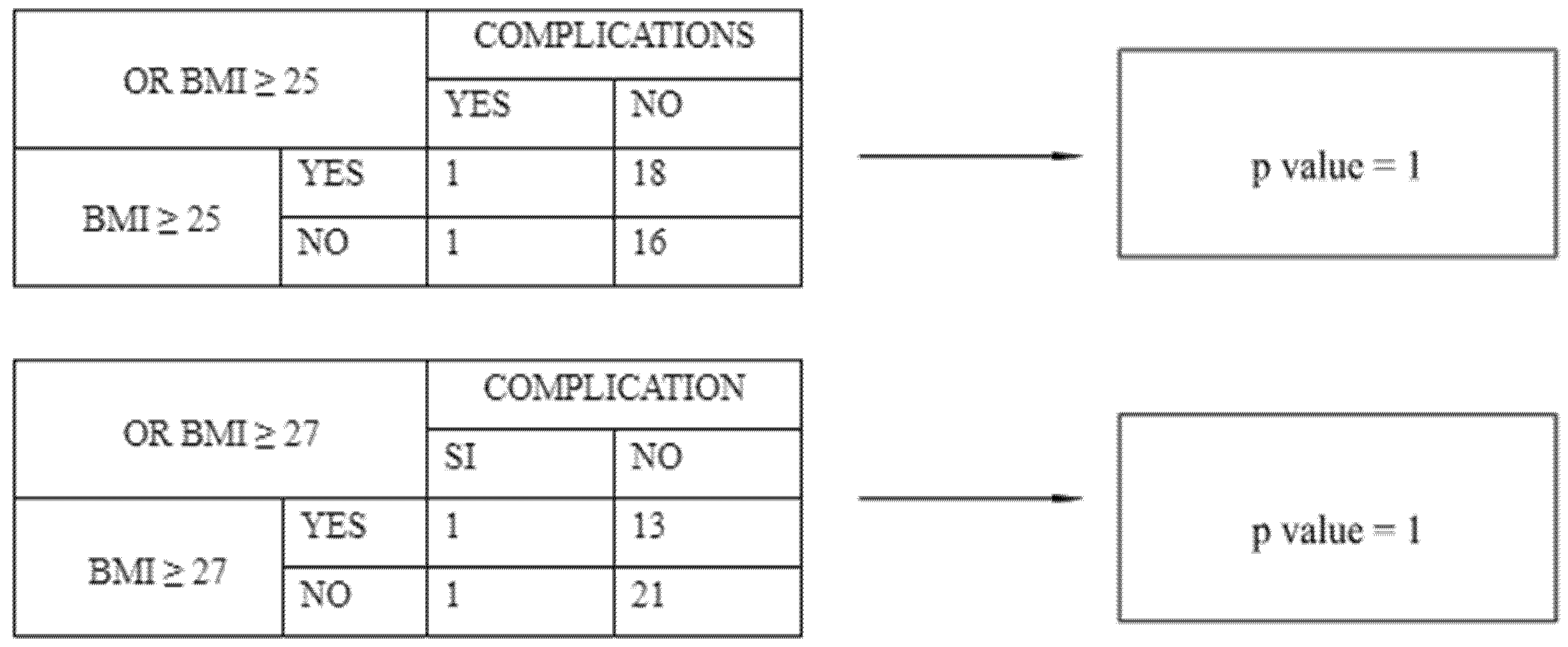
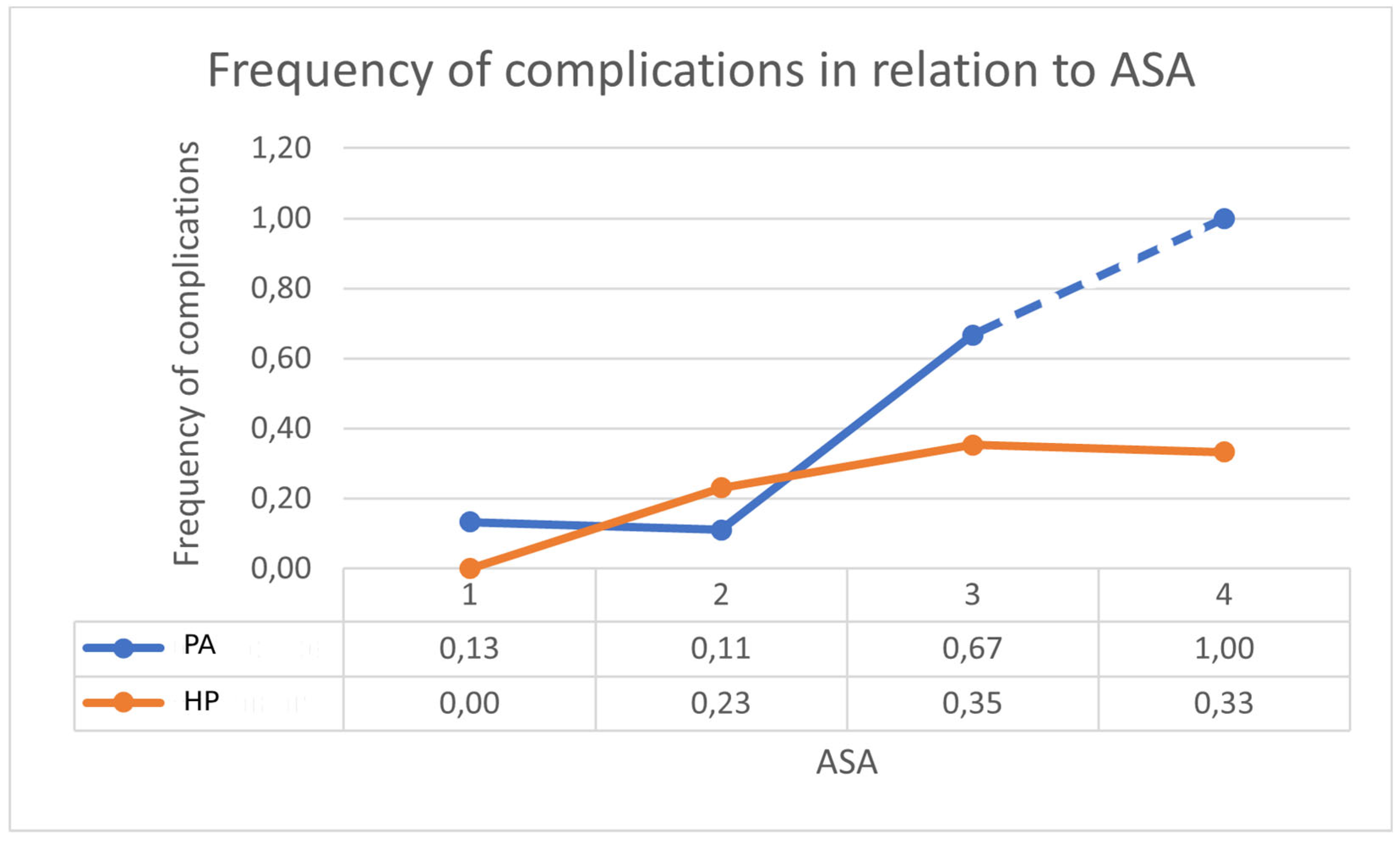
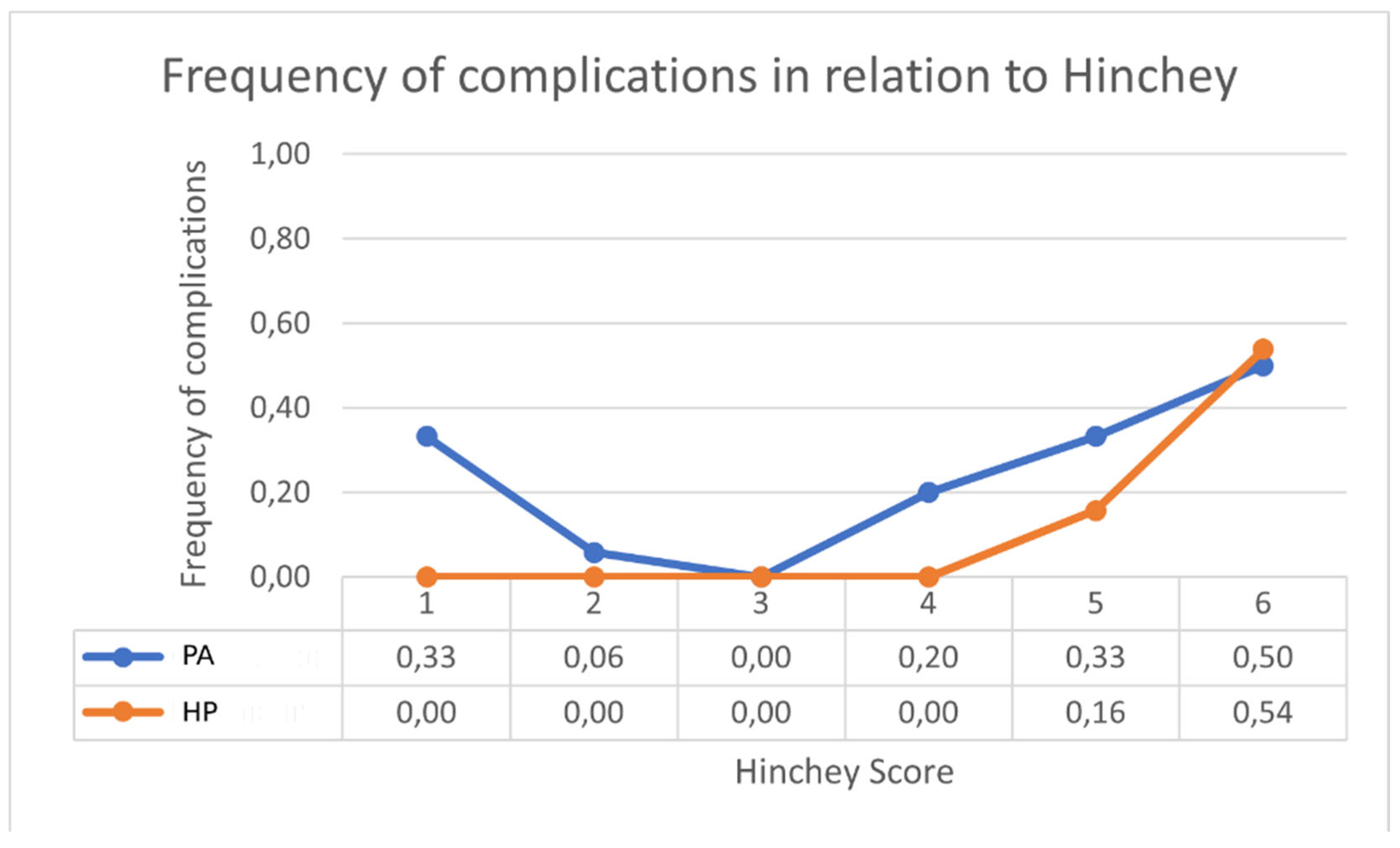
| Characteristics | HP (n = 37) | PA (n = 36) | LPL (n = 18) |
|---|---|---|---|
| Sex ratio (male to female) | 18:19 | 19:17 | 12:6 |
| Age (years, mean ± SD) | 69.89 ± 10.73 | 60.22 ± 12.76 | 61.33 ± 14.33 |
| BMI (kg/m2, mean ± SD) | 26.56 ± 4.77 | 26.66 ± 4.15 | 26.25 ± 4.83 |
| Previous abdominal surgery (n) | |||
| None | 16 | 11 | 8 |
| N° > 1 | 21 | 25 | 10 |
| Previous episodes of diverticulitis (n) | |||
| None | 33 | 11 | 15 |
| N° = 1 | 4 | 14 | 3 |
| N° > 1 | 0 | 11 | 0 |
| Charlson Comorbidity Index (mean ± SD) | 4.45 ± 2.44 | 2.08 ± 1.91 | 2.55 ± 2.93 |
| WBC (109/L, mean ± SD) | 15.11 ± 12.66 | 12.25 ± 14.21 | 17.24 ± 5.72 |
| CRP (mg/L, mean ± SD) | 188.94 ± 152.88 | 60.03 ± 80.22 | 124.85 ± 90.86 |
| American Society of Anesthesiologists (ASA) | |||
| 1 | 4 | 15 | 9 |
| 2 | 13 | 18 | 6 |
| 3 | 17 | 3 | 3 |
| 4 | 3 | 0 | 0 |
| Hinchey classification | |||
| 0 | 0 | 3 | 0 |
| 1a | 1 | 17 | 0 |
| 1b | 0 | 3 | 0 |
| 2 | 4 | 5 | 5 |
| 3 | 19 | 6 | 13 |
| 4 | 13 | 2 | 0 |
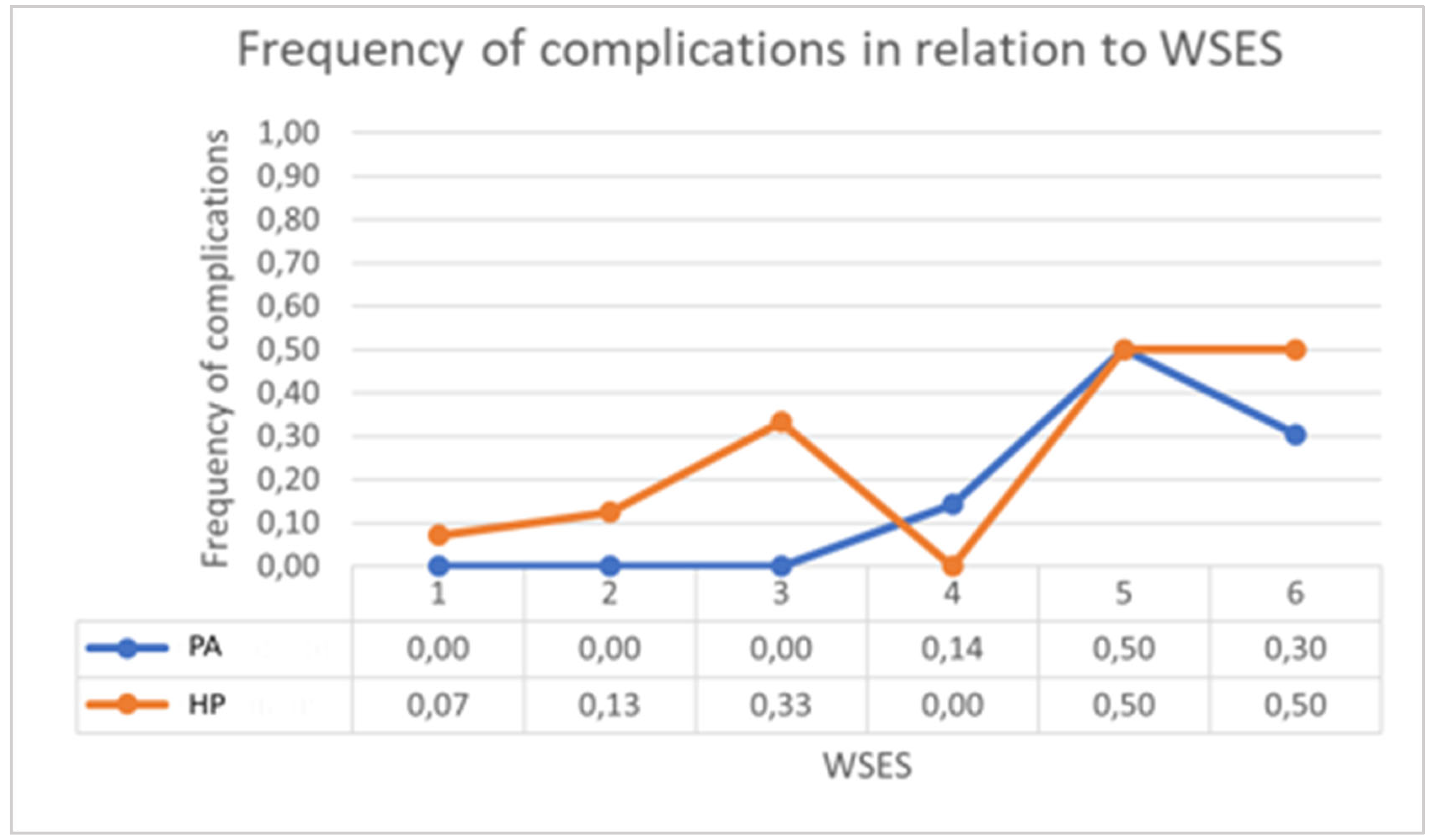
| WSES classification | |||
| 0 | 0 | 14 | 0 |
| 1a | 1 | 8 | 4 |
| 1b | 0 | 3 | 1 |
| 2a | 7 | 2 | 1 |
| 2b | 4 | 3 | 7 |
| 3 | 2 | 2 | 2 |
| 4 | 23 | 4 | 3 |
| Outcome | HP (n = 37) | PA (n = 36) | LPL (n = 18) | p Value (HP vs. PA) | p Value (HP vs. LPL) | p Value (PA vs. LPL) |
|---|---|---|---|---|---|---|
| Severe complications (Clavien–Dindo ≥ 2) | 10 | 6 | 6 | 0.3975 | 0.0199 | 0.0017 |
| Patients alive with stoma | 28 | 1 | 0 | <0.0001 | 0.0004 | 1 |
| Secondary reoperations | 4 | 2 | 9 | 0.6741 | 0.0025 | 0.0003 |
| Antibiotic therapy (days, mean ± SD) | 13.24 ± 7.91 | 12.83 ± 13.37 | 19.72 ± 19.74 | 0.8730 | 0.0874 | 0.1348 |
| Operative time (minutes, mean ± SD) | 149.29 ± 43.27 | 179 ± 59.05 | 68.94 ± 20.69 | 0.0137 | <0.0001 | <0.0001 |
| Canalization (days, mean ± SD) | 4.18 ± 2.69 | 2.33 ± 1.65 | 4 ± 9.38 | <0.001 | 0.9097 | 0.3019 |
| Total hospital stay (days, mean ± SD) | 19.86 ± 26.21 | 10.08 ± 8.55 | 14.33 ± 14.78 | 0.0361 | 0.4098 | 0.1861 |
| Diverticulitis recurrence | 0 | 0 | 5 | 1 | 0.0025 | 0.0027 |
| Readmissions | 0 | 0 | 5 | 1 | 0.0025 | 0.0027 |
| Complications | HP (n = 10) | PA (n = 6) | LPL (n =11) | p Value (HP vs. LPL) | p Value (PA vs. LPL) | p Value (HP vs. PA) |
|---|---|---|---|---|---|---|
| American Society of Anesthesiologists (ASA) | ||||||
| 1 | 0/4 | 2/15 | 4/9 | 0.2280 | 0.1501 | 1 |
| 2 | 3/13 | 2/18 | 5/6 | 0.0408 | 0.0027 | 0.6254 |
| 3 | 6/17 | 2/3 | 2/3 | 0.2154 | 1 | 0.5368 |
| 4 | 1/3 | 0 | 0 | 1 | 1 | 1 |
| Hinchey Classification | ||||||
| 0 | 0/0 | 1/3 | 0 | 1 | 1 | 1 |
| 1a | 0/1 | 1/17 | 0 | 1 | 1 | 1 |
| 1b | 0/0 | 0/3 | 0 | 1 | 1 | 1 |
| 2 | 0/4 | 1/5 | 3/5 | 0.1667 | 1 | 1 |
| 3 | 3/19 | 2/6 | 8/13 | 0.0205 | 0.3498 | 0.5623 |
| 4 | 7/13 | 1/2 | 0 | 1 | 1 | 1 |
| WSES Classification | ||||||
| 0 | 0/0 | 1/14 | 0 | 1 | 1 | 1 |
| 1a | 0/1 | 1/8 | 3/4 | 0.4000 | 0.0667 | 1 |
| 1b | 0/0 | 1/3 | 1/1 | 1 | 1 | 1 |
| 2a | 1/7 | 0/2 | 0/1 | 1 | 1 | 1 |
| 2b | 1/4 | 0/3 | 4/7 | 0.5455 | 0.2000 | 1 |
| 3 | 1/2 | 1/2 | 1/2 | 1 | 1 | 1 |
| 4 | 7/23 | 2/4 | 2/3 | 0.2677 | 1 | 0.5815 |
| Characteristic | HP + PA (n = 73) | LPL (n = 18) | p Value |
|---|---|---|---|
| Sex ratio (male to female) | 37:36 | 12:6 | 0.2939 |
| Age (years, mean ± SD) | 65.12 ± 12.62 | 61.33 ± 14.33 | 0.2698 |
| BMI (kg/m2, mean ± SD) | 26.61 ± 4.44 | 26.25 ± 4.83 | 0.7629 |
| Previous abdominal surgery (n) | |||
| None | 27 | 8 | 0.5962 |
| N° > 1 | 46 | 10 | 0.5962 |
| Previous episodes of diverticulitis (n) | |||
| None | 44 | 15 | 0.0976 |
| N° = 1 | 18 | 3 | 0.5507 |
| N° > 1 | 11 | 0 | 0.1127 |
| Charlson Comorbidity Index (mean ± SD) | 3.28 ± 2.49 | 2.55 ± 2.93 | 0.2842 |
| WBC (109/L, mean ± SD) | 13.70 ± 13.43 | 17.24 ± 5.72 | 0.2786 |
| CRP (mg/L, mean ± SD) | 125.39 ± 137.94 | 124.85 ± 90.86 | 0.9874 |
| American Society of Anesthesiologists (ASA) | |||
| 1 | 19 | 9 | 0.0842 |
| 2 | 31 | 6 | 0.5958 |
| 3 | 20 | 3 | 0.5456 |
| 4 | 3 | 0 | 1 |
| Hinchey classification | |||
| 0 | 3 | 0 | 1 |
| 1a | 18 | 0 | 0.0185 |
| 1b | 3 | 0 | 1 |
| 2 | 9 | 5 | 0.1415 |
| 3 | 25 | 13 | 0.0065 |
| 4 | 15 | 0 | 0.0358 |
| WSES classification | |||
| 0 | 14 | 0 | 0.0636 |
| 1a | 9 | 4 | 0.2790 |
| 1b | 3 | 1 | 1 |
| 2a | 9 | 1 | 0.6800 |
| 2b | 7 | 7 | 0.0056 |
| 3 | 4 | 2 | 0.3390 |
| 4 | 27 | 3 | 0.1605 |
| Outcome | HP + PA (n = 73) | LPL (n = 18) | p Value |
|---|---|---|---|
| Severe complications (Clavien–Dindo ≥ 2) | 16 | 11 | 0.0028 |
| Patients alive with stoma | 29 | 0 | 0.0005 |
| Secondary reoperations | 6 | 9 | 0.0002 |
| Duration of antibiotic therapy (days, mean ± SD) | 13.04 ± 10.84 | 19.72 ± 19.74 | 0.0543 |
| Operative time (minutes, mean ± SD) | 164.37 ± 53.55 | 68.94 ± 20.69 | <0.0001 |
| Canalization (days, mean ± SD) | 3.27 ± 2.47 | 4 ± 9.38 | 0.5557 |
| Total hospital stay (days, mean ± SD) | 15.04 ± 20.08 | 14.33 ± 14.78 | 0.8888 |
| Diverticulitis recurrence | 0 | 5 | 0.0002 |
| Readmissions | 0 | 5 | 0.0002 |
| Complications | HP + PA (n = 16) | LPL (n = 11) | p Value |
|---|---|---|---|
| American Society of Anesthesiologists (ASA) | |||
| 1 | 2/19 | 4/9 | 0.0638 |
| 2 | 5/31 | 5/6 | 0.0030 |
| 3 | 8/20 | 2/3 | 0.5596 |
| 4 | 1/3 | 0 | 1 |
| Hinchey classification | |||
| 0 | 1/3 | 0 | 1 |
| 1a | 1/18 | 0 | 1 |
| 1b | 0/3 | 0 | 1 |
| 2 | 1/9 | 3/5 | 0.0949 |
| 3 | 5/25 | 8/13 | 0.0279 |
| 4 | 8/15 | 0 | 1 |
| WSES classification | |||
| 0 | 1/14 | 0 | 1 |
| 1a | 1/9 | 3/4 | 0.0517 |
| 1b | 1/3 | 1/1 | 1 |
| 2a | 1/9 | 0/1 | 1 |
| 2b | 1/7 | 4/7 | 0.2657 |
| 3 | 2/4 | 1/2 | 1 |
| 4 | 9/27 | 2/3 | 0.5367 |
| NON-COMPLICATED (n = 9) | Characteristic | COMPLICATED (n = 9) | p Value |
|---|---|---|---|
| 6:3 | Sex ratio (male to female) | 6:3 | 1 |
| 64.55 ± 18.29 | Age (years, mean ± SD) | 58.11 ± 8.86 | 0.3556 |
| 23.98 ± 2.92 | BMI (kg/m2, mean ± SD) | 28.53 ± 5.41 | 0.0419 |
| Previous abdominal surgery (n) | |||
| 5 | None | 3 | 0.6372 |
| 4 | N° > 1 | 6 | 0.6372 |
| Previous episodes of diverticulitis (n) | |||
| 7 | None | 8 | 1 |
| 2 | N° = 1 | 1 | 1 |
| 0 | N° > 1 | 0 | 1 |
| 2.00 ± 1.87 | Charlson Comorbidity Index (mean ± SD) | 3.11 ± 3.75 | 0.4386 |
| 17.92 ± 5.67 | WBC (109/L, mean ± SD) | 16.56 ± 6.03 | 0.6286 |
| 123.25 ± 92.04 | CRP (mg/L, mean ± SD) | 126.45 ± 95.21 | 0.9431 |
| American Society of Anesthesiologists (ASA) | |||
| 3 | 1 | 4 | 1 |
| 5 | 2 | 3 | 0.6372 |
| 1 | 3 | 2 | 1 |
| 0 | 4 | 0 | 1 |
| Hinchey classification | |||
| 0 | 0 | 0 | 1 |
| 0 | 1a | 0 | 1 |
| 0 | 1b | 0 | 1 |
| 2 | 2 | 3 | 1 |
| 7 | 3 | 6 | 1 |
| 0 | 4 | 0 | 1 |
| WSES classification | |||
| 0 | 0 | 0 | 1 |
| 2 | 1a | 2 | 1 |
| 0 | 1b | 1 | 1 |
| 1 | 2a | 0 | 1 |
| 3 | 2b | 3 | 1 |
| 2 | 3 | 1 | 1 |
| 1 | 4 | 2 | 1 |
| 67.55 ± 14.62 | Operative time (minutes, mean ± SD) | 70.33 ± 26.30 | 0.7854 |
| 0 | Use of immunosuppressive drugs | 2 | 0.4706 |
| 4 | Smoker | 1 | 0.2941 |
| 2.89 ± 1.36 | Reintroduction of foods (days, mean ± SD) | 3.50 ± 1.41 | 0.3791 |
| NON-COMPLICATED (n = 32) | Characteristic | COMPLICATED (n = 5) | p Value |
|---|---|---|---|
| 15:17 | Sex ratio (male to female) | 3:2 | 0.6599 |
| 69.53 ± 10.89 | Age (years, mean ± SD) | 72.20 ± 10.47 | 0.6122 |
| 26.49 ± 4.68 | BMI (kg/m2, mean ± SD) | 26.92 ± 5.98 | 0.8556 |
| Previous abdominal surgery (n) | |||
| 13 | None | 3 | 0.6339 |
| 19 | N° > 1 | 2 | 0.6339 |
| Previous episodes of diverticulitis (n) | |||
| 30 | None | 3 | 0.0800 |
| 2 | N° = 1 | 2 | 0.0800 |
| 0 | N° > 1 | 0 | 0.0800 |
| 4.25 ± 2.47 | Charlson Comorbidity Index (mean ± SD) | 5.80 ± 1.92 | 0.1913 |
| 29.60 ± 84.49 | WBC (109/L, mean ± SD) | 10.25 ± 4.89 | 0.6160 |
| 177.39 ± 138.30 | CRP (mg/L, mean ± SD) | 263.18 ± 232.73 | 0.2487 |
| American Society of Anesthesiologists (ASA) | |||
| 4 | 1 | 0 | 1 |
| 12 | 2 | 1 | 0.6378 |
| 14 | 3 | 3 | 0.6443 |
| 2 | 4 | 1 | 0.3616 |
| Hinchey classification | |||
| 0 | 0 | 0 | 1 |
| 1 | 1a | 0 | 1 |
| 0 | 1b | 0 | 1 |
| 4 | 2 | 0 | 1 |
| 18 | 3 | 1 | 0.1797 |
| 9 | 4 | 4 | 0.0423 |
| WSES classification | |||
| 0 | 0 | 0 | 1 |
| 1 | 1a | 0 | 1 |
| 0 | 1b | 0 | 1 |
| 7 | 2a | 0 | 1 |
| 4 | 2b | 0 | 1 |
| 1 | 3 | 1 | 0.2553 |
| 19 | 4 | 4 | 0.6303 |
| 146.56 ± 43.27 | Operative time (minutes, mean ± SD) | 166.80 ± 43.52 | 0.3377 |
| NON-COMPLICATED (n = 34) | Characteristic | COMPLICATED (n = 2) | p Value |
|---|---|---|---|
| 18:16 | Sex ratio (male to female) | 1:1 | 1 |
| 60.35 ± 12.98 | Age (years, mean ± SD) | 58.00 ± 7.07 | 0.8028 |
| 26.67 ± 4.24 | BMI (kg/m2, mean ± SD) | 26.91 ± 2.35 | 0.9364 |
| Previous abdominal surgery (n) | |||
| 11 | None | 0 | 1 |
| 23 | N° > 1 | 2 | 1 |
| Previous episodes of diverticulitis (n) | |||
| 11 | None | 0 | 1 |
| 14 | N° = 1 | 0 | 1 |
| 9 | N° > 1 | 2 | 1 |
| 2.00 ± 1.93 | Charlson Comorbidity Index (mean ± SD) | 3.50 ± 0.70 | 0.2889 |
| 12.23 ± 14.63 | WBC (109/L, mean ± SD) | 12.50 ± 0.70 | 0.9800 |
| 61 ± 82.02 | CRP (mg/L, mean ± SD) | 43.65 ± 51.40 | 0.7710 |
| American Society of Anesthesiologists (ASA) | |||
| 13 | 1 | 1 | 1 |
| 18 | 2 | 0 | 0.4857 |
| 1 | 3 | 1 | 0.1095 |
| 0 | 4 | 0 | 1 |
| Hinchey classification | |||
| 2 | 0 | 1 | 0.1619 |
| 16 | 1a | 1 | 1 |
| 3 | 1b | 0 | 1 |
| 5 | 2 | 0 | 1 |
| 6 | 3 | 0 | 1 |
| 2 | 4 | 0 | 1 |
| WSES classification | |||
| 13 | 0 | 1 | 1 |
| 7 | 1a | 1 | 0.4000 |
| 3 | 1b | 0 | 1 |
| 2 | 2a | 0 | 1 |
| 3 | 2b | 0 | 1 |
| 2 | 3 | 0 | 1 |
| 4 | 4 | 0 | 1 |
| 178.41 ± 59.99 | Operative time (minutes, mean ± SD) | 204.50 ± 44.54 | 0.5514 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inversini, D.; El Adla, S.; Vigezzi, A.; Gianazza, S.; Morabito, M.; Rizzi, A.; Palillo, A.; Ietto, G.; Carcano, G. Surgical Decision-Making for the Treatment of Acute Diverticulitis: A Single-Center Retrospective Study. Emerg. Care Med. 2025, 2, 33. https://doi.org/10.3390/ecm2030033
Inversini D, El Adla S, Vigezzi A, Gianazza S, Morabito M, Rizzi A, Palillo A, Ietto G, Carcano G. Surgical Decision-Making for the Treatment of Acute Diverticulitis: A Single-Center Retrospective Study. Emergency Care and Medicine. 2025; 2(3):33. https://doi.org/10.3390/ecm2030033
Chicago/Turabian StyleInversini, Davide, Sara El Adla, Andrea Vigezzi, Simone Gianazza, Marika Morabito, Andrea Rizzi, Andrea Palillo, Giuseppe Ietto, and Giulio Carcano. 2025. "Surgical Decision-Making for the Treatment of Acute Diverticulitis: A Single-Center Retrospective Study" Emergency Care and Medicine 2, no. 3: 33. https://doi.org/10.3390/ecm2030033
APA StyleInversini, D., El Adla, S., Vigezzi, A., Gianazza, S., Morabito, M., Rizzi, A., Palillo, A., Ietto, G., & Carcano, G. (2025). Surgical Decision-Making for the Treatment of Acute Diverticulitis: A Single-Center Retrospective Study. Emergency Care and Medicine, 2(3), 33. https://doi.org/10.3390/ecm2030033







