The Patagonian Mara Dolichotis patagonum (Zimmermann, 1780) (Rodentia, Caviomorpha, Caviidae) in the Late Pleistocene of Northern Uruguay: Body Mass, Paleoenvironmental and Biogeographical Connotations
Abstract
1. Introduction
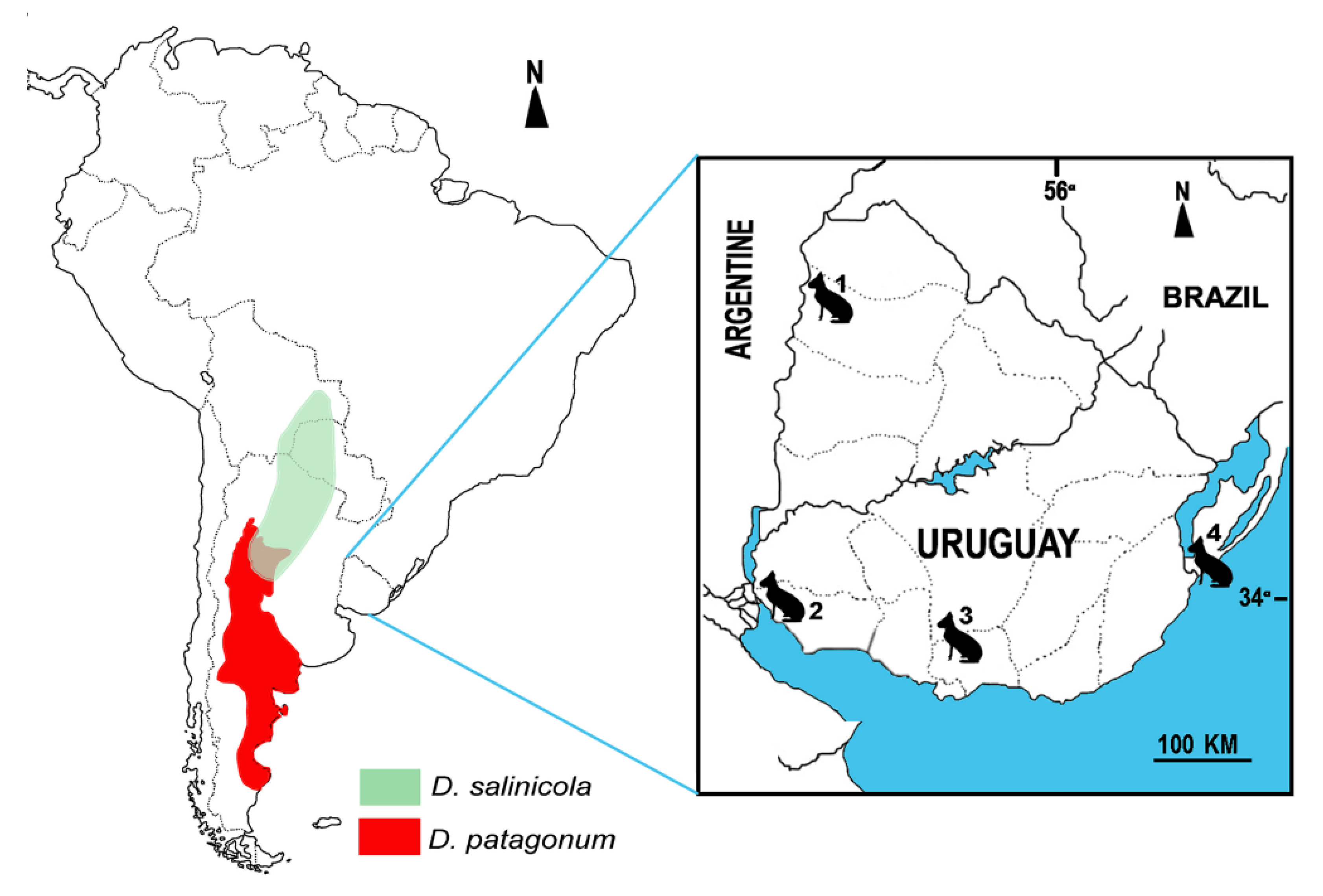
2. Geographic and Geological Setting
3. Materials and Methods
4. Results
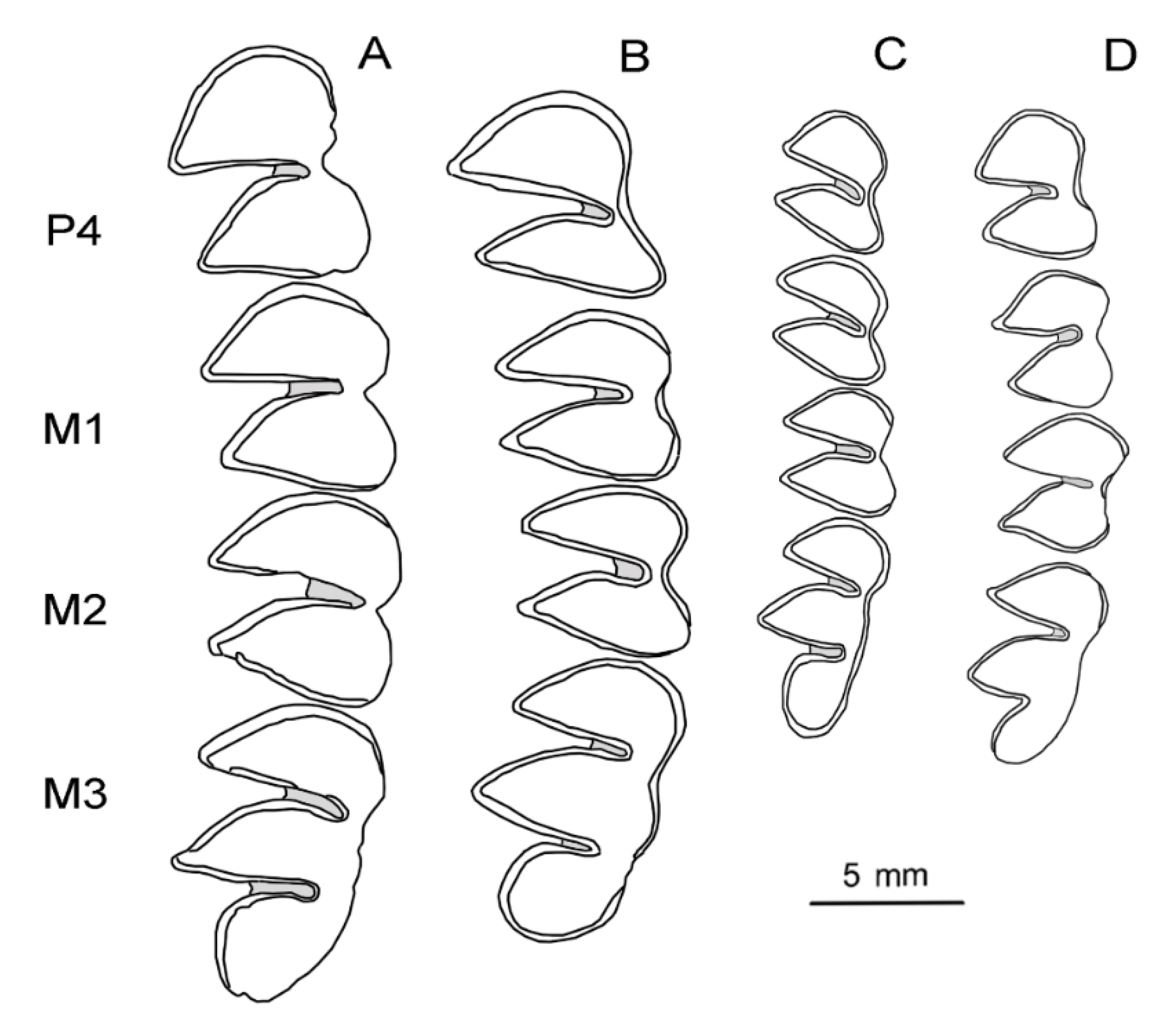
4.1. Systematic Paleontology
4.2. Body Mass Estimates
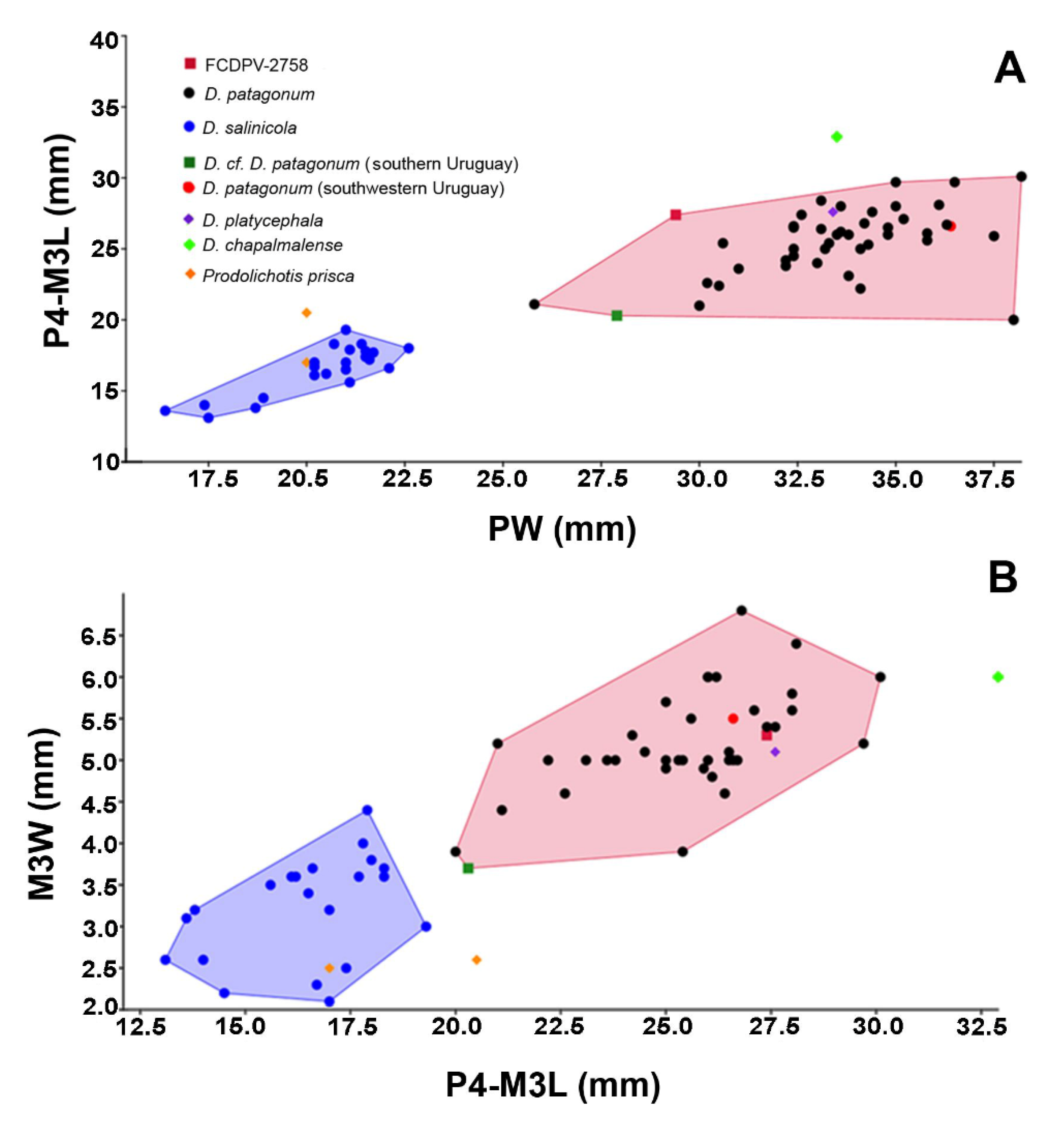
4.3. Scatter Plots and Principal Component Analyses
5. Discussion
5.1. Body Mass
5.2. Comparative Morphology
5.3. Paleoenvironment and Geographic Distribution
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campos, C.M.; Tognelli, M.F.; Ojeda, R.A. Dolichotis patagonum. Mamm. Species 2001, 652, 1–5. [Google Scholar] [CrossRef]
- Dunnum, J. Family Caviidae G. Fischer, 1817. In Mammals of South America. Rodent, 1st ed.; Patton, J., Pardiñas, U., D’Elía, G., Eds.; University of Chicago Press: Chicago, IL, USA, 2015; Volume 2, pp. 690–726. [Google Scholar]
- Alonso, V.; Udrizar, D.; Giannoni, S.M.; Campos, C. Dolichotis patagonum. In Categorización 2019 de los Mamíferos de Argentina Según su Riesgo de Extinción; Lista Roja de los Mamíferos de Argentina, SAyDS-Sarem, Eds.; 2019; p. 337. Available online: https://cma.sarem.org.ar/es/especie-nativa/dolichotis-patagonum (accessed on 20 November 2023). [CrossRef]
- Roach, N. Dolichotis patagonum. IUCN Red List Threat. Species 2016. Available online: https://www.iucnredlist.org/species/6785/22190337 (accessed on 20 November 2023).
- Bernal, N. Dolichotis salinicola. IUCN Red List Threat. Species 2016. Available online: https://www.iucnredlist.org/species/6786/22190451 (accessed on 20 November 2023).
- Calcaterra, A. Dos roedores fósiles nuevos para Uruguay y confirmación de otro. Com. Paleont. Mus. Hist. Nat. Montev. 1972, 1, 11–20. [Google Scholar]
- Ubilla, M.; Perea, D.; Rinderknecht, A.; Corona, A. Pleistocene mammals from Uruguay: Biostratigraphic, biogeographic and environmental connotations. In Quaternario de Rio Grande do Sul. Integrando Conhecimentos, 1st ed.; Ribeiro, A., Girardi, S., Saldanha, C., Eds.; Monografías Sociedade Brasileira de Paleontología: Porto Alegre, Brazil, 2009; pp. 217–230. [Google Scholar]
- Kerber, L.; Pereira, R.; Vucetich, M.; Ribeiro, A.; Pereira, J. Chinchillidae and Dolichotinae rodents (Rodentia: Hystricognathi, Caviomorpha) from the late Pleistocene of southern Brazil. Rev. Bras. Paleontol. 2011, 14, 229–238. [Google Scholar] [CrossRef]
- Madozzo-Jaén, M.; Pérez, M.; Deschamps, C. The oldest species of Dolichotis (Rodentia, Hystricognathi) from the Pliocene of Argentina: Redescription and taxonomic status of “Orthomyctera” chapalmalense. J. Mamm. Evol. 2021, 28, 995–1013. [Google Scholar] [CrossRef]
- Campo, D.; Caraballo, D.; Cassini, G.; Lucero, S.; Teta, P. Integrative taxonomy of extant maras supports the recognition of the genera Pediolagus and Dolichotis within the Dolichotinae (Rodentia, Caviidae). J. Mamm. 2020, 101, 817–834. [Google Scholar] [CrossRef]
- Madozzo-Jaén, M. Systematic and phylogeny of Prodolichotis prisca (Caviidae, Dolichotinae) from the northwest of Argentina (late Miocene-early Pliocene): Advances in the knowledge of evolutionary history of maras. Comptes Rendus Palevol. 2019, 18, 33–50. [Google Scholar] [CrossRef]
- Gervais, H.; Ameghino, F. Les Mammiferes Fossiles de la América Meridional; Savy e Igon: Paris, France, 1880; pp. 1–225. [Google Scholar]
- Ubilla, M.; Perea, D.; Goso, C.; Lorenzo, N. Late Pleistocene vertebrates from northern Uruguay: Tools for biostratigraphic, climatic and environmental reconstruction. Quat. Internat. 2004, 114, 129–142. [Google Scholar] [CrossRef]
- Ubilla, M.; Corona, A.; Rinderknecht, A.; Perea, D.; Verde, M. Marine isotope Stage 3 (MIS 3) and continental beds of northern Uruguay (Sopas Formation): Paleontology, chronology and climate. In Marine Isotope Stage 3 in Southern South America, 60 ka B.P.-30 ka B.P., 1st ed.; Gasparini, G., Rabassa, J., Deschamps, C., Tonni, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 183–205. [Google Scholar]
- Antón, D. Evolución Geomorfológica del Norte del Uruguay; Dirección de Suelos y Fertilizantes, Ministerio de Agricultura y Pesca: Montevideo, República Oriental del Uruguay, 1975; Unpublished Work. [Google Scholar]
- Ubilla, M.; Morosi, E.; Gasparini, G.; Rinderknecht, A. Brasiliochoerus stenocephalus (Lund in Reinhardt, 1880), a large extinct peccary in late Pleistocene beds of Uruguay: Comparative, isotopic and paleoecological studies. J. S. Am. Earth Sci. 2023, 129, 104531. [Google Scholar] [CrossRef]
- Martínez, S.; Rojas, A. Quaternary continental mollusks from northern Uruguay: Distribution and paleoecology. Quat. Internat. 2004, 114, 123–128. [Google Scholar] [CrossRef]
- Cabrera, F.; Montenegro, F.; Badín, A.; Ubilla, M.; Martínez, S. Continental mollusk assemblages from the Quaternary of Uruguay. J. S. Am. Earth Sci. 2023, 130, 104575. [Google Scholar] [CrossRef]
- Verde, M.; Ubilla, M.; Jiménez, J.; Genisse, J. A new earthworm trace fossil from palaeosols: Aestivation chambers from the late Pleistocene Sopas Formation of Uruguay. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 243, 339–347. [Google Scholar] [CrossRef]
- Gasparini, G.; Ubilla, M.; Tonni, E.P. The Chacoan peccary, Catagonus wagneri (Mammalia, Tayassuidae) in the late Pleistocene (northern Uruguay, South America): Palaeoecological and palaeobiogeographic considerations. Hist. Biol. 2013, 25, 679–690. [Google Scholar] [CrossRef]
- Jones, W.; Rinderknecht, A.; Montenegro, F.; Vezzosi, R.; Ubilla, M. First report of large cathartids (Aves, Cathartidae) from the late Pleistocene of Uruguay. J. S. Am. Earth Sci. 2020, 107, 102946. [Google Scholar] [CrossRef]
- Manzuetti, A.; Ubilla, M.; Jones, W.; Perea, D.; Prevosti, F. The ocelot Leopardus pardalis (Linnaeus, 1758) (Carnivora, Felidae) in the late Pleistocene of Uruguay. Hist. Biol. 2022, 35, 108–115. [Google Scholar] [CrossRef]
- Manzuetti, A.; Ubilla, M.; Jones, W.; Montenegro, F.; Perea, D. The otter Lontra Gray, 1843 (Mustelidae, Lutrinae) in the late Pleistocene-early Holocene of Uruguay. Ann. Paléontol. 2023, 109, 102633. [Google Scholar] [CrossRef]
- Cione, A.L.; Gasparini, G.M.; Soibelzon, E.; Soibelzon, L.; Tonni, E.P. The Great American Biotic Interchange. A South American Perspective, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–97. [Google Scholar]
- Cohen, K.M.; Finney, S.C.; Gibbard, P.L.; Fan, J.-X. The ICS international chronostratigraphic Chart. Episodes 2013, 36, 199–204, Updated 2022. [Google Scholar] [CrossRef]
- Ubilla, M.; Perea, D. Vertebrados del Cuaternario continental de Uruguay: Cronología y ambientes. Paleontol. Electr. APA 2023, 23, 231–240. [Google Scholar] [CrossRef]
- Morosi, E.; Ubilla, M. Feeding and environmental studies on late Pleistocene horses in mid-latitudes of South America (northern Uruguay). Quat. Sci. Rev. 2019, 225, 106025. [Google Scholar] [CrossRef]
- Corona, A.; Ubilla, M.; Perea, D. New records and diet reconstruction using dental microwear analysis for Neolicaphrium recens Frenguelli, 1921 (Litopterna, Proterotheriidae). Andean Geol. 2019, 46, 153–167. [Google Scholar] [CrossRef]
- Ubilla, M.; Rinderknecht, A. Comparative analysis of Galea (Rodentia, Caviidae) and expanded diagnosis of Galea ortodonta Ubilla & Rinderknecht, 2001 (Late Pleistocene, Uruguay). Geobios 2014, 47, 255–269. [Google Scholar]
- Ubilla, M.; Rinderknecht, A. Lagostomus maximus (Desmarest) (Rodentia, Chinchillidae), the extant plains vizcacha in the late Pleistocene of Uruguay. Alcheringa 2016, 40, 354–365. [Google Scholar] [CrossRef]
- Engelman, R.K. Occipital condyle width (OCW) is a highly accurate predictor of body mass in therian mammals. BMC Biol. 2022, 20, 37. [Google Scholar] [CrossRef]
- Bertrand, O.C.; Schillaci, M.A.; Silcox, M.T. Cranial dimensions as estimators of body mass and locomotor habits in extant and fossil rodents. J. Vertebr. Paleontol. 2015, 36, e1014905. [Google Scholar] [CrossRef]
- Millien, V. The largest among the smallest: The body mass of the giant rodent Josephoartigasia monesi. Proc. R. Soc. B. 2008, 275, 1953–1955. [Google Scholar] [CrossRef]
- Boivin, M.; Álvarez, A.; Ercoli, M.D.; Moyano, S.R.; Salgado-Ahumada, J.S.; Ortiz, A.M.; Cassini, G.H. Body mass estimation from cheek tooth measurements in extinct caviomorphs (Ctenohystrica, Hystricognathi): The importance of predictor, reference sample and method. J. Mamm. Evol. 2024, 31, 43. [Google Scholar] [CrossRef]
- Ubilla, M.; Rinderknecht, A. A late Miocene Dolichotinae (Mammalia, Rodentia, Caviidae) from Uruguay, with comments about the relationships of some related fossil species. Mastozool. Neotrop. 2003, 10, 293–302. [Google Scholar]
- Rovereto, C. Los estratos araucanos y sus fósiles. An. Mus. Hist. Nat. Buenos Aires 1914, 25, 1–249. [Google Scholar]
- Kraglievich, L. Diagnosis osteológico dental de los géneros vivientes de la subfamilia Caviinae. An. Mus. Hist. Nat. Buenos Aires 1930, 36, 59–96. [Google Scholar]
- Madozzo-Jaén, M.; Pérez, M. Redescription of a small Caviidae (Rodentia: Hystricognathi) from the Neogene of northwestern Argentina and its systematic implications. C. R. Palevol. 2024, 23, 269–292. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1. [Google Scholar]
- Ameghino, F. Los mamíferos fósiles de la República Argentina. Acta Acad. Nac. Cienc. Córdoba 1889, 6, XXXII+1–1026. [Google Scholar]
- Gorchow, J. Dolichotis salinicola. Animal Diversity Web, University of Michigan (On-Line). 2011. Available online: https://animaldiversity.org/accounts/Dolichotis_salinicola/#BC9848C1-10AC-45DB-B2AA-6CBAD0A5C85F (accessed on 14 December 2023).
- Baldi, R. Breeding success of the endemic mara Dolichotis patagonum in relation to habitat selection: Conservation implications. J. Arid Environ. 2007, 68, 9–19. [Google Scholar] [CrossRef]
- Zar, J. Biostatistical Analysis, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2010; pp. 1–944. Available online: https://bayesmath.com/wp-content/uploads/2021/05/Jerrold-H.-Zar-Biostatistical-Analysis-5th-Edition-Prentice-Hall-2009.pdf (accessed on 15 February 2023).
- Rencher, A. Methods of Multivariate Analysis, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2002; pp. 1–708. [Google Scholar]
- Brassey, C.A. Body-mass estimation in Paleontology: A review of volumetric techniques. Paleontol. Soc. Papers 2017, 22, 133–156. Available online: https://www.cambridge.org/core/journals/the-paleontological-society-papers/article/abs/bodymass-estimation-in-paleontology-a-review-of-volumetric-techniques/16BA5B00CB196283C6D07A24AABAABBA (accessed on 10 February 2023). [CrossRef]
- Janis, C.M. Correlation of cranial and dental variables with body size in ungulates and macropodoids. In Body Size in Mammalian Paleobiology: Estimation and Biological Implications, 1st ed.; Damuth, J., MacFadden, B.J., Eds.; Cambridge University Press: Cambridge, UK, 1990; pp. 255–299. [Google Scholar]
- Moncunill-Solé, B.; Jordana, X.; Marín-Moratalla, N.; Moyá-Solá, S.; Köhler, M. How large are the extinct giant insular rodents? New body mass estimations from teeth and bones. Integrat. Zool. 2014, 9, 197–219. [Google Scholar] [CrossRef]
- Moncunill-Solé, B.; Quintana, J.; Jordana, X.; Engelbrektsson, P.; Köhler, M. The weight of fossil leporids and ochotonids: Body mass estimation model for the Order Lagomorpha. J. Zool. 2015, 295, 269–278. [Google Scholar] [CrossRef]
- Damuth, J.; MacFadden, B.J. Introduction: Body size and its estimation. In Body Size in Mammalian Paleobiology: Estimation and Biological Implications, 1st ed.; Damuth, J., MacFadden, B., Eds.; Cambridge University Press: Cambridge, UK, 1990; pp. 1–10. [Google Scholar]
- Prevosti, F.; Romano, C.; Pardiñas, U.; Forasiepi, A.M.; Hemming, S.; Bonini, R.; Candela, A.M.; Cerdeño, E.; Madozzo Jaén, M.C.; Ortiz, P.E.; et al. New radiometric 40Ar-39Ar dates and faunistic analyses refine evolutionary dinamics of Neogene vertebrates assemblages in Southern South America. Sci. Rep. 2021, 11, 9830. [Google Scholar] [CrossRef]
- Brown, A.; Martínez Ortiz, M.; Acerbi, J.; Corcuera, J. La Situación Ambiental Argentina 2005; Fundación Vida Silvestre: Buenos Aires, Argentina, 2006; pp. 1–587. [Google Scholar]
- Brown, A. Ecoregiones. 2018. Available online: https://www.arcgis.com/apps/mapviewer/index.html?layers=eb24b243af3c47ab950c558f1e45d91c (accessed on 1 July 2024).
- Kufner, M.B.; Sbriller, A.P. Composición botánica de la dieta del mara (Dolichotis patagonum) y del ganado bovino en el Monte mendocino. Rev. Arg. Prod. An. 1987, 7, 255–264. [Google Scholar]
- Rosati, V.; Bucher, E. Seasonal diet of the Chacoan Cavy (Pediolagus salinicola) in the western Chaco, Argentina. Mammalia 1992, 56, 567–574. [Google Scholar] [CrossRef]
- Sombra, M.; Mangione, A. Obsessed with grasses? The case or mara Dolichotis patagonum (Caviidae, Rodentia). Rev. Chilena Hist. Nat. 2005, 78, 401–408. [Google Scholar] [CrossRef]
- Rodríguez, D.; Dacar, M. Composición de la dieta de la mara (Dolichotis patagonum) en el sudeste del Monte Pampeano (La Pampa, Argentina). Mastozool. Neotrop. 2008, 15, 215–220. [Google Scholar]
- Puig, S.; Cona, M.; Videla, F.; Méndez, E. Diet of the Mara (Dolichotis patagonum), food availability and effects of an extended drought in northern Patagonia (Mendoza, Argentina). Mamm. Biol. 2010, 75, 389–398. [Google Scholar] [CrossRef]
- APN. Fichas Ecorregiones; Administración de Parques Nacionales: Buenos Aires, Argentina, 2024; Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.sib.gob.ar/portal/wp-content/uploads/2021/10/fichas_ecorregiones.pdf&ved=2ahUKEwjHk_K58aeNAxU7GLkGHZx3AHwQFnoECBkQAQ&usg=AOvVaw3Pu5zJyQnJTUgH_QT8WQ_j (accessed on 20 November 2023).
- Elissamburu, A.; Vizcaíno, S. Limb proportions and adaptations in caviomorph rodents (Rodentia, Caviomorpha). J. Zool. Lond. 2004, 262, 145–159. [Google Scholar] [CrossRef]
- Vasallo, A.; Rocha-Barboza, O. Limb bone stress in the mara Dolichotis patagonum (Caviomorpha, Caviidae, Dolichotinae). Hystrix 2020, 31, 35–39. [Google Scholar]
- Tambussi, C.; Ubilla, M.; Acosta, C.; Perea, D. Fossil records and palaeoenvironmental implications of Chloephaga picta (Gmelin, 1789) (Magellan Goose) and Cariama cristata (Linnaeus, 1766) (Seriema) from the Late Pleistocene of Uruguay. N. Jarb. Geol. Palaeontol. Mh. 2005, 5, 257–268. [Google Scholar] [CrossRef]
- Perea, D.; Toriño, P.; Rego, N.; Vezzosi, R.; Montenegro, F. Chaetophractus villosus (Desmarest, 1804) (Xenarthra: Euphractinae) in Uruguay (Upper Pleistocene): Taxon age, biogeography, and paleoclimatic implications. J. Mamm. Evol. 2022, 29, 773–782. [Google Scholar] [CrossRef]
- Álvarez, B. Los mamíferos fósiles del Cuaternario de Arroyo Toropí, Corrientes (Argentina). Ameghiniana 1974, 11, 295–311. [Google Scholar]
- Francia, A.; Zurita, A.; Orfeo, O.; Miño-Boilini, A.R.; Erra, G.; Zacarías, S.; Rodríguez-Bualó, M.; Alcaraz, M.A.; Lutz, A.I. Paleontología y geología del Pleistoceno de la provincia de Corrientes, Argentina. Acta Geol. Lill. 2019, 26, 165–192. [Google Scholar]



| Variable | Measurements | Predictive Equation | Body Mass Estimation (g) | |
|---|---|---|---|---|
| 1 | SL | 136.6 mm | log M = 3.9519 log X − 4.2316 | 16,125 |
| 2 | CTL | 27.4 mm | log M = 3.1047 log X − 0.5796 | 7659 |
| 3 | CTW | 5.6 mm | log M = 3.3134 log X + 0.98322 | 2899 |
| 4 | COL | 12.5 mm | log M = 4.1781 log X − 0.77376 | 6445 |
| 5 | COW | 5.1 mm | log M = 3.6294 log X + 0.92033 | 3079 |
| 6 | FMH | 16.6 mm | log M = 5.1475 log X − 1.6249 | 45,249 |
| 7 | FMW | 11.8 mm | log M = 4.6018 log X − 1.5143 | 2620 |
| 8 | PAL-I | 69.4 mm | log M = 3.3273 log X − 2.0254 | 12,629 |
| 9 | CTL × CTW | 153.4 mm2 | log M = 1.6137 log X + 0.1606 | 4874 |
| 10 | skull | 119.8 mm | log M = 3.49 log X − 3.332 | 8532 |
| 11 | UTRW | 27.4 mm | log M = 2.70 log X − 0.038 | 6981 |
| 12 | upper T | 5.4 mm | log M = 2.45 log X + 2.080 | 7488 |
| 13 | OCW | 20.9 mm | ln M = 7.8157 (ln X)2/3 − 8.2573 | 3440 |
| 14 | LVS | 109.2 mm | log M = 3.225 log X − 2.797 | 5974 |
| 15 | AR | 158.1 mm2 | log M = 1.493 log X + 0.371 | 4508 |
| 16 | LR | 27.4 mm | log M = 2.916 log X − 0.335 | 7202 |
| 17 | WR | 6.0 mm | log M = 3.029 log X + 1.143 | 3162 |
| Variable | Measurements Dolichotis patagonum (ROMM 90847) * | Body Mass Estimation of FCDPV-2758 (g) |
|---|---|---|
| SL | 127.69 mm | 10,957 |
| CTL | 26.73 mm | 9640 |
| CTW | 6.29 mm | 6316 |
| COL | 14.73 mm | 5469 |
| COW | 9.18 mm | 1535 |
| FMH | 17.45 mm | 7705 |
| FMW | 10.27 mm | 13,576 |
| PAL-I | 70.24 mm | 8633 |
| CTL × CTW | 168.10 mm2 | 7802 |
| Body mass | 8950 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ubilla, M.; Ghizzoni, M.; Rinderknecht, A. The Patagonian Mara Dolichotis patagonum (Zimmermann, 1780) (Rodentia, Caviomorpha, Caviidae) in the Late Pleistocene of Northern Uruguay: Body Mass, Paleoenvironmental and Biogeographical Connotations. Foss. Stud. 2025, 3, 7. https://doi.org/10.3390/fossils3020007
Ubilla M, Ghizzoni M, Rinderknecht A. The Patagonian Mara Dolichotis patagonum (Zimmermann, 1780) (Rodentia, Caviomorpha, Caviidae) in the Late Pleistocene of Northern Uruguay: Body Mass, Paleoenvironmental and Biogeographical Connotations. Fossil Studies. 2025; 3(2):7. https://doi.org/10.3390/fossils3020007
Chicago/Turabian StyleUbilla, Martín, Martín Ghizzoni, and Andrés Rinderknecht. 2025. "The Patagonian Mara Dolichotis patagonum (Zimmermann, 1780) (Rodentia, Caviomorpha, Caviidae) in the Late Pleistocene of Northern Uruguay: Body Mass, Paleoenvironmental and Biogeographical Connotations" Fossil Studies 3, no. 2: 7. https://doi.org/10.3390/fossils3020007
APA StyleUbilla, M., Ghizzoni, M., & Rinderknecht, A. (2025). The Patagonian Mara Dolichotis patagonum (Zimmermann, 1780) (Rodentia, Caviomorpha, Caviidae) in the Late Pleistocene of Northern Uruguay: Body Mass, Paleoenvironmental and Biogeographical Connotations. Fossil Studies, 3(2), 7. https://doi.org/10.3390/fossils3020007






