1. Introduction
Cranial kinesis is a hallmark of avian cranial evolution, enabling a remarkable range of skull mobility that underlies key functional, ecological, and evolutionary innovations in Neornithes. Following [
1,
2] cranial kinesis can be defined as the presence of movements between portions of the skull at intracranial joints. In Neornithes, cranial kinesis can involve several distinct types of intracranial movement, including prokinesis (movement between the neurocranium and the facial skeleton at the craniofacial hinge), neurokinesis (movement between the neurocranium and the palate at the basipterygoid articulation), streptostyly (mobility of the quadrate bone), rhynchokinesis (flexibility within the upper beak), and streptognathism (lateromedial movement of the lower jaw). However, in most discussions of avian cranial kinesis, the term typically refers to prokinesis.
The morphofunctional complex that acts in prokinesis consists of several bones joined by mobile unions or zonae flexoriae [
3,
4,
5,
6]. These mobile areas include the otic synovial joint, the quadratum–pterygoid and quadratum–arcus jugalis articulations, pterygopalatine articulation, basal synovial joint (when present), the zona flexoria arcus jugalis, zona flexoria palatina, and the zona flexoria craniofacialis [
7]. These mobile points rotate mainly by the action of the musculus protractor pterygoidei et quadrati, which originates on the interorbital septum and inserts on the mesial surface of the os quadratum and on the dorsal surface of the os pterygoideum [
8,
9]. When this muscle contracts, it pushes the os quadratum forward (which rotates at the level of the otic synovial joint), pushing forward the pterygopalatine bones and the jugal arch. Thanks to the ventral zonae flexoriae (palatina et arcus jugalis), the upper jaw can rotate relative to the jugal arch and the palatine bones [
4,
10,
11]. This rotation is dorsal due to the rostral thrust of the palatines and jugal arch and the presence of the dorsal flexion zone, the zona flexoria craniofacialis. Altogether, the coordinated action of contraction of the protractors and the consequent rotation at the flexion zones results in upper jaw elevation, allowing a greater oral opening or gape. It has been stated that m. depressor mandibulae also play a role, in that lowering the mandible also pushes the os quadratum rostrally, further engaging the push-rod system to elevate the upper jaw [
4,
5].
A widely accepted framework identifies four anatomical criteria required to assess the presence of cranial kinesis [
1]: (1) an otic synovial articulation (quadratosquamosal articulation); (2) a basal synovial articulation (basipterygoid articulation); (3) the presence of protractor muscles, (4) permissive kinematic linkages. According to the fulfillment of one or several of these criteria, the authors recognize three states. The “Partially kinetically competent” state includes fulfillment of criteria 1, 2 or 3, but not 4, and lacks in vivo validation. The “Fully kinetically competent” state includes all four criteria, also without in vivo validation. The “Kinetic” state requires that all criteria are fulfilled and that there is in vivo validation. The authors also acknowledge the possible existence of a fourth state, “Akinetic”, but they note that the term is ambiguous, as it may encompass partially or fully kinetically competent states. In birds, criteria 1 and 3 are consistently fulfilled, but criteria 2 and 4 may not be.
The case of terror birds (Cariamiformes, Phorusrhacidae) is of wide interest, as they represent one of the most iconic groups of birds of the South American avifauna, occupying the ecological role of apex terrestrial predators during much of the Cenozoic. Phorusrhacids were mid- to large-body-size birds that filled the apex predatory niche [
12,
13] during most of the Cenozoic (middle Eocene to upper Pleistocene [
14,
15]). Phylogenetically related to extant seriemas (Cariamiformes, Cariamidae), they had poor-to-absent flying capabilities, but were highly cursorial [
16,
17]. While some authors propose a sister relationship between Cariamidae and Phorusrhacidae [
18,
19], recent phylogenetic analyses proposed a sister relationship between
Bathornis+Cariamidae and Phorusrhacidae [
20] or between
Ameghinornis+Phorusrhacidae, with a successive sister relationship with
Bathornis and Cariamoidea (a taxon that includes Cariamidae,
Dynamopterus, and
Elaphrocnemus, see [
21]).
The characteristically high, narrow, and elongated beaks of phorusrhacids, often ending in a pronounced distal hook, have long been considered as a distinctive trait. However, unlike other Neornithes, terror birds lost the ability to rotate their upper beaks dorsoventrally, that is, they lack the cranial kinesis typical of most modern birds. This cranial immobility, unknown in other predatory avians, represents a derived condition within this clade. It is thought to have enabled terror birds to subdue their prey through powerful vertical strikes delivered with their beaks [
12,
13].
Here, we present an extended anatomical description of the cranial regions associated with the loss of kinesis in phorusrhacids. Our aim is to investigate the evolution of this trait within a phylogenetic framework in order to assess potential relationships between cranial morphology, trophic adaptations, and diversification of the group. By integrating anatomical and evolutionary perspectives, this study contributes to a better understanding of the functional and ecological implications of cranial immobility in this iconic clade of extinct predatory birds.
4. Discussion
Some anatomical features of the Phorusrhacidae are congruent with kinetic capabilities—such as streptostyly and the presence of protractor muscles—reflecting a retained kinetic heritage. However, other derived traits, such as the absence of zonae flexoriae, clearly certify that phorusrhacids were incapable of mobilizing the maxilla (i.e., of performing prokinesis). In other words, phorusrhacids were akinetic.
The function of the processus basipterygoideus and its contact with the os pterygoideum, forming a presumably synovial basal joint, remains difficult to establish [
4]. However, it has been hypothesized that these processes serve as guides [
10] or as stops during kinetic movements [
4,
10,
34]. Gussekloo and Bout [
35] proposed that the processus basipterygoidei in Palaeognathae restrict caudal movements of ossa pterygoidei. Consequently, these processi prevent depression of the maxilla below its resting position, which occurs during contraction of the pterygoideus muscles and musculus pseudotemporalis profundus [
4,
10,
27,
36]. The peculiar and intimate association that exists between the components of the basal joint—the os pterygoideum and the processus basipterygoideus—in phorusrhacids is difficult to explain. This feature has not been recorded in other Cariamiformes (absent in Cariamidae, not preserved in
Bathornis). Although it is plausible that it was absent in basal Cariamiformes, its absence in extant Cariamidae could alternatively represent a cariamid apomorphy. It is noteworthy that seemingly “open” and synovial basal joints occur in various akinetic archosaur clades, leading [
1] to suggest that the basal joint may plesiomorphically represent a growth zone rather than a kinetic joint. Basal joints and basipterygoid processes seem to have arisen multiple times in Neornithines. However, the presence of a patent basal joint in phorusrhacids appears to be related to the immobilization of the palate, a phenomenon likely associated with their evolution of akinesis.
The function of the protractor musculature in the phorusrhacids is also complex to elucidate. Holliday and Witmer [
1] demonstrated that, similar to the basal joint, protractor muscles were widely present in archosaur clades that are almost universally considered akinetic; this pattern also holds true for theropods along the stem leading to birds. They proposed that the presence of these muscles, that do not produce movement, may have evolved instead to moderate and redirect forces generated by the jaw adductor musculature. Following the loss of certain bones in the avian lineages (e.g., postorbital, ectopterygoid) as part of a suite of morphological changes near the origin of birds—including expansion of the brain, reorientation of jaw muscles (see [
2])—kinematic linkages became permissive of cranial kinesis, allowing the protractor musculature to assume an active role in powering prokinesis.
The basic avian prokinetic apparatus was already well established by the time Cariamiformes, including phorusrhacids, evolved. Therefore, the presence of protractor muscles in phorusrhacids represents a relic of their kinetic ancestry. The loss of cranial kinesis in phorusrhacids, despite the retention of the protractor musculature, suggests that these muscles may have reverted to their ancestral functions observed in more distant akinetic non-avian ancestors. Such functions could include shock absorption, impact mitigation, or compensating for forces exerted by the external adductor muscles, consistent with the estimated large bite force in phorusrhacids. Moreno et al. [
37] suggested that the musculus protractor pterygoideus et quadrati controls part of the strain experienced by the os pterygoideum during contraction of the pterygoid musculature. Given that the pterygoid muscles of phorusrhacids are highly developed [
25], it plausible to hypothesize that the musculus protractor pterygoideus et quadrati functioned as a shock dissipator, compensating any strain or stress generated by the contraction of the pterygoid muscles or even the total bite force.
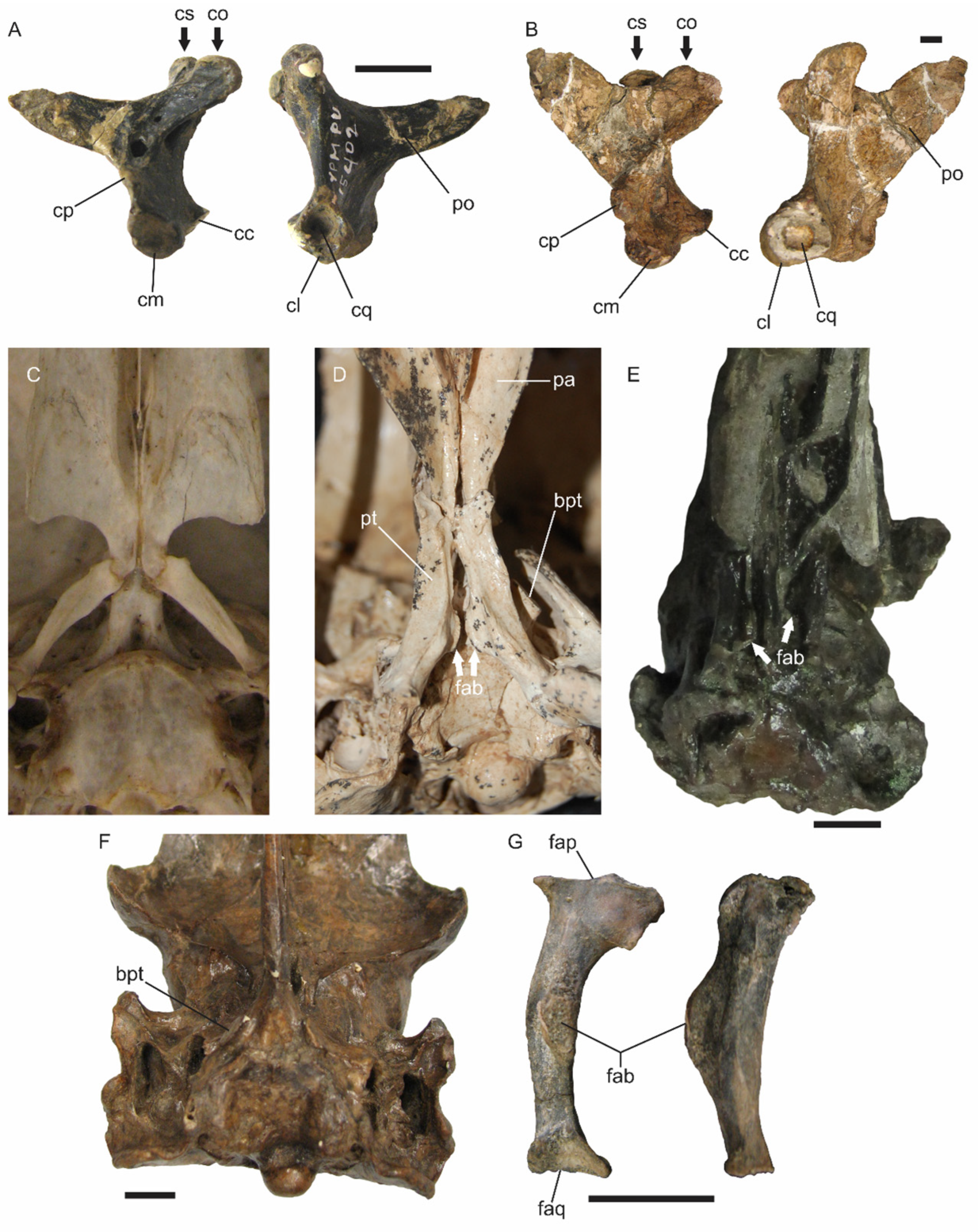
The nature of the articulations between skull bones is critically important for understanding the mechanical behavior of the skull, as the connections either permit or restrict intracranial movements [
1] and influence the distribution of stress and deformation [
38,
39]. In phorusrhacids, the absence of zonae flexoriae indicates that no form of cranial kinesis is possible. In morphotype II skulls (see [
12]), this condition is further reinforced by the presence of additional rigid elements: the processus orbitalis of the os lacrimale is fused with the os lacrimale communicans [
13], constituting a structural column that links the os lacrimale with the arcus jugalis. In
Andalgalornis, a medial lip at the contact area with the arcus jugalis ensures a rigid articulation. In this taxon, the os lacrimale also fuses cranially with both the os nasale and os frontale, while in
Kelenken the processus supraorbitalis of the os lacrimale is completely fused with the os frontale [
30]. A similar condition is observed in
Bathornis grallator, where the os lacrimale is fused with the os frontale and the os ectethmoidale, forming a solid structural unit [
19].
According to [
4], the coupling produced by the ligamentum postorbitale, when present, prevents depression of the lower jaw beyond its resting (closed-mouth) position unless the upper jaw is elevated. In other words, its absence allows independent movements of the upper and lower jaws. However, the validity of this coupling mechanism has been debated and even refuted by [
6]. Several species possessing this ligament are able of independent movement of the jaw and maxilla (see [
40,
41]), and experimental data show that the ligament offers minimal resistance to mandibular depression when the maxilla is held stationary [
42]. Zusi [
5] argued that, even when the upper jaw is fixed, the minor caudal displacement of the mandible resulting from contraction of the m. depressor mandibulae is sufficient to reduce system coordination, thereby allowing increased mandibular depression. The author also proposed that this ligament may serve functions beyond jaw coupling: specifically, it could act to stabilize and protect the os quadratum–mandibular joint by maintaining both surfaces in close apposition [
5,
6]. The predatory behavior of phorusrhacids supports this stabilizing function, as the powerful bite forces exerted on active prey could otherwise compromise the integrity of the beak [
13].
4.1. The Phorusrhacids as Akinetic Neornithes
As [
1] pointed out, the term “akinetic” can be ambiguous, as it may encompass more than one of the kinetic states they recognized. It is therefore necessary to distinguish between “morphologically or anatomically akinetic” and “functionally akinetic”. This conceptual distinction was briefly outlined, though not fully developed, by [
4], and more recently mentioned by [
43] to characterize cranial function in
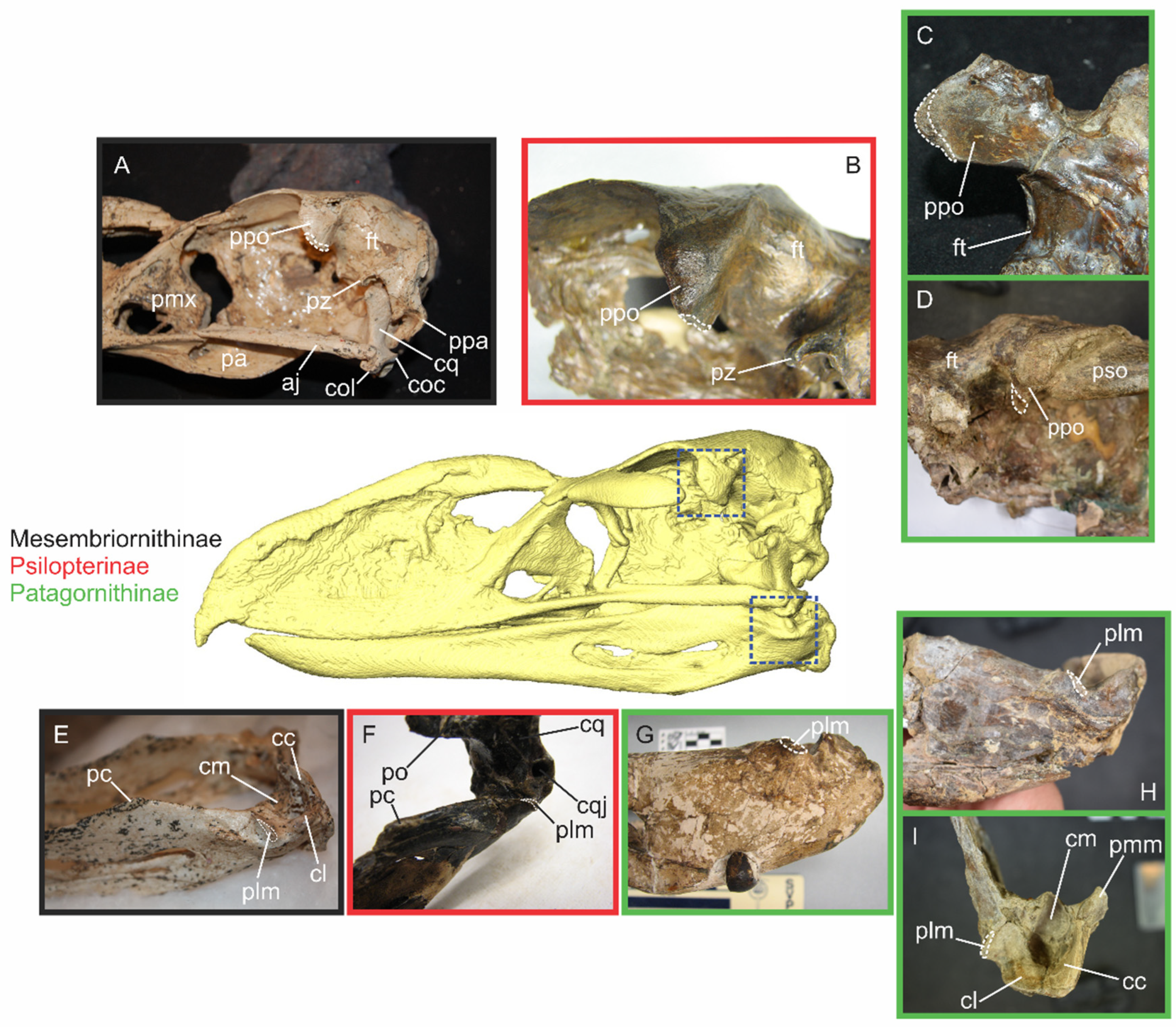
Tyrannosaurus. Phorusrhacids represent an excellent example for this differentiation: although some intracranial articulations that might allow movements are retained (e.g., os squamosum–os quadratum and os pterygoideum–os palatinum articulations), most of the mobile cranial regions present in Neornithes are absent in phorusrhacids. This includes the zonae flexoriae arcus jugalis and palatina et craniofacialis. Due to extensive bone fusion and thickening, these regions are anatomically akinetic, incapable of any cranial movement. On the other hand, the streptostylic os quadratum is theoretically capable of rotating and therefore is anatomically kinetic.
According to [
1]’s classification of kinetic states, phorusrhacids may be considered “partially kinetically competent”, as they fulfill criteria 1, 2, and 3. However, they do not meet criterion 4, which requires the presence of permissive kinematic joints. This last criterion is ultimately exclusive, as it directly relates to the dorsoventral movement of the upper jaw characteristic of prokinesis. In smaller phorusrhacids, the palate itself is the limiting structure, incapable of flexing rostrally to allow movement of the upper jaw. In larger phorusrhacids, both the palate and the upper jaw are rigid: the latter cannot flex dorsally due to the absence of the zona flexoria craniofacialis. This condition is best described as “functionally akinetic”, because—although certain skull elements may be anatomically capable of performing kinesis—the system as a whole is unable to execute such motions, and the kinetically competent joints remain immobile. This functional immobility characterizes all known phorusrhacid skulls, which in this sense, are akinetic. The term “partially kinetically competent” may lead to confusion, as it does not explicitly indicate which elements are theoretically mobile, despite ultimately referring to an akinetic system.
Here, the term “akinetic” is used in reference to both functional and anatomical immobility, depending on the scale of analysis (i.e., individual elements vs. the entire skull). A skull that is anatomically akinetic, with no mobile components, is by necessity functionally akinetic. But a functionally akinetic skull may still contain individual joints that could move if they are not mechanically constrained by other elements, thereby rendering the system immobile as a whole. In any case, within Neornithes, the term “akinetic” is appropriately applied to skulls that lack the ability to perform prokinetic movements, and this is the sense that the term is used in here.
4.2. The Functional Significance of Akinesis in Phorusrhacids
The loss of cranial kinesis in phorusrhacids is a remarkable derived feature, but the interpretation of this loss is complicated by the uncertainties about the functional role of cranial kinesis. It is possible that there is no single explanation for its evolution and function in all Neognathae [
31]. Bock [
4] suggested six potential roles for avian cranial kinesis: (1) passive maintenance of the jaw in closed position, (2) an increase in opening force in “gapers”, (3) maintenance of the primary visual orientation axis, (4) faster closure of the beak, (5) a shock absorption mechanism, (6) an increase in the attachment area for jaw musculature. Posteriorly, Zusi [
5,
11] proposed that the fundamental advantage of cranial kinesis is the potential diversity and versatility that it gives to the beak (maxilla and mandible) as a manipulative tool. Hoese and Westneat [
41] proposed that kinesis could be linked to singing ability. Zweers et al. [
28] related kinesis to the development of a greater strength of mandibular closure due to a better use of the horizontal component of the pterygoid musculature, which is highly developed in Neornithes. However, they also postulated that the kinetic mechanism could be the result of selective pressures during the development of flight: larger ocular and cerebral development and a lightening of the skull due to development of dermatocranial fenestration, as well as large nostrils, would be exaptations for cranial kinesis. Following this hypothesis, Bout and Zweers [
6] stated that the adaptive meaning of avian cranial kinesis remains uncertain and that it could be the result of the cranial design, especially associated with the enlargement of the eye. Finally, Wilken et al. [
2] recently found that cranial kinesis in birds is actually the result of the expansion of the neurocranium (as a result of the brain increment) which led to a change in jaw arrangement which influenced intracranial hinges by reorienting the protractor muscles in order to maximize the transmission of forces through the os pterygoideum.
One of the trade-offs of cranial kinesis is its relationship with bite force: although kinesis increases versatility for manipulating food items, it reduces the bite force compared with an akinetic system [
6,
35,
39]. Cranial kinesis constrains the efficiency of the mandibular muscles and therefore limits the potential for generating high bite forces. Any morphological adaptation that increases the rigidity of the mandibular apparatus, such as the ossification or transformation of flexion zones into immobile regions, will consequently enhance the theoretical maximum bite force [
44,
45]. Akinetic skulls are capable of producing bite forces up to 1.3 times greater than those of kinetic avian skulls [
6]. These considerations are consistent with both the muscular reconstructions and the high bite forces estimated for phorusrhacids [
25], suggesting that the jaw closure mechanism in these birds was optimized for bite force generation at the expense of closure speed.
Phorusrhacids, particularly those with a morphotype II skull—the so-called “terror bird” type sensu [
12]—exhibit a highly rigid cranial architecture associated with functional akinesis. This condition has profound biomechanical implications, including the capacity to deliver stronger bite forces and a simplification of food-handling mechanics. Importantly, these advantages are not strictly dependent on body size: the rigidity of the morphotype II skull likely enabled greater bite forces than those achievable by phorusrhacids with a morphotype I (“psilopterine”-type) skull [
12], which is comparatively less robust.
4.3. Evolution of Cranial Akinesis in Phorusrhacids
While [
46] proposed that the transformation of cranial flexion zones into syndesmoses or diarthroses is typically associated with increased body size—as exemplified by
Gastornis giganteus (Gastornithiformes)—Phorusrhacidae exhibit the opposite pattern. In this lineage, akinesis is not a consequence of size alone, but rather the result of a secondary transformation from a kinetically capable skull. Moreover, within Phorusrhacidae, the degree of cranial rigidity appears to correlate positively with size, such that the largest taxa display the most structurally reinforced and akinetic skulls (
Figure 5). This reversal highlights the unique evolutionary pathway of phorusrhacids, in which increased cranial stiffness may have been favored to maximize bite force and stabilize the skull during predatory strikes.
The primitive trait of Cariamiformes is the presence of cranial kinesis (i.e., prokinesis), meaning that the ancestor of all these taxa was capable of dorsal flexion of the upper beak. Akinesis is, therefore, a derived trait of the Phorusrhacidae. Both
Bathornis and members of Cariamidae exhibit skull morphologies consistent with prokinetic capabilities. Although Cariamidae lack processus basipterygoidei—and their presence cannot be confirmed in
Bathornis [
19]—the emergence of constraints on palatal mobility likely began in the common ancestor of
Bathornis, Cariamidae, and Phorusrhacidae. It appears probable that presence of the processus basipterygoidei, one of the proposed synapomorphies of Phorusrhacidae [
18], evolved prior to the complete fusion of the palatal flexion zones. The processes would have played a key role in restricting palatal retraction during contraction of the pterygoid musculature—which is especially developed in phorusrhacids—even at a time when limited motion of the zonae flexoriae was still possible. This suggests that the evolution of these processes was an early step toward the progressive akinesis observed in the lineage.
In Phorusrhacidae, the bones that constitute the zona flexoria palatina and the zona flexoria arcus jugalis are completely fused, creating rigid unions that preclude any degree palatal movement. This complete ossification and immobilization of flexion zones constitutes a synapomorphy of the Phorusrhacidae clade [
18]. When compared with the condition observed in Cariamidae (see [
29]), it is likely that the zona flexoria palatina was the first to be suppressed. Subsequently, as the arcus jugalis became more robust and extended, it likely established broader contact with the os palatinum and os maxillare, resulting in the loss of the zona flexoria arcus jugalis.
Psilopterinae and Mesembriornithinae retain the zona flexoria craniofacialis, and the contact between the os lacrimale and the arcus jugalis is mediated by a novel element, the os lacrimale communicans (also present in Cariamidae). The condition becomes even more derived in the larger Phorusrhacidae such as Patagornithinae and Phorusrhacinae, which not only lack both palatal flexion zones, but also lose the zona flexoria craniofacialis, fully abolishing any prokinetic mobility, an apomorphic condition within the clade. These taxa are also characterized by another derived trait: the processus orbitalis of the os lacrimale forms a firm contact with the arcus jugalis through its fusion with the os lacrimale communicans [
12,
13]. In
Andalgalornis and
Kelenken, this ossification pattern is taken even further, with the os lacrimale fusing with the ossa nasale and frontale, making a completely immobile and rigid structure. Thus, phorusrhacids show a unique evolutionary reversal: from ancestrally kinetic skulls to secondarily akinetic architectures, where increasing rigidity correlates with both phylogenetic position and body size, culminating in some of the most robust cranial constructions among Neornithes.
5. Concluding Remarks
Cariamiformes represent a unique lineage of Neornithes that exhibits parallel trends toward terrestriality, body mass enlargement, and progressive cranial immobilization. While the association between body enlargement and terrestriality has already been noted [
17], the link between body mass enlargement and the evolution of akinesis is particularly striking. Skull immobilization seems to have arisen through different anatomical solutions across the group: while seriemas present extensive contact between the palatine bones and the jugal bars,
Bathornis shows fusion of the lacrimals–ecthetmoid–frontals, and the processus maxillopalatinus with the processus maxillaris of the os palatinum.
Nevertheless, this trend reaches its most extreme expression in phorusrhacids, where the suppression of cranial flexion zones is most pronounced in the largest taxa. This condition seems to be closely related to their unique predatory hunting strategy: active prey was dispatched with powerful, hatchet-like strokes delivered with the beak [
13,
47]. Cranial akinesis likely evolved in tandem with this behavior, enabling and reinforcing a feeding mechanism that departs radically from other Neornithes.
It is plausible to propose that akinesis not only permitted the evolution of this peculiar predatory behavior but also played a key role in the ecological success and adaptive radiation of phorusrhacids. Since no other group of Neornithes possesses such extreme cranial modifications, Phorusrhacidae stand as the only truly akinetic clade among crown birds. In summary, the terror birds exemplify how form and function can co-evolve in unexpected ways—transforming a kinetic ancestral skull into a rigid predatory apparatus, and turning a constraint into a singular evolutionary opportunity.