1. Introduction
Trichobezoars are conglomerates of hair and other indigestible materials, and they represent a rare yet intriguing entity encountered in clinical practice. They may arise in association with psychiatric disorders such as trichotillomania and trichophagia. While they commonly manifest as a mass within the stomach or small intestine, their presentation can vary widely, leading to diagnostic challenges and potential mimicry of other gastrointestinal conditions, including intestinal obstruction or malabsorption and even inflammatory bowel disease (IBD). The diagnostic workup for IBD often involves a combination of clinical assessment, endoscopic evaluation, and histopathological examination of tissue samples. However, in this unique instance, the clinical presentation of trichobezoar may closely resemble that of IBD, leading to diagnostic confusion and potential delays in appropriate management. Herein, a unique case of an indigestible trichobezoar masquerading as inflammatory bowel disease has been reported, along with a comprehensive review of the existing literature on trichobezoars, focusing on cases with atypical presentations.
The aim of this manuscript is to highlight the importance of considering uncommon etiologies in the differential diagnosis of gastrointestinal disorders and to elucidate the clinical features that may aid in distinguishing trichobezoars from inflammatory bowel disease.
2. Case Description
A 15-year-old girl was admitted to our Pediatric Emergency Department (PED) with a four-week history of abdominal discomfort, anorexia, diarrhea, emesis, weight loss, and low-grade fever.
The patient had previously been admitted to a non-referral, spoke hospital where blood tests showed microcytic hypochromic anemia with a normal white blood cell count and slightly increased C-Reactive Protein levels.
Table 1 shows the main blood chemistry tests.
Abdominal ultrasonography revealed no pathological findings with no inflammation or thickening of the intestinal loops. At our evaluation in the PED, physical examination was unremarkable, except for epigastric tenderness. Parents reported an eight kg weight loss in a few weeks. Blood tests confirmed iron deficiency anemia and hypoalbuminemia. Abdominal ultrasonography was repeated to confirm the absence of pathological findings. Abdominal radiography revealed no free air or hydroaerial levels. Broad-spectrum stool cultures were negative; thus, IBD was suspected, and the patient was hospitalized. In the following days, the patient showed no clinical improvement, with persistent post-prandial vomiting, non-hematic diarrhea, tenesmus, anorexia due to nausea and early satiety, and intermittent low-grade fever with a maximum peak of 37.8 °C daily.
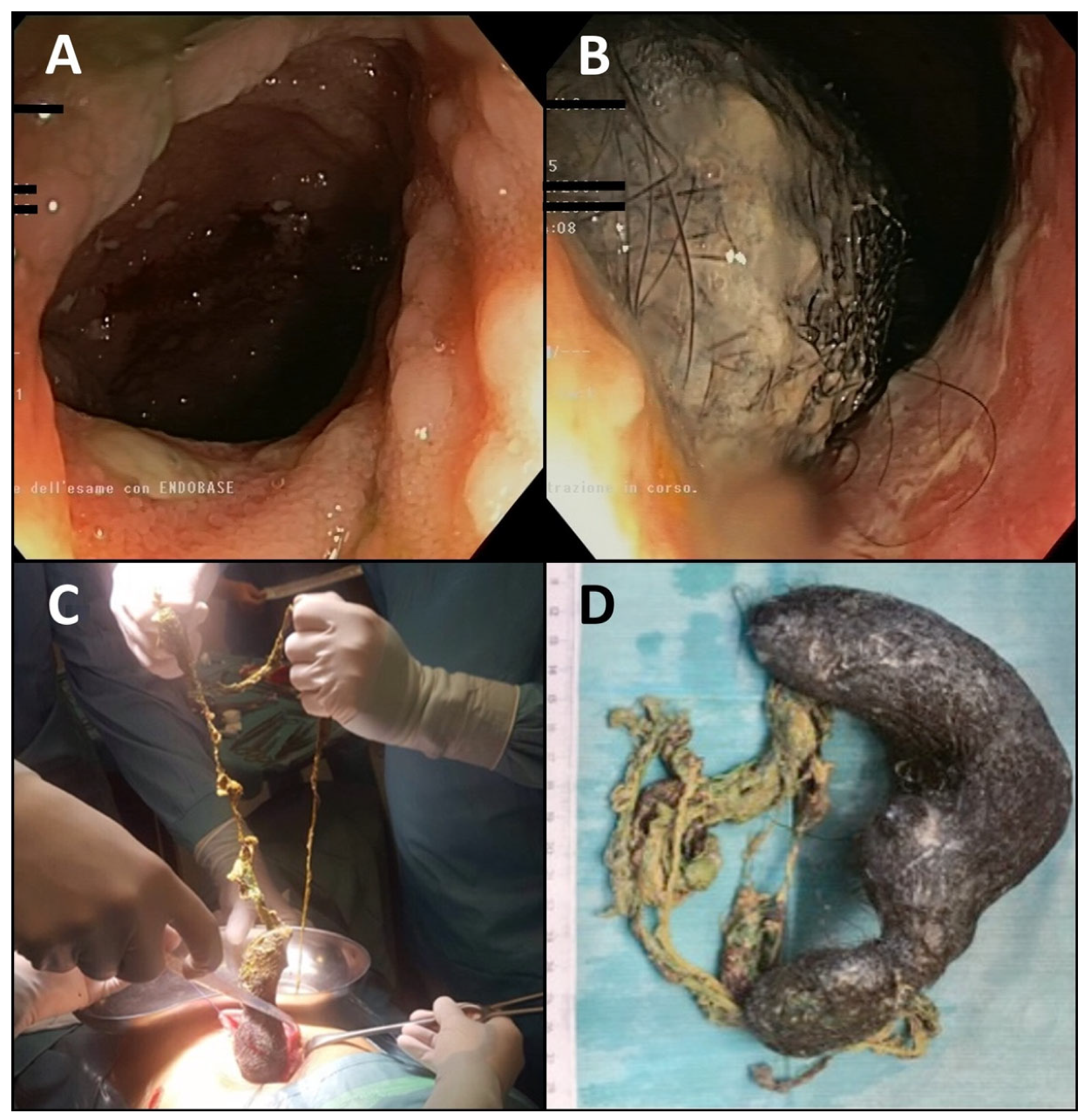
Therefore, complete endoscopic investigation was considered mandatory. The patient initially refused esophagogastroduodenoscopy (EGDS) due to fear and rejection of the endoscopic tube in the mouth, even with conscious sedation; thus, only colonoscopy with a 25 cm retrograde ileoscope was performed, with evidence of normal-appearing mucosa with diffuse presence of skipping ileal ulcers covered by fibrin (
Figure 1A). The ileocecal valve presented a single ulcer covered by fibrin, while the colonic mucosa from the cecum to the rectum appeared normal.
In suspicion of Crohn’s Disease, the patient was convinced to undergo EGDS under general anesthesia to complete the diagnostic pathway, incidentally revealing a huge trichobezoar with spread gastric erosions covered by fibrin clots (
Figure 1B). Endoscopic or laparoscopic removal was considered unfeasible; thus, a 10 cm incision laparotomy of the anterior gastric wall was performed (
Figure 1C) and a huge gastric-shaped trichobezoar extending into the jejunum was removed (
Figure 1D).
The postoperative course was uneventful, and the histological examination of ileal, colonic, and rectal biopsies was negative for IBD. Biopsies corresponding to the ileal lesions showed focal, moderately active inflammation with no architectural disorders and no granuloma, compatible with non-specific ileitis. Infectious causes such as yersinia, campylobacter, and tuberculosis infections were previously excluded.
The patient was discharged with a psychiatric follow-up recommendation showing discontinued trichotillomania and trichophagy at the six-month evaluation. She reported complete resolution of gastrointestinal symptoms but refused an endoscopic follow-up one year later.
3. Materials and Methods
A systematic literature search was conducted using the Pubmed database to identify relevant articles. The search process was independently conducted by four researchers to ensure comprehensive coverage and included keywords related to “Inflammatory Bowel Disease”, “Endoscopy”, “Bezoar”, “Trichobezoar” and “Rapunzel Syndrome” in pediatric patients, and articles published within the last 10 years were included in the search. Search terms were selected based on their relevance to the research question and were combined using Boolean operators. Filters were applied to limit the search to articles written in English and published in peer-reviewed journals. Additionally, manual searches were performed by screening reference lists of relevant articles. Duplicates were identified and removed using reference management software, and any discrepancies in article selection were resolved through consensus. The final selection of articles was based on their relevance to the research question and the quality of evidence presented, and finally data extraction was performed independently by four reviewers and cross-checked for accuracy.
4. Discussion
4.1. The Nature of Bezoars
Bezoars are intraluminal conglomerates of indigestible foreign material trapped within the gastrointestinal tract, usually in the stomach [
1].
Four primary types of bezoars have been described based on the nature of the forming materials, which are always non-food items taken orally, accidentally or intentionally [
2,
3]. Phytobezoars, the most common type, contain plant and vegetable substances, such as shells, seeds, and fruit remnants; trichobezoars are mainly composed of ingested hair; pharmacobezoars are conglomerates of medications; and lactobezoars are undigested masses of concentrated infant milk formula and mucus. Other possible and bizarre materials described include paper, plastic, ceramics, metals, and parasitic worms (ascaris) [
4,
5,
6,
7,
8].
Trichobezoars are associated with underlying psychiatric disorders and are common in young female adolescents in the second decade of life, as our patient, between 13 and 20 years of age [
9,
10]. The most common psychiatric comorbidities are emotional disturbances, pica, obsessive compulsive disorder (OCD), attention-deficit/hyperactivity disorder (ADHD), depression, and anorexia nervosa [
11,
12].
Trichotillomania, which is the compulsive urgency to pull out hair, and tricophagia, defined as the compulsive necessity of eating one’s hair, are the most common psychiatric conditions associated with trichobezoars, but it has been estimated that only 1% of patients with trichophagia will develop a trichobezoar [
13,
14].
Hair cannot be digested because of its smooth and slippery external surface, which makes it enzyme-resistant and permits it to escape peristaltic propulsion. Therefore, continuous ingestion of hair leads to its accumulation in the gastric mucosa folds and may eventually congregate into a mass bonding together with food remnants and mucus [
4]. Gastric acid denatures hair proteins and oxidizes the hair, giving trichobezoars a dark color [
15].
All case reports reviewed by Naik et al. in 2007 were female, except for one. The explanation resides in the tradition of females more frequently having long hair, and the only male patient reported eating his sister’s hair [
16].
4.2. Symptoms and Complications of Bezoars
Bezoars can be asymptomatic or present with various gastrointestinal symptoms. Whatever their nature, bezoars can cause two main types of enteric diseases: intestinal obstruction or malabsorption [
1,
17,
18,
19,
20]. The clinical presentation is associated with the size and presence of complications and if a bezoar is not promptly recognized, the risk of complications increases. Because of large stomach capacity, gastric bezoars do not show any symptoms until they become very bulky, and the most common symptoms are abdominal pain, discomfort or fullness, anorexia, and difficulty swallowing [
1,
10]. Halitosis is caused by bacterial colonization and undigested fat fermentation [
17]. Moreover, symptoms related to gastrointestinal bleeding, such as hematemesis, bloody stool or melena, anemia, and fainting, are secondary to the development of ulcers in the gastric mucosa due to intraluminal pressure induced by the bezoar, leading to necrosis [
18].
Prolonged misdiagnosis of gastrointestinal bleeding may lead to iron deficiency anemia, leading to a symptom cohort mimicking IBSs, as we also reported [
1]. Obstructive jaundice, cholangitis, malabsorption, pancreatitis, and protein-losing enteropathy have been described as infrequent presentations of gastric trichobezoar [
19,
20].
At clinical evaluation, 85% of patients present with a well-defined, firm, and mobile mass in the epigastrium (Lamerton’s sign), while 20% may present signs of peritonitis [
20]. Most patients may deny a tendency toward trichotillomania or trichophagia, even when specifically asked, but alopecia may be a clue to confirm the diagnosis [
15,
21].
As previously reported, in most cases, the trichobezoar is confined within the stomach but sometimes may extend through the pylorus into the jejunum, ileum, or even colon, leading to a condition known as Rapunzel Syndrome (RS), which was first described in 1968 by Vaughan et al. [
15,
18,
19,
22,
23]. Moreover, part of the tail can accidentally break and migrate into the small intestine, leading to intestinal obstruction [
2,
17]. Although different definitions have been proposed, the proper features qualifying RS are the presence of a trichobezoar with a tail extending at least to the jejunum and an association with symptoms suggestive of intestinal obstruction [
15].
Rarely, trichobezoar tail migration or enlargement may lead to complete intestinal obstruction and perforation, with a critical presentation of acute abdomen with abdominal distension, vomiting, hypotension, mental disorientation, and shock [
24].
4.3. Diagnosis of Bezoars
Currently, the gold standard for the diagnosis of trichobezoars is upper gastrointestinal tract endoscopy [
9]. Modern high-definition endoscopes provide direct visualization of masses, facilitating the detection of gastric or upper gastrointestinal bezoars. Usually, a trichobezoar presents as a single black mass located in the gastric fundus; however, sometimes it may present as multiple masses [
1]. Diagnosis can be confirmed by radiological investigations. Abdominal radiography may show a gastric-shaped partially opacified area that can be mistaken for a food-filled stomach and, eventually, air-fluid levels suggestive of intestinal obstruction. Abdominal ultrasound can evidentiate an intragastric, heterogeneous lesion, while a contrast meal may reveal a speckled heterogeneous mass or a gastric filling defect of contrast; the latter can contribute to the differential diagnosis of intestinal diverticuli, adenomas, or malignancies [
25]. Detailed emergency investigations or preoperative assessments of intestinal obstruction or perforation are not possible based only on these methods [
26].
Contrast-enhanced computed tomography (CT) is a superior radiological investigation showing high sensitivity (up to 90%) and specificity (up to 60%) in the identification of a trichobezoar and definition of its extension, which is a characteristic well-defined mottled intraluminal mass containing air [
1,
11,
25]. A CT scan may evidentiate the presence of multiple lesions, clarify differential diagnosis with other causes of intestinal obstruction, and evaluate the conditions of the intestinal loops. Finally, CT scans permit accurate preoperative planning [
27].
4.4. Management of Bezoars
The treatment of trichobezoar depends on its location, volume, extent, and associated disease. Conservatively managed cases usually present a poor outcome; hence, endoscopic or surgical intervention within 48 h of diagnosis is recommended [
1,
10]. Chemical dissolution, endoscopic fragmentation and removal, laparotomy, and laparoscopic surgery are available treatment options. In cases of acute abdomen due to intestinal obstruction, bezoar removal must be delayed until the patient is stabilized [
28].
Chemical dissolution, which can be used on all types of gastric bezoars and has demonstrated high rates of success in the management of phytobezoars, both with Coca Cola
® (Coca-Cola Company, Atlanta, GA, USA) and enzymatic agents such as cellulase, showed poor results in the management of trichobezoars even after prolonged treatment [
1,
29,
30].
Endoscopic fragmentation and removal of bezoars is another successful option for the management of bezoars, even of great size, but is generally ineffective for trichobezoars because of the high density of the hair conglomerate [
1]. Only one case report of a trichobezoar fragmented with an electrosurgical knife has been reported [
31]. However, an endoscopic approach is possible for small-sized trichobezoars, even if reports of successful endoscopic removal of trichobezoars are scarce and vastly outnumbered by case reports documenting unsuccessful attempts [
32]. Although not a therapeutic option, endoscopy is a reliable diagnostic instrument to differentiate between trichobezoars and foreign bodies that can be fragmented and removed [
33,
34].
Surgical removal is an inevitable therapeutic option for cases presenting with ileus, intestinal ischemia, or perforation and for patients with refractory bezoars. The most common surgical technique is enterotomy and removal of the mass, while the milking technique, making the mass advance proximally towards the stomach or distally through the ileocecal valve, has also been performed [
1]. In cases of intestinal ischemia or perforation, segmental resection with anastomosis or stoma is mandatory [
28]. Due to the very high success rate, possibility of exploring the whole gastrointestinal tract, short operative time, and reduced number of complications, laparotomic surgery is still the preferred treatment method, particularly in cases of large trichobezoars, as in our case report [
33,
35]. A laparoscopic approach is even possible and is, at present, increasingly being reported, also with a robot-assisted approach [
36,
37,
38,
39]. Moreover, the advantages of using a minimally invasive approach include better cosmetic results, fewer postoperative complications, and reduced admittance time [
17].
Conventional laparotomy is the only valid treatment option for patients with RS [
40]. To date, there has been no case report of Rapunzel Syndrome removed completely with an endoscopic approach; moreover, endoscopic removal of this type of bezoar is not recommended because of the risk of tail migration in the small intestine with consequent obstruction. Removal of the tail requires careful manipulation of the bowel to prevent perforation, and multiple enterotomies may be needed [
33].
Although postoperative surveillance has been suggested to reduce the risk of recurrence, there is a lack of guidelines available in the literature. Ultrasonography or endoscopy at the 6th, 12th, and 24th months has been proposed for the follow-up of these patients [
40,
41].
4.5. Bezoars and Psychiatric Disorders
Due to the increasing incidence of neuropsychiatric disorders among children and adolescents during the SARS-CoV-2 pandemic and the relationship between psychiatric disorders and trichobezoars, an increase in the number of diagnoses of trichobezoars has been observed during the last few years [
42]. Moreover, psychosocial stressors, familial dynamics, and potential genetic predispositions may play intricate roles and warrant further investigation [
43].
Psychiatric evaluation and counseling should be performed to prevent recurrences, and specific conditions such as trichotillomania and tricophagia must be managed with cognitive behavioral therapy as a primary intervention to modify negative thought patterns and behaviors. A pediatric nutritionist can also be involved in addressing alimentary behaviors correctly. Pharmacological interventions with selective serotonin reuptake inhibitors may be considered in severe cases or when comorbidities such as anxiety or depression are present [
44].
5. Conclusions
Trichobezoars are aggregates of hair trapped in the gastrointestinal tract that typically occur in female adolescents with psychiatric diseases [
1,
33]. They may lead to complications such as intestinal obstruction or perforation, gastric and intestinal ulceration, protein-losing enteropathy, sideropenic anemia, and mimicking IBSs and often require surgical removal [
18].
In recent years, due to the SARS-CoV-2 pandemic, the incidence of neuropsychiatric disorders among adolescents has increased enormously; therefore, there could also be an increase in trichobezoars, so a high clinical suspicion is mandatory [
42].
Interdisciplinary collaboration between psychiatrists, nutritionists, pediatricians, and pediatric surgeons is crucial not only during the acute phase but also for long-term management and prevention of recurrence [
45].
Author Contributions
Conceptualization, T.B. and D.F.; Methodology, T.B. and D.F.; Literature Review, D.F., B.L., E.F. and V.A.; Clinical Management, T.B., C.F., P.G. and G.M.; Writing—original draft, D.F. and F.P.; Writing—review and editing, T.B., P.G. and G.M.: Supervision, E.P., P.G. and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The study did not need Internal Review Board approval as it was a purely review manuscript. However, the institute’s ethics committee had previously approved all COVID-19 pandemic-related data collection (Regione Liguria Ethical Board; IRB#370/2020, 1 July 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Paschos, K. Pathophysiological and clinical aspects of the diagnosis and treatment of bezoars. Ann. Gastroenterol. 2019, 32, 224–232. [Google Scholar] [CrossRef]
- Yeh, J.; Saul, T.; Gingrich, A.; Wassermann, J. Bezoar. J. Emerg. Med. 2013, 45, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Dorterler, M.; Günendi, T.; Çakmak, M.; Shermatova, S. Bezoar types in children and aetiological factors affecting bezoar formation: A single-centre retrospective study. Afr. J. Paediatr. Surg. 2023, 20, 8–11. [Google Scholar] [CrossRef]
- Iwamuro, M.; Okada, H.; Matsueda, K.; Inaba, T.; Kusumoto, C.; Imagawa, A.; Yamamoto, K. Review of the diagnosis and management of gastrointestinal bezoars. World J. Gastrointest. Endosc. 2015, 7, 336–345. [Google Scholar] [CrossRef]
- Kumar, G.S.; Amar, V.; Ramesh, B.; Abbey, R.K. Bizarre Metal Bezoar: A Case Report. Indian J. Surg. 2012, 75, 356–358. [Google Scholar] [CrossRef]
- Barron, M.M.; Steerman, P. Gummi Bear Bezoar: A case report. J. Emerg. Med. 1989, 7, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.D.; Schachter, P.; Katz, M.; Bilik, R.; Avigad, I. A bizarre bezoar: Case report and review of the literature. Pediatr. Surg. Int. 1998, 14, 218–219. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.-P. Esophageal space-occupying lesion caused by Ascaris lumbricoides. World J. Gastroenterol. 2012, 18, 1552–1554. [Google Scholar] [CrossRef]
- Gonuguntla, V.; Joshi, D.-D. Rapunzel Syndrome: A Comprehensive Review of an Unusual Case of Trichobezoar. Clin. Med. Res. 2009, 7, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Aybar, A.; Safta, A.M. Endoscopic removal of a gastric trichobezoar in a pediatric patient. Gastrointest. Endosc. 2011, 74, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Park, J. Treatment of large gastric trichobezoar in children: Two case reports and literature review. Medicine 2023, 102, e33589. [Google Scholar] [CrossRef]
- Cohen, L.J.; Stein, D.J.; Simeon, D.; Spadaccini, E.; Rosen, J.; Aronowitz, B.; Hollander, E. Clinical profile, comorbidity, and treatment history in 123 hair pullers: A survey study. J. Clin. Psychiatry 1995, 56, 319–326. [Google Scholar] [PubMed]
- Melo, D.F.; Lima, C.d.S.; Piraccini, B.M.; Tosti, A. Trichotillomania: What Do We Know So Far? Skin Appendage Disord. 2021, 8, 1–7. [Google Scholar] [CrossRef]
- Veloso, N.; Silva, J.D.; Gonçalves, L.; Medeiros, I.; Godinho, R.; Viveiros, C. Trichotillomania and trichophagia: The causes of Rapunzel syndrome. Rev. Esp. Enferm. Dig. 2013, 105, 103–104. [Google Scholar] [CrossRef]
- Vaughan, E.D.; Sawyers, J.L.; Scott, H.W. The Rapunzel syndrome. An unusual complication of intestinal bezoar. Surgery 1968, 63, 339–343. [Google Scholar] [PubMed]
- Hirugade, S.T.; Talpallikar, M.C.; Deshpande, A.V.; Gavali, J.S.; Borwankar, S.S. Rapunzel syndrome with a long tail. Indian J. Pediatr. 2001, 68, 895–896. [Google Scholar] [CrossRef]
- Nour, I.; Alatef, M.A.; Megahed, A.; Yahia, S.; Wahba, Y.; Shabaan, A.E. Rapunzel syndrome (gastric trichobezoar), a rare presentation with generalised oedema: Case report and review of the literature. Ann. Trop. Paediatr. 2017, 39, 76–78. [Google Scholar] [CrossRef]
- Abourazzak, S.; Jerrar, I.O.; Idrissi, M.L.; Hida, M. Rapunzel syndrome complicated with pancreatitis, intussusception and intestinal perforation. BMJ Case Rep. 2022, 15, e247005. [Google Scholar] [CrossRef]
- Naik, S.; Gupta, V.; Naik, S.; Rangole, A.; Chaudhary, A.K.; Jain, P.; Sharma, A.K. Rapunzel Syndrome Reviewed and Redefined. Dig. Surg. 2007, 24, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, H.; Filston, H.C. Obstructive jaundice due to gastric trichobezoar. J. Pediatr. Surg. 1976, 11, 103–104. [Google Scholar] [CrossRef]
- Sah, D.E.; Koo, J.; Price, V.H. Trichotillomania. Dermatol. Ther. 2008, 21, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.; Chaoui, Y.; Mountasser, M.; Sabbah, F.; Raiss, M.; Hrora, A.; Alaoui, M.; Ahallat, M.; Chaouch, S.; Ouazzani, H. Giant gastric trichobezoar in a young female with Rapunzel syndrome: Case report. Pan Afr. Med. J. 2017, 27, 252. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, F.; Liu, D.; Fang, Y.; Li, F. The diagnosis and treatment of Rapunzel syndrome. Acta Radiol. Open 2016, 5, 2058460115627660. [Google Scholar] [CrossRef]
- Altintoprak, F.; Dikicier, E.; Deveci, U.; Cakmak, G.; Yalkin, O.; Yucel, M.; Akbulut, G.; Dilek, O.N. Intestinal obstruction due to bezoars: A retrospective clinical study. Eur. J. Trauma Emerg. Surg. 2012, 38, 569–575. [Google Scholar] [CrossRef]
- Mirza, M.B.; Talat, N.; Saleem, M. Gastrointestinal trichobezoar: An experience with 17 cases. J. Pediatr. Surg. 2020, 55, 2504–2509. [Google Scholar] [CrossRef]
- Barrows, A.; Vachon, T.; Campin, R.C.; Ignacio, R.C. Trichobezoars Detected and Treated Based on Plain Radiography. Mil. Med. 2015, 180, e1136–e1138. [Google Scholar] [CrossRef]
- Wang, P.-Y. Bezoar-induced small bowel obstruction: Clinical characteristics and diagnostic value of multi-slice spiral computed tomography. World J. Gastroenterol. 2015, 21, 9774–9784. [Google Scholar] [CrossRef] [PubMed]
- Dikicier, E. Intestinal obstruction due to phytobezoars: An update. World J. Clin. Cases 2015, 3, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Ladas, S.D.; Kamberoglou, D.; Karamanolis, G.; Vlachogiannakos, J.; Zouboulis-Vafiadis, I. Systematic review: Coca-Cola can effectively dissolve gastric phytobezoars as a first-line treatment. Aliment. Pharmacol. Ther. 2012, 37, 169–173. [Google Scholar] [CrossRef]
- Gold, M.; Patteson, T.E.; Green, G.I. Cellulase bezoar injection: A new endoscopic technique. Gastrointest. Endosc. 1976, 22, 200–202. [Google Scholar] [CrossRef]
- Wang, Y.T.; Gou, Y.W.; Ye, F.; Liu, Y.L.; Hou, G.F.; Ishrat, I.; Zhang, K.G. Endoscopic retrieval of a huge gastric trichobezoar using a polypectomy snare and an electrosurgical knife: A case report. J. Dig. Dis. 2021, 23, 54–56. [Google Scholar] [CrossRef]
- Ahmad, Z.; Sharma, A.; Ahmed, M.; Vatti, V. Trichobezoar Causing Gastric Perforation: A Case Report. Iran. J. Med. Sci. 2016, 41, 67–70. [Google Scholar] [PubMed]
- Gorter, R.R.; Kneepkens, C.M.F.; Mattens, E.C.J.L.; Aronson, D.C.; Heij, H.A. Management of trichobezoar: Case report and literature review. Pediatr. Surg. Int. 2010, 26, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, R.; Masuda, S.; Fujita, S.; Akiyama, N. Trichobezoar effectively treated with direct endoscopic injection of Coca-Cola: A case report. DEN Open 2023, 4, e283. [Google Scholar] [CrossRef]
- Middleton, E.; Macksey, L.F.; Phillips, J.D. Rapunzel syndrome in a pediatric patient: A case report. AANA J. 2012, 80, 115–119. [Google Scholar] [PubMed]
- Zmudzinski, M.; Hayashi, A. Laparoscopic removal of massive pediatric gastric trichobezoars: A brief report. Am. J. Surg. 2020, 219, 810–812. [Google Scholar] [CrossRef] [PubMed]
- E Al-Mulla, A.; Altabeekh, A.; Al-Jafar, A.; Dashti, S. Successful laparoscopic extraction of trichobezoar due to Rapunzel syndrome: First reported case in Kuwait. J. Surg. Case Rep. 2021, 2021, rjab532. [Google Scholar] [CrossRef] [PubMed]
- Lisi, D.; D’Ovidio, V.; Lucidi, C. A Giant Trichobezoar Diagnosed by Ultrasound, Confirmed by Upper Endoscopy, and Managed by Minimally Invasive Laparoscopic Surgery. Clin. Gastroenterol. Hepatol. 2020, 19, e125. [Google Scholar] [CrossRef] [PubMed]
- Serpa, E.; Luciano, E.; Pacheco, F.; Ghanem, M. Robotic-assisted extraction of giant gastric trichobezoar: A case report. J. Surg. Case Rep. 2022, 2022, rjac472. [Google Scholar] [CrossRef]
- Lyons, R.; Ismaili, G.; Devine, M.; Malik, H. Rapunzel syndrome causing partial gastric outlet obstruction requiring emergency laparotomy. BMJ Case Rep. 2020, 13, e232904. [Google Scholar] [CrossRef]
- Chin, X.; Ng, J.Y. Acute Presentation of Rapunzel Syndrome and a Review of Bezoars. Cureus 2021, 13, e20785. [Google Scholar] [CrossRef] [PubMed]
- Bozzola, E.; Ferrara, P.; Spina, G.; Villani, A.; Roversi, M.; Raponi, M.; Corsello, G.; Staiano, A.; Chiarelli, F. The pandemic within the pandemic: The surge of neuropsychological disorders in Italian children during the COVID-19 era. Ital. J. Pediatr. 2022, 48, 126. [Google Scholar] [CrossRef]
- López-Álvarez, L.M.; Moreno-Castellanos, M.A.; Álvarez-Acuña, A.M.; Echeverri-Mejía, C.; Narvaez-Rojas, A.; Lozada-Martínez, I.D. Huge gastric trichobezoar caused by involuntary nocturnal trichophagia and trichotillomania in a female adolescent. Int. J. Surg. Case Rep. 2022, 99, 107627. [Google Scholar] [CrossRef]
- Ahmed, M.; Habib, M.; Memon, H.; Ahmad, R.R.; Chaudhary, M.A. Trichotillomania, Trichophagia and Trichobezoar in a Male Paediatric Patient: A Case Report and Literature Review. Int. J. Surg. Case Rep. 2024, 117, 109520. [Google Scholar] [CrossRef] [PubMed]
- Snorrason, I.; Ricketts, E.J.; Stein, A.T.; Björgvinsson, T. Trichophagia and trichobezoar in trichotillomania: A narrative mini-review with clinical recommendations. J. Obs. -Compuls. Relat. Disord. 2021, 31, 100680. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).