Abstract
Background: Treatment of a flexor tendon sheath infection of the thumb usually involves prompt surgical irrigation and debridement (ID). There are few descriptions of this procedure despite the unique anatomy of the thumb flexor sheath. The aim of this study was to investigate thumb flexor sheath ID and explore the relevant anatomy. Methods: The current ID technique was performed on eight embalmed cadaveric hands. Coloured latex was injected into the sheath, and the surrounding region was dissected. Outcomes of interest were the distribution of latex, the success of the procedure, and the anatomy of the radial bursa. Results: Latex was successfully injected into all specimens, although A1 pulley stenosis caused significant resistance to flow. Latex filled the radial bursa (four specimens), reached the distal boundary of the transverse carpal ligament (three), or did not pass the A1 pulley (one); in addition, latex was found in the deep spaces of the hand and wrist (five specimens). The radial bursa was located at a median (range) of 33.2 (23.9–34.5) mm proximal to the carpometacarpal joint and at 7.8 (0–14.0) mm distal to the distal border of the pronator quadratus. Conclusion: These findings contribute to the existing body of knowledge on the anatomy of the thumb flexor sheath and radial bursa, and will help guide hand surgeons to perform thorough ID for infection. A modified surgical technique is presented, which may help further inform the treatment of pyogenic flexor tenosynovitis and other serious hand conditions.
1. Introduction
Pyogenic flexor tenosynovitis of the hand can be devastating and must be diagnosed and treated promptly in order to avoid a poor clinical outcome [1]. Treatment of this condition will usually consist of surgical irrigation and debridement (ID), combined with antibiotic therapy [1]. Knowledge of the anatomy of the flexor sheath is essential for diagnosis, as well as to help guide surgeons with the placement of their incisions and the surgical procedure [1]. Compared to the other digits, the thumb is unique with respect to the composition of both its fibrous flexor sheath (arrangement and number of pulleys) and synovial tendon sheath. This synovial sheath consistently communicates with the adjacent radial bursa and, to a lesser extent, the space of Parona, the midpalmar space, and the ulnar bursa [2,3,4,5,6,7,8,9,10]. These anatomical particularities should be considered when planning the optimal surgical approach.
There is a paucity of literature supporting the ideal surgical technique for the ID of the flexor sheath of the thumb and radial bursa, and a contemporary review of pyogenic tenosynovitis [11] does not address this subject. The most comprehensive recent account is provided by Hoppenfeld et al. (2021) [12], although this method is originally attributed to the earlier work of Kanavel in 1939 [6]. While not easily found in contemporary digital databases, the ID procedure was also described by Neviaser in 1978 [13].
In order to perform the ID of the flexor sheath of the thumb and the radial bursa, it is important to understand the bursal anatomy, including any anatomical variations. However, there is a limited understanding of this structure, and no anatomical studies have recently been published on this topic.
Therefore, the purpose of this study was to trial the surgical technique described by Hoppenfield et al. [12] and to explore the anatomy of the flexor sheath and radial bursa of the thumb in cadaveric specimens. This information is not readily accessible in the literature, and it is anticipated that the findings of this study will be of interest to hand surgeons, providing a valuable resource that can be used to guide treatment of thumb flexor tenosynovitis.
2. Materials and Methods
2.1. Specimens
Eight embalmed upper limb specimens (two left, six right; six males, two females; median age, 81.5 years) from individuals who had bequeathed their bodies to the Department of Anatomy, University of Otago under the Human Tissues Act (2008) were used in this study. The specimens included the elbow joint, forearm, wrist, and hand, and were obtained from cadavers embalmed with Crosado mix (60.2% ethanol [95%], 15.1% glycerine, 15.1% water, 7.6% phenoxytol [90%], 2.0% formalin [37%]) [14]. No antemortem data were available in relation to hand function and/or surgery of the individuals included in this study, but none had a history of systemic musculoskeletal or neurological conditions, or obvious hand or thumb osteoarthritis or deformities. Prior to starting this project, consultation was undertaken with the Ngāi Tahu Research Consultation Committee and ethical approval was obtained from the Human Ethics Committee, University of Otago (reference number H22/058).
2.2. Injection of the Sheath and Bursa
The surgical technique described by Hoppenfeld [12] was utilised to replicate the irrigation of the thumb flexor sheath (Figure 1). The protocol was initially trialled in one specimen, the data of which were not included in the results.
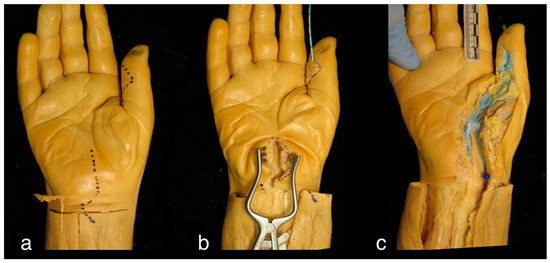
Figure 1.
Right cadaveric hand. (a) Location of incisions over the interphalangeal joint of the thumb and the carpal tunnel; (b) Technique for latex injection using a paediatric feeding tube to inject latex distal to proximal, and a retractor placed in the carpal tunnel to expose the radial bursa; (c) For the purposes of this study, anatomical dissection was undertaken to reveal the flexor sheath and radial bursa, and to assess the distribution of the injected latex.
Firstly, the flexor sheath was opened distally utilising an incision over the flexor crease of the interphalangeal joint. Then, proximally, a standard carpal tunnel approach was used to expose the radial bursa surrounding the flexor pollicis longus (FPL) tendon. A metallic fistula probe was passed into the sheath from distal to proximal in order to provide dilation and to facilitate the passage of the irrigation fluid (i.e., latex).
To replicate the irrigation technique used for pyogenic flexor tenosynovitis and to enhance tissue differentiation, between 5–10 mL of latex coloured with blue dye was injected into the sheath with the use of a 10 mL syringe attached to either an 18 gauge blunt tip needle or a paediatric feeding tube. The metacarpophalangeal joint was extended during latex injection as this was found to help facilitate flow. Latex was injected under pressure until no further flow was possible or until there was overflow from the distal incision. Observations were made during each phase of the procedure, and notes were taken relating to any difficulties that were encountered. The latex was then left for several days to solidify in order to facilitate dissection.
2.3. Dissection and Measurement
The skin and fascia overlying the thumb and palm were removed, and the muscles of the thenar eminence were released from their origins and related nerve branches were transected, including the recurrent branch of the median nerve which passes across the radial bursa. Reflection of the transverse carpal ligament enabled the tracking of the FPL tendon and sheath proximally, as well as the identification of the proximal extent of the radial bursa. In instances where latex did not fill the bursa, fine-toothed forceps were inserted into the sheath and gently moved proximally in order to identify the location of the blind end of the bursa. The flexor digitorum superficialis and profundus tendons were resected and reflected to expose the FPL and pronator quadratus.
Observations relating to the distribution of latex were taken, including whether there was communication between the flexor sheath of thumb and the radial bursa or between the radial bursa and other potential spaces of the hand. The proximal extent of the radial bursa was marked with a pin, and measurements relative to this landmark were taken with digital callipers (SC02 point digital callipers; Tresna, Germany, accuracy ±0.03 mm) as follows: the distance to the distal border of the pronator quadratus (mm; along the line of the FPL tendon), and to the radial aspect of the carpometacarpal (CMC) joint of the thumb (mm). Each measurement was taken twice (SW) on separate occasions. The median distances were calculated from these data.
3. Results
Latex was successfully injected into the FPL sheath in all eight specimens—it extended into and filled the radial bursa in four, reached the level of the distal boundary of the transverse carpal ligament in three, and did not pass the A1 pulley in one. Relative to the radial aspect of the CMC joint, the radial bursa was located at a median (range) of 33.2 (23.9–34.5) mm proximally and was 7.5 (0–14.0) mm distal to the distal border of the pronator quadratus (Figure 2). Its close proximity to the median nerve was also observed (Figure 3).
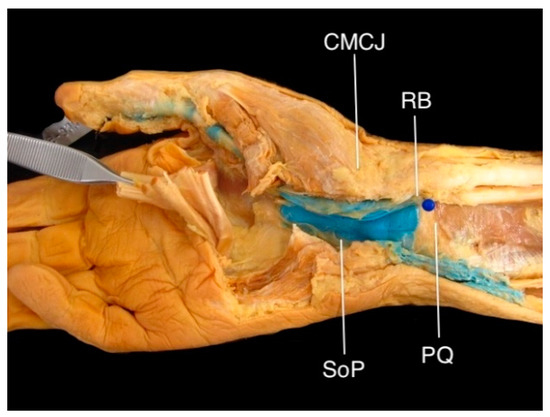
Figure 2.
The radial bursa (RB) in a right cadaveric hand, injected with blue latex. The proximal aspect of the radial bursa (blue pin) was located approximately 7.5 mm distal to the distal border of pronator quadratus (PQ) and 32 mm proximal to the carpometacarpal joint (CMCJ). Note that this specimen shows communication with the space of Parona (SoP).
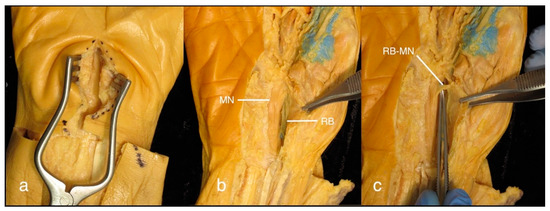
Figure 3.
(a) Right cadaveric hand showing the surgeon’s view of the approach to the radial bursa lying deep within the carpal tunnel; (b) The median nerve (MN), lying ulnar and superficial to the radial bursa (RB). Gentle retraction of the median nerve is required to gain access, and insertion of a fistula probe from distal or proximal may help with the identification of the bursa; (c) The recurrent branch of the median nerve (RB-MN) lies superficial to the radial bursa at the level of the thenar eminence; an open approach at this level is not recommended.
A difficulty experienced with all specimens was the significant resistance that was experienced when injecting the latex. Based on how far the surgical equipment was able to be passed into the flexor sheath, this was attributed to the stenosis of the sheath at the level of the A1 pulley, which was also observed during dissection (although not quantified) (Figure 4).
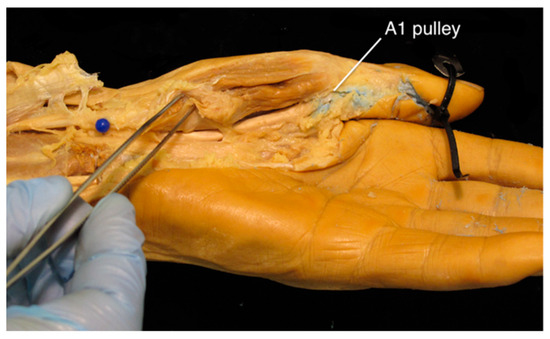
Figure 4.
Left cadaveric hand showing the obstruction of latex at the level of the A1 pulley. To thoroughly irrigate the thumb flexor sheath or the radial bursa, an A1 pulley release is recommended.
Various amounts of latex were also evident in extrasynovial spaces, such as within the subcutaneous fat and in the plane adjacent to the FPL tendon sheath, likely originating from piercing the sheath with the surgical equipment. An overflow of latex from the distal incision was also common. As such, it was difficult to accurately measure how much latex was injected, but across the specimens, a median volume of 8 mL (range of 5–15 mL) was used.
In five specimens, latex was found in the deep spaces of the hand and wrist (Figure 5), with various distribution patterns as follows: in two, the latex was confined to the space of Parona, in the region of the carpal tunnel; and in three, it spread into the deep spaces of the palm, extending as far ulnarly as the middle (n = 1) or fourth (n = 2) metacarpal. In one of these latter specimens, there was a clear septum of connective tissue that ensured the latex did not communicate with the ulnar bursa.
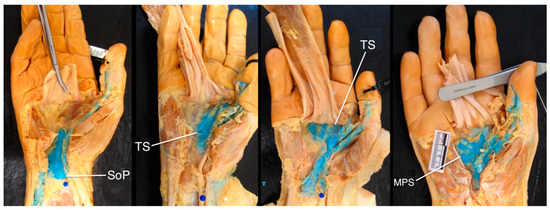
Figure 5.
Communication between the radial bursa and other spaces in the hand, highlighting anatomical variation. Consider the spread of infection to other spaces in the hand, including the space of Parona (SoP), thenar space (TS), and midpalmar space (MPS).
4. Discussion
To our knowledge, this is the first study to specifically explore the method described by Hoppenfeld [12] for the ID of the flexor sheath of the thumb and radial bursa. Seven out of eight specimens showed communication between the flexor sheath of the thumb and the radial bursa, as indicated by the presence of latex in the bursa following injection within the sheath distally. This result validates previously described surgical techniques [6,12,13].
A key finding in this study is that the A1 pulley was consistently stenotic and caused significant resistance to latex flow in all specimens, thus leading to difficulty in filling the tendon sheath from distal to proximal. Based on this, it is possible that this stenosis would limit the flow required to attain a thorough irrigation of the tendon sheath on a patient in vivo. Therefore, we recommend considering an A1 pulley release [15] at the level of the first MCP joint [16] with the subsequent irrigation of the tendon sheath proximally and distally.
The bursa is the most radial structure in the carpal tunnel, and is positioned deep in the surgical wound, with the median nerve in close proximity [17,18]. Given this relationship, care is required to access the bursa at the time of surgery. Regarding the proximal exposure of the radial bursa, this study shows that it extends a median of 33.2 mm proximal to the CMC joint and is in close proximity to the distal border of pronator quadratus. These findings support the notion that a thorough ID of the radial bursa should involve exposure at the level of the carpal tunnel. We advise against an open surgical approach at the level where the bursa runs deep to the thenar eminence as the recurrent branch of the median nerve crosses the bursa superficially [17,18] and is, therefore, at high risk of transection.
Given the variability of the proximal extension of the radial bursa, surgeons could consider utilising an additional open approach at the level of the distal radius. A separate incision should be used to avoid injury to the palmar cutaneous branch of the median nerve. An approach through the fascia deep to flexor carpi radialis tendon will give access to the bursa and the space of Parona at the level of the distal radius [19].
The flexion and extension of the thumb can aid in identifying the bursa as it overlies the FPL tendon. In addition, retrograde insertion of a fistula probe along the tendon sheath to help identify the bursa in the carpal tunnel was recommended by Hoppenfeld [12]. This is a practical concept; however, in this study, it was too difficult to pass the probe across the A1 pulley. A pulley release may facilitate this technique.
This study revealed the propagation of latex and communication between the radial bursa and the deep spaces of the hand in five out of eight specimens. It is important to be aware of this anatomical variation. We suggest having a low threshold for the exploration of the other spaces of the hand, such as the space of Parona [10] and the midpalmar or thenar space. Careful physical examination with clinical findings, suggestive of proximal infections like acute carpal tunnel syndrome, pain on palpation of the volar wrist, or forearm pain, should alert the surgeon to this. Imagery such as ultrasound or magnetic resonance imaging may be beneficial to appreciate the full extent of infection when planning a thorough surgical ID [18].
The use of elderly embalmed cadaveric specimens, which demonstrate different properties to living patients, is a limitation of this study. Embalming can cause some tissue shrinkage and drying [14,20], although the embalming process used in the current study results in less shrinkage than standard formaldehyde preparations [14]. As such, the combination of age and tissue changes may have resulted in the joint stiffness that was noted in the specimens, potentially affecting the injection of latex by causing tighter anatomical spaces. Latex was useful for injection and dissection; however, as it was viscous, using a liquid like saline may have exhibited different fluid dynamics and potentially increased the flow beneath the A1 pulley. The location of the proximal extent of the bursa may also be influenced by hand size, and using normalised data from a larger number of specimens would be required to better appreciate this.
Taking into account the anatomical findings and results of the simulated injections conducted in this study, we propose a modified surgical technique for the ID of the flexor sheath of the thumb and radial bursa—the key steps are summarised in Table 1 and Figure 6.

Table 1.
Proposed modified surgical technique for the irrigation and débridement of the FPL tendon and radial bursa.
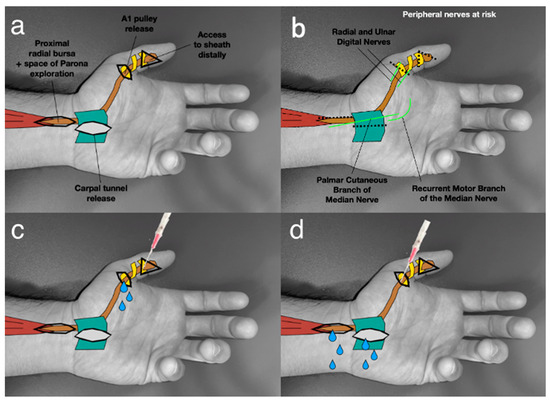
Figure 6.
A modification of Hoppenfeld’s [12] surgical technique for the irrigation and débridement of the FPL flexor sheath and radial bursa. (a) This method includes an A1 pulley release. (b) Note the nerves at risk of injury when planning skin incisions. If utilising an approach to the radial bursa proximal to the carpal tunnel, a separate incision should be made, avoiding injury to the palmar cutaneous branch of the median nerve. (c) Irrigation of the thumb flexor sheath between the IPJ and A1 pulley. (d) Irrigation of the radial bursa between the A1 pulley and proximal approaches at the carpal tunnel and distal forearm.
5. Conclusions
The findings of this study contribute to the existing body of knowledge on the anatomy of the flexor sheath of the thumb and the radial bursa, helping to guide hand surgeons wishing to perform thorough ID for infection. This study highlights the extension of the radial bursa proximal to the carpal tunnel, as well as potential communication with other spaces within the hand. Stenosis at the A1 pulley requires consideration, and a pulley release may be necessary if difficulty is encountered when achieving high flow irrigation. Based on these findings, a modified surgical technique is presented which may help further inform the treatment of pyogenic flexor tenosynovitis and other serious hand conditions.
Author Contributions
Conceptualization, J.W.G. and L.L.; methodology, J.W.G. and S.J.W.; formal analysis, S.J.W.; investigation, J.W.G., L.L. and S.J.W.; resources, S.J.W.; data curation, S.J.W.; writing—original draft preparation, J.W.G.; writing—review and editing, L.L. and S.J.W.; supervision, L.L.; project administration, J.W.G. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Institutional Review Board Statement
Prior to starting this project ethical approval was obtained from the Human Ethics Committee, University of Otago (reference number H22/058, 23 May 2022).
Informed Consent Statement
Informed consent was not relevant for this study.
Data Availability Statement
The data is available on request from the authors.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Draeger, R.W.; Bynum, D.K., Jr. Flexor tendon sheath infections of the hand. J. Am. Acad. Orthop. Surg. 2012, 20, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Jebson, P. Deep subfascial space infections. Hand Clin. 1998, 14, 557–566. [Google Scholar] [CrossRef]
- Aguiar, R.O.C.; Gasparetto, E.L.; Escuissato, D.L.; Marchiori, E.; Trudell, D.J.; Haghighi, P.; Resnick, D. Radial and ulnar bursae of the wrist: Cadaver investigation of regional anatomy with ultrasonographic-guided tenography and MR imaging. Skelet. Radiol. 2006, 35, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.R.; Botte, M.J. Surgical Anatomy of the Hand and Upper Extremity, 1st ed.; Lippincott Williams & Wilkins: Philadelphia, UK, 2003; pp. 532–666. [Google Scholar]
- Doyle, J.R.; Blythe, W.F. Anatomy of the flexor tendon sheath and pulleys of the thumb. J. Hand Surg. 1977, 2, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Kanavel, A.B. Infections of the Hand: A Guide to the Surgical Treatment of Acute and Chronic Suppurative Processes in the Fingers, Hands, and Forearm, 7th ed.; Lea & Febiger: Philadelphia, UK, 1939; pp. 81–387. [Google Scholar]
- Resnick, D. Roentgenographic anatomy of the tendon sheaths of the hand and wrist: Tenography. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1975, 124, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E. The retinacular system of the hand. In Functional and Surgical Anatomy of the Hand, 3rd ed.; J.B. Lippincott: Philadelphia, UK, 1965; pp. 277–279. [Google Scholar]
- Scheldrup, E. Tendon sheath patterns in hand. Mod. Med. 1952, 20, 92–93. [Google Scholar]
- Sharma, K.S. Space of Parona infections: Experience in management and outcomes in a regional hand centre. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Hermena, S.; Tiwari, V. Pyogenic Flexor Tenosynovitis; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hoppenfeld, S.; DeBoer, P.; Buckley, R. Surgical Exposures in Orthopaedics: The Anatomic Approach, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, UK, 2021; pp. 270–271. [Google Scholar]
- Neviaser, R.J. Closed tendon sheath irrigation for pyogenic flexor tenosynovitis. J. Hand Surg. 1978, 3, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Crosado, B.; Löffler, S.; Ondruschka, B.; Zhang, M.; Zwirner, J.; Hammer, N. Phenoxyethanol-based embalming for anatomy teaching: An 18 years’ experience with Crosado embalming at the University of Otago in New Zealand. Anat. Sci. Educ. 2020, 13, 778–793. [Google Scholar] [CrossRef] [PubMed]
- Guler, F.; Kose, O.; Ercan, E.C.; Turan, A.; Canbora, K. Open versus percutaneous release for the treatment of trigger thumb. Orthopedics 2013, 36, 1290–1294. [Google Scholar] [CrossRef] [PubMed]
- Standring, S. (Ed.) Gray’s Anatomy. The Anatomical Basis of Clinical Practice, 42nd ed.; Elsevier: New York, NY, USA, 2020. [Google Scholar]
- Rigopoulos, N.; Dailiana, Z.H.; Varitimidis, S.; Malizos, K.N. Closed-space hand infections: Diagnostic and treatment considerations. Orthop. Rev. 2012, 4, 83–87. [Google Scholar] [CrossRef]
- Malizos, K.N.; Papadopoulou, Z.K.; Ziogkou, A.N.; Rigopoulos, N.; Athanaselis, E.D.; Varitimidis, S.E.; Dailiana, Z.C. Infections of deep hand and wrist compartments. Microorganisms 2020, 9, 838. [Google Scholar] [CrossRef]
- Protopsaltis, T.S.; Ruch, D.S. Volar approach to distal radius fractures. J. Hand Surg. 2008, 33, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.; Albert, M.; Nicholson, H. The fascicular anatomy and peak force capabilities of the sternocleidomastoid muscle. Surg. Radiol. Anat. 2017, 39, 629–645. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).