Abstract
Tyrannosaurs are among the most intensively studied and best-known dinosaurs. Despite this, their relationships and systematics are highly controversial. An ongoing debate concerns the validity of Nanotyrannus lancensis, interpreted either as a distinct genus of small-bodied tyrannosaur or a juvenile of Tyrannosaurus rex. We examine multiple lines of evidence and show that the evidence strongly supports recognition of Nanotyrannus as a distinct species for the following reasons: 1. High diversity of tyrannosaurs and predatory dinosaurs supports the idea that multiple tyrannosaurids inhabited the late Maastrichtian of Laramidia; 2. Nanotyrannus lacks characters supporting referral to Tyrannosaurus or Tyrannosaurinae but differs from T. rex in >150 morphological characters, while intermediate forms combining the features of Nanotyrannus and T. rex are unknown; 3. Histology shows specimens of Nanotyrannus showing (i) skeletal fusions, (ii) mature skull bone textures, (iii) slow growth rates relative to T. rex, (iv) decelerating growth in their final years of life, and (v) growth curves predicting adult masses of ~1500 kg or less, showing these animals are subadults and young adults, not juvenile Tyrannosaurus; 4. growth series of other tyrannosaurids, including Tarbosaurus and Gorgosaurus, do not show morphological changes proposed for a Nanotyrannus–Tyrannosaurus growth series, and deriving Tyrannosaurus from Nanotyrannus requires several changes inconsistent with known patterns of dinosaur development; 5. Juvenile T. rex exist, showing diagnostic features of Tyrannosaurus; 6. Phylogenetic analysis suggests that Nanotyrannus may lie outside Tyrannosauridae. Tyrannosaur diversity before the K-Pg extinction is higher than previously appreciated. The challenges inherent in diagnosing species based on fossils mean paleontologists may be systematically underestimating the diversity of ancient ecosystems.
1. Introduction
1.1. The Tyrannosaurs, the Most Iconic of All Dinosaurs
The first small, primitive tyrannosaurs evolved in the Late Jurassic of Laurasia and then diversified in the early Cretaceous []. Initially, tyrannosaurs were largely subordinate to larger-bodied megalosauroid and allosauroid [,] predators. However, by the end of the Cretaceous, tyrannosaurs became larger and more specialized [,,], evolving giant forms with massive skulls and reduced forelimbs. Tyrannosaur evolution culminated in the late Maastrichtian, with the appearance of the giant Tyrannosaurus rex [,,]. T. rex was among the last of the tyrannosaurs and the largest tyrannosaur, perhaps the largest terrestrial predator of all time.
Tyrannosaurs are among the best-known and most intensively studied groups of dinosaurs [,,]. Dozens of skeletons are known, representing almost thirty species [,,]. Of these, far and away the most well-known, intensively studied species is Tyrannosaurus rex. Multiple studies have examined the evolution [,,], development [,,], locomotion [], feeding [,,,], and systematics [,,,,] of T. rex. It is one of the most well-known fossil organisms, and probably more thoroughly studied than most living species.
Despite this, much remains unknown. Remarkably, one of the most fundamental problems—how many tyrannosaur species are represented by fossils assigned to Tyrannosaurus—remains highly controversial. Among the most persistent issues concerns whether the latest Maastrichtian tyrannosaurs of western North America represent one species, showing remarkable variation through development, or whether small specimens represent a distinct lineage of small-bodied tyrannosaurs. This issue is of interest because this basic problem—classifying fossils into species—underpins our efforts to understand the evolution and extinction of fossil species, their geographic ranges, their growth, and their biology. That such a well-known, intensively studied animal remains so controversial is remarkable, and raises fundamental questions about the reliability of the taxonomies forming the foundation of paleontology.
1.2. Abbreviations
AMNH, American Museum of Natural History, New York, NY, USA; BHI, Black Hills Institute of Geological Research, Hill City, SD, USA; BMNH, British Museum of Natural History, London, UK; BMRP, Burpee Museum of Natural History, Rockford, IL, USA; CM, Carnegie Museum, Pittsburgh, PA, USA; CMNH, Cleveland Museum of Natural History, Cleveland, OH, USA; DDM, Dinosaur Discovery Museum, Kenosha, WI, USA; FMNH, Field Museum of Natural History, Chicago, IL, USA; LACM, Natural History Museum, Los Angeles, CA, USA; KU, University of Kansas, Lawrence, Kansas; MOR, Museum of the Rockies, Bozeman, MT, USA; NHMUK, York Natural History Museum, London, England; NMMNH New Mexico Museum of Natural History and Science, Albuquerque, NM, USA; PIN, Paleontological Institute, Russian Academy of Science, Moscow, Russia; RSM, Royal Saskatchewan Museum, Eastend, Saskatchewan, Canada; SDSM, South Dakota School of Mines and Technology, Rapid City, SD, USA; HRS, Hanson Research Station, Newcastle, WY, USA; TMM, Texas Memorial Museum, Austin, TX, USA; TMP, Royal Tyrrell Museum of Palaeontology, Drumheller, Alberta, Canada; UALVP, University of Alberta Laboratory for Vertebrate Paleontology, Edmonton, Alberta, Canada; UCMP, University of California Berkeley, Berkeley, CA, USA; UMNH, University of Utah Museum of Natural History; USNM, United States National Museum, Smithsonian Institution, Washington, DC, USA; UWBM, University of Washington Burke Museum, Seattle, Washington, DC, USA; UWGM, Geology Museum, Madison, WI, USA; ZPAL, Institute of Paleobiology, Polish Academy of Sciences, Warsaw, Poland.
1.3. Tyrannosaurus rex
Historically, a single tyrannosaur species has been recognized from the latest Maastrichtian of western North America, Tyrannosaurus rex. Named by Henry Fairfield Osborn in 1905 [], T. rex has a convoluted history (Table 1). Like many dinosaurs discovered in the ‘Dinosaur Rush’ of the late 19th and early 20th centuries, it was part of the ‘Bone Wars’, the scientific rivalry between Othniel Charles Marsh and Edward Drinker Cope [], although it would not be well-known or studied in detail until many years later.

Table 1.
Taxonomy of Tyrannosaurus and late Maastrichtian Tyrannosaurini.
Among the first discoveries that can be referred to as Tyrannosaurus is an isolated fourth metatarsal, USNM 2110, collected by J. B. Hatcher in 1890 from the late Maastrichtian-aged Lance Formation of Wyoming []. Later that year, Marsh named the fossil as an ornithomimid, Ornithomimus grandis []. Two years later, in 1892, Cope collected a pair of huge vertebrae from the Hell Creek Formation of South Dakota, which he described and named Manospondylus gigas []. Both animals, being giant tyrannosaurs, probably belong to Tyrannosaurus but are not diagnostic to species level, so these names are considered invalid.
A few years later, Barnum Brown collected a pair of tyrannosaur skeletons for the American Museum of Natural History in New York. The first, AMNH 5866 (sold to the British Museum, now BMNH R7995), was collected in 1900 from the Lance Formation of Wyoming. He collected the second, AMNH 973 (sold to the Carnegie Museum, now CM 9380), in 1902 from the uppermost Hell Creek Formation of Montana [].
In 1905, Osborn described the first skeleton, AMNH 5866, as Dynamosaurus imperiosus, and the second, AMNH 973, as Tyrannosaurus rex []. Following further preparation and study, Osborn concluded in 1906 that the two animals were “generically if not specifically identical” []. Because Dynamosaurus and Tyrannosaurus were published in the same paper, ICZN rules let Osborn, as the first reviser, choose which name to retain. Unsurprisingly, he retained the now-iconic name Tyrannosaurus rex [].
Since Osborn, Tyrannosaurus has generally been considered to contain a single species [,,,,], T. rex. However, even setting aside the controversial specimens later assigned to Nanotyrannus, significant differences exist between specimens unambiguously assigned to Tyrannosaurus [,]. The possibility of multiple species has been raised several times [,].
Larson [] cites a communication with Bakker as the source of this idea and, following Bakker, proposed that T. rex could be divided into T. rex and “T. x”, which differed in subtle details such as the shape of the second dentary tooth, tooth count, and the size of the lacrimal pneumatic foramen. Recently, Paul et al. [] recognized three species of Tyrannosaurus: T. rex, T. imperator, and T. regina; these do not neatly conform to the T. rex and T. “x” of Larson, given that Larson [] considered the holotype of T. imperator, FMNH PR 2081, the famed Sue specimen, to represent T. rex. The existence of multiple species is plausible, given the extensive variation seen in the genus [,] and the existence of species-level turnover within the Hell Creek Formation [], but it remains controversial []. We consider this hypothesis viable but in need of further study.
1.4. Nanotyrannus
The systematics of late Maastrichtian tyrannosaurs were further complicated by the naming of Gorgosaurus lancensis, later renamed Nanotyrannus lancensis, from the late Maastrichtian beds that produced T. rex. In 1942, a field party from the Cleveland Museum of Natural History discovered a small tyrannosaur skull (Figure 1) in the Hell Creek Formation of Montana []. The skull, CMNH 7541, was described and named by Charles Gilmore in a paper published posthumously in 1946 [].
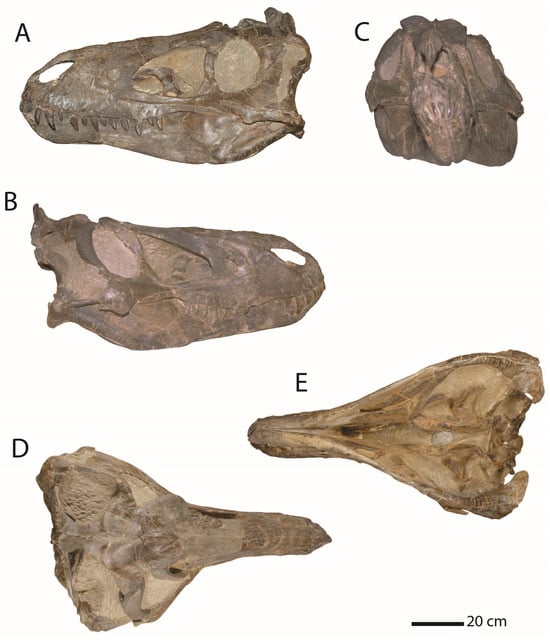
Figure 1.
Holotype of Nanotyrannus lancensis, CMNH 7541. In (A), left lateral view; (B), right lateral view; (C), anterior view; (D), dorsal view; (E), ventral view. Scale = 20 cm.
The skull differs markedly from Tyrannosaurus in its proportions (Figure 2). Gilmore compared the animal to Gorgosaurus libratus from the Campanian of Canada and, noting similarities, argued that the skull was referable to Gorgosaurus []. He described the skull as a distinct species, Gorgosaurus lancensis, primarily based on the long period of time separating the two []. Since then, the status of Nanotyrannus has proven controversial.
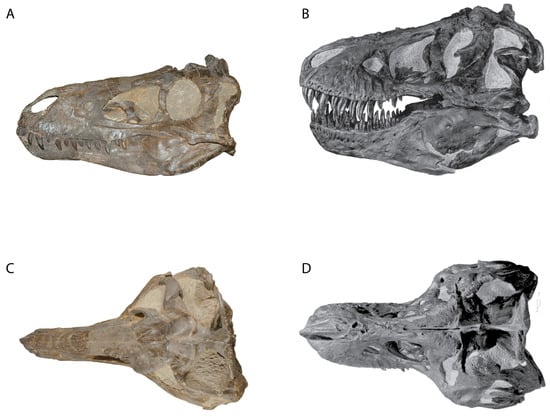
Figure 2.
Comparison of Nanotyrannus lancensis CMNH 7541 (A,C) and Tyrannosaurus AMNH 5027 (B,D) (not to scale).
Rozhdestvensky [], based on a growth series of Tarbosaurus, suggested that Gorgosaurus lancensis might represent a juvenile T. rex [], while Russell [] regarded G. lancensis as an adult.
A paper by Bakker, Williams, and Currie in 1988 [] would support Gilmore’s recognition of the animal as a distinct species. Bakker and colleagues, however, argued that Gorgosaurus lancensis was not only distinct from T. rex but distinct from Gorgosaurus and represented a deeper diverging lineage of tyrannosaur []. This would put it outside of Tyrannosauridae as currently defined (i.e., Tyrannosaurus rex + Albertosaurus sarcophagus). If so, CMNH 7541 would represent a non-tyrannosaurid member of Tyrannosauroidea. Accordingly, Bakker et al. created a new genus, renaming CMNH 7541 Nanotyrannus lancensis [].
The taxonomy of Nanotyrannus is further complicated by the discovery of a small tyrannosaur near Jordan, Montana [,,]. The “Jordan theropod” has unserrated premaxillary teeth with a chisel-shaped tip, similar to those described as Aublysodon by Leidy [] from the Campanian of Montana. Molnar and Carpenter referred the Jordan theropod to Aublysodon []. Paul (1988) described the Jordan theropod as a new species of Aublysodon, Aublysodon molnari []. It was later named as a distinct genus, Stygivenator, by Olshevsky []. Stygivenator molnari is similar to the type of Nanotyrannus in overall morphology, raising the possibility that it is synonymous with Nanotyrannus (in which case it also could be a juvenile T. rex). However, there are subtle, potentially significant differences in the shape of the teeth, maxillae, and dentaries (see Discussion).
Subsequently, Carpenter [] suggested that the holotype of Nanotyrannus lancensis might be immature and, following Rozhdestvensky, interpreted it as a juvenile T. rex. Further study by Carr [] agreed with Rozhdestvensky and Carpenter’s identification of the animal as immature. Evidence for immaturity comes from the existence of striated texture of the surficial bone, [] which is typical of young, rapidly growing dinosaurs, including extant birds [,] (but see below).
In recent years, the interpretation of Nanotyrannus as a juvenile Tyrannosaurus has been widely adopted [,,,,] but not universally accepted. Critically, we will argue, it is unclear that these animals are in fact juveniles, or that they show features referable to T. rex. Carr identified 13 characters in the Nanotyrannus type that supposedly support referral to T. rex []. These characters are problematic because many (if not all) are widely distributed in Tyrannosauridae [] or appear to be absent from Nanotyrannus (see Discussion).
Meanwhile, others have contested the referral to Tyrannosaurus. Currie [] suggested that the difference in tooth count between the animals is of taxonomic significance, arguing that theropods do not show large changes in tooth count over ontogeny. Larson [] provided the most comprehensive case for recognizing Nanotyrannus as distinct, cataloging characters potentially differentiating the two []. He also argued that a new and larger animal showing the Nanotyrannus morphology, the “Jane” specimen [], represented an adult of the species []. Larson [] also reported a new skeleton, part of the “Dueling Dinosaurs”, noting features such as elongated forelimbs, suggesting Nanotyrannus was distinct from T. rex [].
Witmer and Ridgeley [,] noted extensive differences between the holotypes of Nanotyrannus and Tyrannosaurus. They found many characters difficult to ascribe to ontogeny but remained agnostic about the taxon’s validity []. Schmerge and Rothschild [] noted a lateral groove on the dentary as potentially supporting Nanotyrannus as distinct, a conclusion disputed by Brusatte et al. [].
Woodward et al. [] sectioned bones of putative Nanotyrannus, including “Jane” and “Petey” BMRP 2006.4.4, interpreting them as juveniles of Tyrannosaurus. They noted that the bones lack an external fundamental system, a characteristic of old, slow-growing adults, and argued the histology is consistent with the animals representing juvenile Tyrannosaurus. However, a more recent analysis suggested that the growth trajectory of the second animal, BMRP 2006.4.4, could not be linked to the growth curves of T. rex [].
Finally, Carr [] attempted to synthesize the known data to assemble specimens assigned to Nanotyrannus and Tyrannosaurus into a hypothetical ‘growth curve’.
The taxonomy of Nanotyrannus is, therefore, as complicated as that of T. rex (Table 2), and its status currently remains unresolved: was Nanotyrannus a juvenile of Tyrannosaurus or a distinct lineage of tyrannosaur (Figure 3)?

Table 2.
Current taxonomy of Nanotyrannus lancensis and N. lancensis-like fossils.
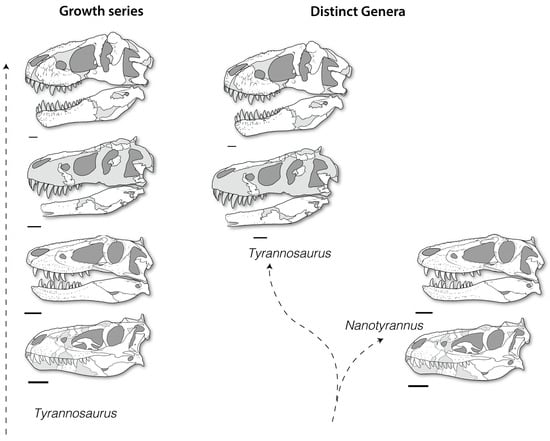
Figure 3.
Competing hypotheses. Nanotyrannus as either juveniles of Tyrannosaurus or as a distinct lineage of small-bodied tyrannosaur. Scale = 10 cm.
1.5. Purpose and Approach of This Paper
The debate over Nanotyrannus has been considered resolved by some, but important questions remain. Our goal here is to synthesize all available evidence concerning the taxonomic status of Nanotyrannus. Rather than focusing on a single line of evidence, such as a growth series or histology, our approach is to synthesize multiple different lines of evidence to provide a robust inference.
A single line of argumentation and analysis, even one that appears conclusive, could be incorrect due to biases or errors in the data. It could also arrive at incorrect conclusions due to errors in the analysis of the data, choice of models used, or interpretation. It is less likely that multiple lines of evidence will independently point to the wrong conclusions, an approach referred to as triangulation of conclusions. As Galileo argued, ‘two truths can never contradict each other’, i.e., all lines of evidence are necessarily consilient with the correct hypothesis and must agree with each other.
We consider the following lines of evidence:
- Patterns of diversity in Tyrannosauridae and other predators. The frequent coexistence of two species of tyrannosaur suggests that this pattern is the rule; the existence of a distinct taxon alongside Tyrannosaurus is expected from known patterns of dinosaur diversity.
- Morphological differences between Nanotyrannus and Tyrannosaurus and lack of intermediates. The hypothesis that the animals represent a growth series of a single species predicts intermediate forms linking the two. However, dozens of characters in almost every skull bone distinguish Nanotyrannus and Tyrannosaurus, while no intermediate forms are known.
- Developmental patterns seen in other tyrannosaurs. The hypothesized synonymy of Nanotyrannus and Tyrannosaurus suggests that Nanotyrannus will resemble the juveniles of other tyrannosaur species. However, the major changes in morphology proposed for the Nanotyrannus–Tyrannosaurus growth series are not seen in tyrannosaurs such as Tarbosaurus and Gorgosaurus, arguing against ontogeny as an explanation for morphological differences.
- Adult specimens referable to Nanotyrannus. The hypothesis that Nanotyrannus is a juvenile Tyrannosaurus predicts that all Nanotyrannus will show features associated with immaturity, including immature bone texture and lack of fusion between skeletal elements. Histology is also predicted to show Nanotyrannus specimens rapidly growing to reach adult sizes on the order of ~8000 kg. Nanotyrannus specimens instead show patterns of bone fusion and bone texture consistent with maturity, are slow growing compared to maximal growth rates of T. rex, and show a pattern of decelerating growth in their final years of life, consistent with fairly mature animals. Reconstructed growth curves predict small adult body mass (<2000 kg, more likely ~900–1500 kg).
- Juvenile tyrannosaurs showing diagnostic features of T. rex. The hypothesized Nanotyrannus–Tyrannosaurus growth series requires that small specimens showing features of Tyrannosaurus do not exist. A juvenile skull, slightly larger than Nanotyrannus, and a juvenile frontal, smaller than the holotype, represent juveniles of Tyrannosaurus.
- Phylogenetic analysis of Nanotyrannus. If Nanotyrannus is a juvenile Tyrannosaurus, then phylogenetic analysis with ontogenetically variable characters removed should cause Nanotyrannus to cluster with Tyrannosaurus. The morphology of Nanotyrannus instead places it outside of Tyrannosaurinae and Tyrannosauridae, even when restricting analysis to characters known to be stable during ontogeny.
2. Materials and Methods
2.1. Clustering Analysis
To study patterns of morphological variation, we added a series of specimens of Nanotyrannus and Tyrannosaurus to a character–taxon matrix, coded for 158 morphological characters chosen as potentially differentiating Nanotyrannus from Tyrannosaurus (Supplementary Information S1); body size (estimated skull length > 1 m) was also added as a character. UPGMA clustering analysis was run in PAUP* 4.10 b10 [] to create a tree that joins specimens where branch length is proportional to character difference. A second UPGMA analysis was conducted using the character–taxon matrix of Carr [] with hypothetical “embryo” and “adult” removed. The two datasets were also analyzed using principal coordinates analysis (PCoA), which is used instead of PCA because of its ability to handle missing data.
2.2. Histology and Growth Curves
Midshaft femoral LAG numbers (periosteum arbitrarily assigned a half-LAG value to reflect an incomplete record of annual growth) and circumferences for Sue (FMNH PR 2081) and Petey (BMRP 2006.4.4) were obtained from Cullen et al. []. The periosteal circumference was derived from Campione et al. [] for Sue, and Figure S2C of Woodward et al. [] using the measurement tool in Adobe Photoshop (v. 25.0.0) for Petey. For Jane (BMRP 2002.4.1), LAG numbers were obtained from Woodward et al. []) and either left uncorrected or corrected for split multi-LAGs (as in Cullen et al. []), reducing the number of LAGs counted from nine to six. Given the incomplete transverse section of the midshaft femur in Jane, LAG distance from the endosteal surface was first measured in Photoshop using Figure 4A in Woodward et al. []. Then, the half width (i.e., radius) of the femur at midshaft at death (i.e., including medullary cavity and cortex on one side) was measured as 43.5 mm from Figure S2A in Woodward et al. []. LAG spacing was then used to back-estimate femoral width at the time of deposition of each previous LAG (i.e., femoral radius at death was subtracted by the difference in the radii between the periosteum and the final most LAG from the endosteum, and so on towards the interior LAGs). The LAG circumference was then estimated by approximating the midshaft femoral cross section as a circle (i.e., double femur radius at a given LAG times pi). Masses at each LAG were calculated from the circumference data using Equation (7) of Campione et al. [] for bipeds without correction for the non-circular cross-section. Logistic, Gompertz, logarithmic, and von Bertalanffy growth models were fit to each specimen in R (version 4.1.1). See Supplementary Information S2 for curves and mass estimates (Supplementary Information S2), and Supplementary Information Data for data and Supplementary Information Code for code.
The Zuri pubis (HRS081514), was analyzed similarly, by measuring the LAG spacing from the cortical bone in the pubis thin section shown in Figure 3 of Griffin [] in Photoshop. Next, the growth record of the pubis’ radius was back estimated by scaling and aligning the narrow region of the thin section shown in Figure 3 to the entire transverse section in Figure 6 of Griffin [] and then obtaining the radius of the pubis at the time of death that was parallel to the measured LAG spacing. Models were fit to the data in R.
2.3. Phylogenetic Analysis
We coded Nanotyrannus lancensis into two previously published character–taxon matrices [,] using a composite coding of CMNH 7541, BMRP 2002.4.1, LACM 28471, and the HRS material (Supplementary Information S3, Supplementary Information S4). Coding focused on the skull because the postcrania of BMRP 2002.4.1 are mounted and neither BMRP 2002.4.1 nor BMRP 2006.4.4 are described. Phylogenetic analysis was run in equal-weights parsimony using PAUP* 4.10 b10 [] (a single character, character 504, was assessed to be potentially redundant and excluded). A second set of analyses was conducted using only ontogenetically stable characters, i.e., characters coding the same in Tarbosaurus adults [], subadults [], and young juveniles []. “Raptorex kriegsteini” was excluded from analysis because it appears to represent a juvenile tyrannosaurine, most likely Tarbosaurus [].
3. Results
3.1. Diversity Patterns of Tyrannosaurs and Apex Predators
The first argument for recognizing Nanotyrannus as a distinct taxon is that tyrannosaurids achieved high diversity in the latest Cretaceous (Table 3) and that well-sampled dinosaurian assemblages typically had several large predator species. This point is far from conclusive, but it is an important starting point in considering the evidence. All else being equal, we should expect multiple tyrannosaurs to exist in the latest Maastrichtian of North America, and arguments for the existence of a distinct taxon should, therefore, be considered carefully.
Tyrannosaurs were diverse in the Late Cretaceous of Laurasia [,,,,,,,,,]. Small tyrannosaurs of the Cenomanian [] and Turonian [,] were replaced by large-bodied tyrannosaurs by the Campanian [,]. Multiple lineages evolved, including the gracile Albertosaurinae [] and robust Tyrannosaurinae []. Tyrannosaurs also show endemicity; distinct taxa occur up and down the Western Interior from Mexico [] and the American Southwest [,,,] north into the Northern Great Plains [,] and the High Arctic [], implying high speciation rates.
Strikingly, several well-sampled assemblages supported two distinct tyrannosaurid taxa. The Dinosaur Park Formation assemblage of southern Canada included at least two species, the gracile Gorgosaurus libratus [] and the larger and more robust Daspletosaurus []; similarly, Gorgosaurus and Daspletosaurus coexist in the Judith River Formation []. The Nemegt Formation of Mongolia was home to the small and gracile Alioramus [] and the larger, more robust Tarbosaurus []. Although many formations contain just one species, these formations are generally poorly sampled, producing either a handful of diagnostic remains, or often, a single diagnostic specimen, so their low diversity may be due to sampling. The Horseshoe Canyon Formation is perhaps the only well-sampled formation in western North America to have a single tyrannosaur []. While our sample of faunas is limited and imperfect, it appears that, as often as not, multiple tyrannosaurs coexisted.
Other theropods show similar patterns. Multiple abelisaurids coexisted in the late Maastrichtian of Morocco [], the Campanian-Maastrichtian La Colonia Formation of Argentina [], and the Maastrichtian Lameta Formation of India [,,,]. Several large carnivores coexisted in the Cenomanian Kem Kem beds of Morocco [,] and the Cenomanian Candeleros Formation of Argentina [,]. The Late Jurassic of North America had four medium to giant carnivores—Marshosaurus, Torvosaurus, Allosaurus, and Ceratosaurus. Torvosaurus, Allosaurus, and Ceratosaurus co-occur in the Late Jurassic of Europe, and high diversity is also seen in the Late Jurassic of East Africa [].
Similar patterns occur in mammals and marine vertebrates. Mammalian apex predators are small relative to dinosaurs, but in North America, saber-toothed Smilodon coexisted with American lions, dire wolves [], and the cheetah-like Miracinonyx [], and these would have been joined by puma and jaguars []. In Europe, saber-toothed cats coexisted with cave lions and hyenas []. In Africa, as recently as 1.5 Ma, the saber-toothed cats Dinofelis and Homotherium, lions, leopards, cheetahs, and hyenas coexisted []. The more depauperate predator communities of modern ecosystems are likely due to human-induced [] megafaunal extinctions and give a biased picture of terrestrial predator diversity.
Similarly, marine ecosystems typically have multiple species of apex predators. Several species of large, predatory mosasaurs coexisted in the Maastrichtian [,], and the giant shark Otodus megalodon coexisted with the predatory whale Livyatan []. Modern marine ecosystems, meanwhile, have two different large apex predators, great whites [] and orcas [], as well as smaller apex predators, such as false killer whales and leopard seals [].
It is also common for clades of predators to show size disparity. Felids range from 1.1–1.6 kg (Prionaillurus rubiginosus) to over 300 kg (Panthera tigris) [], and canids range from 1.0–1.5 kg (Vulpes zerda) to 80 kg (Canis lupus); mustelids range from 25–250 g (least weasel, Mustela nivelis) to a maximum of 32 kg (wolverine, Gulo gulo) []. Among varanids, masses range from 16.3 g (Dampier Peninsula monitor, Varanus sparnus) to 80 kg (Komodo dragon, Varanus komodoensis) []. Among birds [], falcons range from 43 g (black-thighed falconet, Microhierax fringillarius) to 1.75 kg (Gyrfalcon, Falco rusticolus); hawks range from 93 g (Pearl Kite, Gampsonyx swainsonii) to 8.2 kg (Cape Griffon, Gyps coprotheres). Many of these size ranges were still larger in the Pleistocene prior to the elimination of larger members of these clades by humans.
Within predator clades, diversity tends to be higher towards the lower end of the mass range; that is, there are fewer species of big cats than small cats, fewer wolves than foxes, many small weasels and ferrets, and just one wolverine []. The reasons for these patterns are unknown, but they imply higher speciation rates at low mass, higher extinction at large size, or both. In light of this, one would expect the diversity of small tyrannosaurs to be higher than for large tyrannosaurs.


Table 3.
Diversity of Tyrannosauroidea in the latest Cretaceous (Campanian-Maastrichtian) of Laramidia, Appalachia, and Asia.
Table 3.
Diversity of Tyrannosauroidea in the latest Cretaceous (Campanian-Maastrichtian) of Laramidia, Appalachia, and Asia.
| Taxon | Age | Formation | Locality | Describer |
|---|---|---|---|---|
| Dryptosauridae | ||||
| Dryptosaurus aquilunguis | Late Maastrichtian | Hornerstown Formation | New Jersey, USA | [] |
| Appalachiosaurus montgomeriensis (?) | Early Campanian | Demopolis Formation | Alabama, USA | [] |
| Alioraminae | ||||
| Alioramus altai | Maastrichtian | Nemegt Formation | Mongolia | [] |
| Alioramus remotus | Maastrichtian | Nemegt Formation | Mongolia | [] |
| Qianzhousaurus sinensis | Maastrichtian | Nanxiong Formation | China | [] |
| Albertosaurinae | ||||
| Albertosaurus sarcophagus | Early Maastrichtian | Horseshoe Canyon Formation | Alberta, Canada | [] |
| Gorgosaurus libratus | Late Campanian | Dinosaur Park Formation | Alberta, Canada | [] |
| Tyrannosaurinae | ||||
| Thanatotheristes degrootorum | Early Campanian | Foremost Formation | Alberta, Canada | [] |
| Daspletosaurus torosus | Middle Campanian | Oldman Formation | Alberta, Canada | [] |
| Daspletosaurus wilsoni | Middle Campanian | Judith River Fm. | Montana, USA | [] |
| Daspletosaurus sp. | Late Campanian | Dinosaur Park Formation | Alberta, Canada | [] |
| Daspletosaurus horneri | Late Campanian | Two Medicine Fm. | Montana, USA | [] |
| Dynamoterror dynastes | Early Campanian | Menefee Fm. | New Mexico, USA | [] |
| Labocania anomala | Late Campanian | La Bocana Roja Formation | Baja California, Mexico | [] |
| Lythronax argestes | Early Campanian | Wahweap Formation | Utah, USA | [] |
| Teratophoneus curriei | Late Campanian | Kaiparowits Formation | Utah, USA | [] |
| Bistahieversor sealeyi | Late Campanian | Kirtland Formation | New Mexico, USA | [] |
| Nanuqsaurus hoglundi | Middle Maastrichtian | Prince Creek Fm. | Alaska, USA | [] |
| Shanshanosaurus huoyanshanensis | Late Cretaceous | Subashi Formation | Xinjiang, China | [] |
| Tarbosaurus bataar | Maastrichtian | Nemegt Formation | Mongolia | [] |
| Zhuchengtyrannus magnus | Campanian | Hongtuya Formation, Wangshi Group | Shandong, China | [] |
| Tyrannosaurus rex | Late Maastrichtian | Hell Creek, Lance, Frenchman, Scollard, North Horn Fms | Alberta and Saskatchewan, Canada; Montana, Wyoming, North Dakota, South Dakota, Colorado, Utah, USA | [] |
Extraordinary claims require extraordinary evidence, but the existence of multiple large predators in the late Maastrichtian of North America would be ordinary. It would be extraordinary to find a single, giant predator and no smaller species. In light of other dinosaur faunas, modern mammal communities, and marine faunas, niche partitioning between large predators is the rule. In no known ecosystem—dinosaurian, mammalian, terrestrial, or marine—did a single, giant species of predator dominate. If T. rex was the only tyrannosaurid in the ecosystem, then this leaves a remarkable gap in size between T. rex, approaching [] or exceeding [] 8000–9000 kg in mass, and the dromaeosaurs Dakotaraptor steini [] and Acheroraptor temertyorum [], which were more than an order of magnitude smaller.
3.2. Morphology of Nanotyrannus and Tyrannosaurus
The two hypotheses make different predictions about the morphology of Nanotyrannus specimens and Tyrannosaurus specimens. Both hypotheses predict that the two will show distinct forms—either a distinct Nanotyrannus morphology and a distinct Tyrannosaurus morphology or a distinct juvenile Tyrannosaurus and adult Tyrannosaurus morphology. If Nanotyrannus is a juvenile, however, the Nanotyrannus morphology and Tyrannosaurus rex morphology must be linked by intermediate forms []. These intermediates should show character states intermediate between the Tyrannosaurus state and the Nanotyrannus state and/or mosaicism, with mixtures of juvenile Nanotyrannus characters and adult T. rex characters. If, however, Nanotyrannus is a distinct taxon, such intermediates will be nonexistent; variation should be discrete, not continuous.
3.2.1. Characters Differentiating Nanotyrannus and Tyrannosaurus
The following list of 158 characters (Table 4) is assembled from previous studies of Nanotyrannus, including the original description by Gilmore [], as well as Bakker et al. [], Witmer and Ridgely [], Larson [], Schmerge and Rothschild [] phylogenetic analyses by Loewen et al. [] and Brusatte and Carr [], and new characters found during this study. The number of characters differentiating the two is remarkable. Diagnostic characters occur in every bone in the skull examined; multiple characters typically diagnose each bone. Even more striking is the absence of clear intermediate fossils linking the two morphotypes: tyrannosaurs either exhibit the Nanotyrannus character states or the Tyrannosaurus character states but never combinations of these states, strongly arguing that they represent two distinct species.

Table 4.
Morphological characters differentiating Nanotyrannus and Tyrannosaurus.
This list is not comprehensive. Some characters were subtle or variable and so excluded pending further study. The list also focuses on cranial characters because casts, specimens, and descriptions are more readily available, but the postcrania also show marked differences, which require more study. Selected characters are illustrated (Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12 and Figure 13) but are not meant to exhaustively catalog all diagnostic characters found.
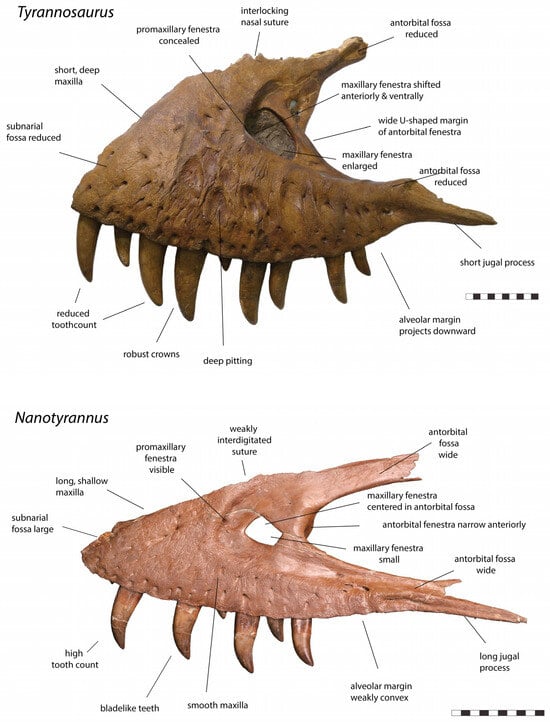
Figure 4.
Left maxilla of Tyrannosaurus rex CM 9380 and right maxilla (reversed) of Nanotyrannus BMRP 2002.4.1 in lateral view, showing anatomical characters differentiating the two. Scale = 10 cm.
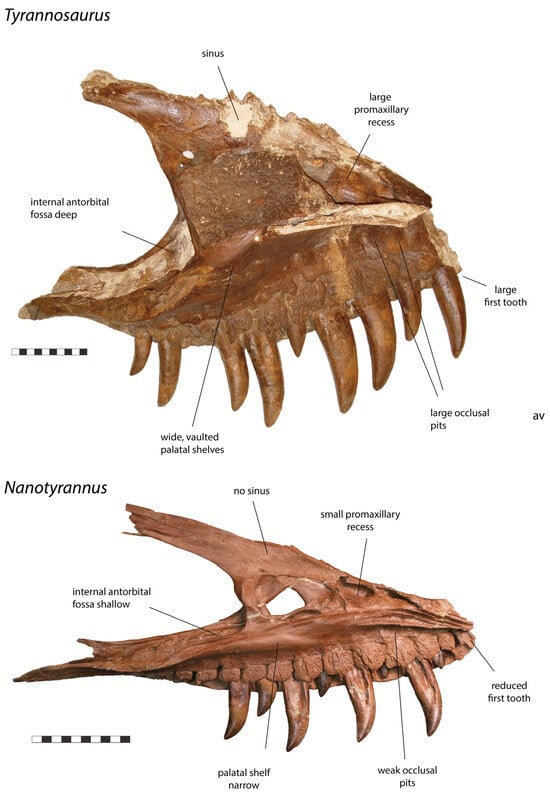
Figure 5.
Left maxilla of Tyrannosaurus rex CM 9380 and right maxilla (reversed) of Nanotyrannus BMRP 2002.4.1, medial view, showing anatomical characters differentiating the two. Scale = 10 cm.
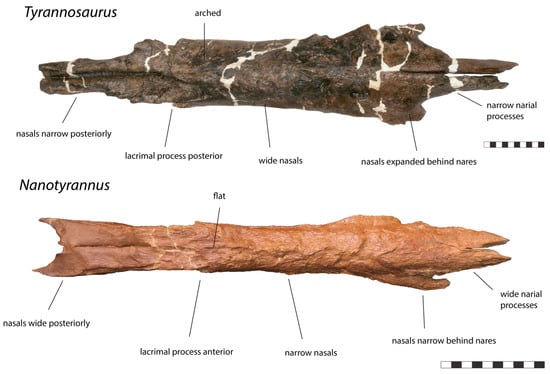
Figure 6.
Nasals of Tyrannosaurus RSM P2523.8 and nasals of Nanotyrannus BMRP 2002.4.1, dorsal view, showing anatomical characters differentiating the two. Scale = 10 cm.
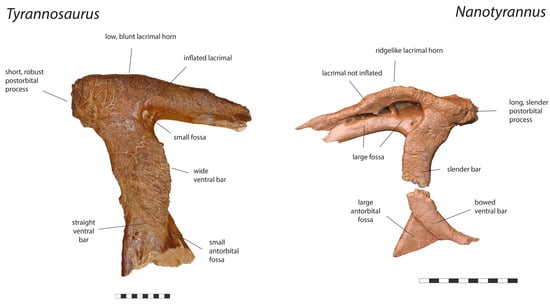
Figure 7.
Right lacrimal of Tyrannosaurus rex holotype CM 9380 and left lacrimal Nanotyrannus BMRP 2002.4.1, lateral view, showing anatomical characters differentiating the two. Scale = 10 cm.

Figure 8.
Right postorbital of Tyrannosaurus rex LACM 150167 and left postorbital Nanotyrannus BMRP 2002.4.1 in lateral view, showing anatomical characters differentiating the two. Scale = 10 cm.
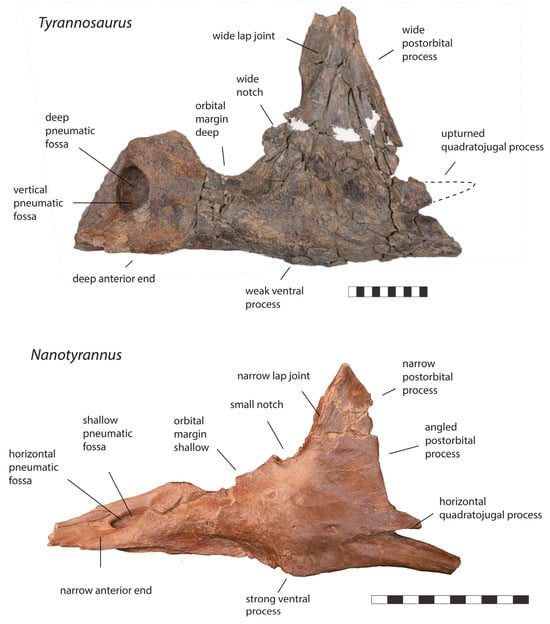
Figure 9.
Left jugal of Tyrannosaurus RSM P2523.8 and left jugal of Nanotyrannus BMRP 2002.4.1 in lateral view, showing anatomical characters differentiating the two. Scale = 10 cm.
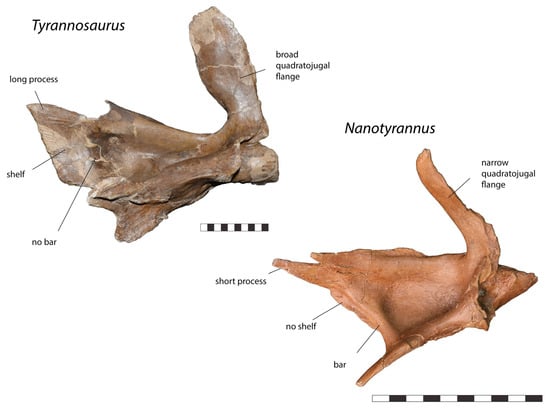
Figure 10.
Right squamosal of Tyrannosaurus CM 9380 and left squamosal (reversed) Nanotyrannus BMRP 2002.4.1 in ventral view, showing anatomical characters differentiating the two. Scale = 10 cm.
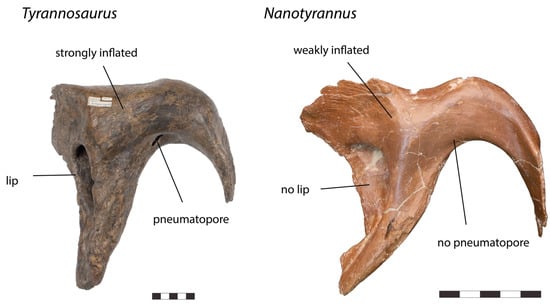
Figure 11.
Left ectopterygoid of Tyrannosaurus RSM P2523.8 and right ectopterygoid (reversed) of Nanotyrannus BMRP 2002.4.1 in lateral view, showing anatomical characters differentiating the two. Scale = 5 cm.
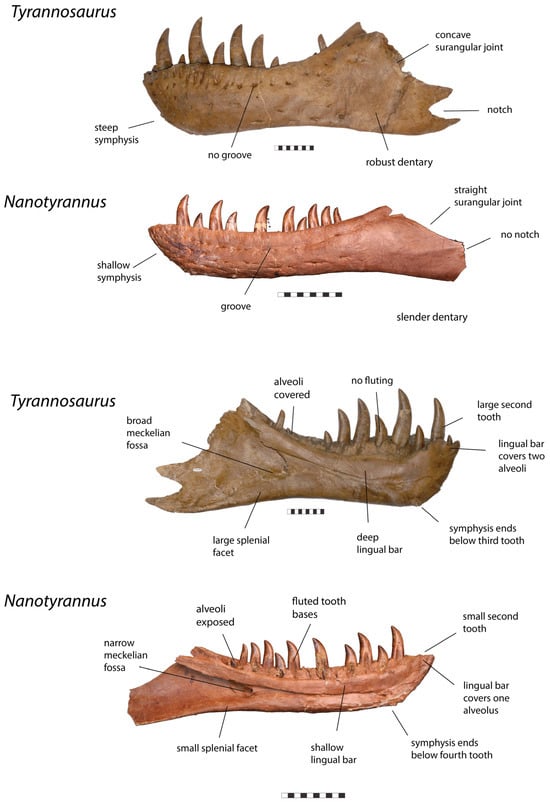
Figure 12.
Left dentary of Tyrannosaurus rex CM 9380 and right dentary (reversed) Nanotyrannus BMRP 2002.4.1 in lateral (top) and medial (below) views, showing anatomical characters differentiating the two. Scale = 10 cm.
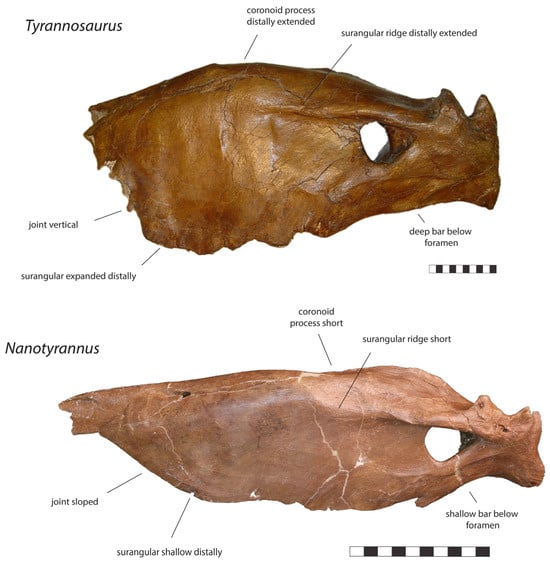
Figure 13.
Left surangular of Tyrannosaurus CM 9380 and right surangular (reversed) Nanotyrannus BMRP 2002.4.1 in lateral views, showing anatomical characters differentiating the two. Scale = 10 cm.
Character List: Characters differentiating Nanotyrannus and Tyrannosaurus. Ontogenetically stable characters = †, Ontogenetically labile characters = *. Nanotyrannus characters are plesiomorphies unless denoted otherwise.
We were able to assess just over half of these characters (80/158) for their stability over the course of development in Tyrannosaurini by examining their expression in juvenile Tarbosaurus [] versus adult Tarbosaurus [,] (or for the frontals, juvenile versus adult Tyrannosaurus: see below). Not all characters are known for juveniles of Tarbosaurus, and a handful of the characters that diagnose Tyrannosaurus do not occur in Tarbosaurus, but of the 50% of characters that could be assessed, just under half (36 characters, 45%) changed over ontogeny, and slightly more than half (44 characters, 55%) were stable, being visible in young juveniles and adults. It is possible that some characters scored here as ontogenetically stable are absent in very young T. rex, but they do not change over the ranges of size relevant to the Nanotyrannus problem. Characters related to the premaxilla, maxilla, and dentition tended to be stable; characters related to the orbit, cranial ornament, and skull roof tended to show ontogenetic change.
This is meant to be a preliminary study; more specimens and a more thorough analysis of the problem are required. However, if some differences could conceivably be explained by ontogeny, not all can be. Furthermore, there is the issue of whether the distribution of characters in the specimens is consistent with this hypothesis.
3.2.2. Character Distribution and Clustering Analysis
The hypothesis that the Nanotyrannus morphology and the Tyrannosaurus morphology represent endpoints of a “growth series” makes a testable prediction about the distribution of the characters distinguishing the two. The “growth series” hypothesis predicts that morphological intermediates must exist between the endpoints of the small Nanotyrannus morphology and the large T. rex morphology. If Nanotyrannus is a juvenile T. rex, then the species should progressively pick up T. rex-like characters. It should exhibit traits intermediate between the two (e.g., a maxilla taller than in Nanotyrannus but lower than in T. rex), or exhibit a mosaic of traits of the two, or some combination of intermediate traits and mosaicism.
However, the traits are strongly clustered and show a discrete rather than continuous distribution, with no clear intermediates known. This distribution of traits is inconsistent with the hypothesis of a growth series.
To visualize these patterns, we performed a clustering analysis. We analyzed the morphological data using a UPGMA clustering analysis (Figure 14) using PAUP* 4.10 b10 to analyze a matrix of 158 anatomical characters coded for Nanotyrannus and Tyrannosaurus. Because a UPGMA tree shows branch lengths as proportional to similarity, it serves to visualize the overall difference between the specimens. If the two represent a growth series, they should form a continuum. Instead, specimens show two discrete clusters, consistent with two separate lineages. A similar pattern (Figure 15) emerges using principle coordinates analysis (PCoA).
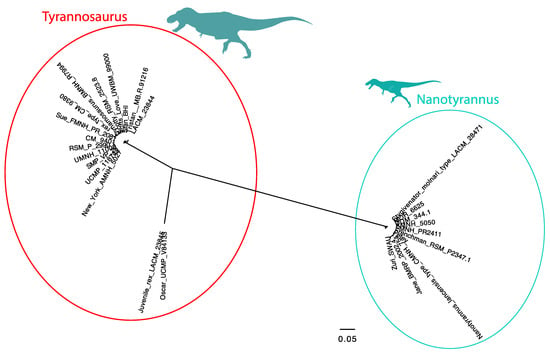
Figure 14.
Unweighted pair group with arithmetic mean (UPGMA) tree showing distinct clustering of Tyrannosaurus and Nanotyrannus, with no intermediate morphotypes using characters identified in this study (Supplementary Information S2). Of 158 potentially diagnostic morphological characters, almost all are invariant, exclusively found in either Nanotyrannus or Tyrannosaurus.
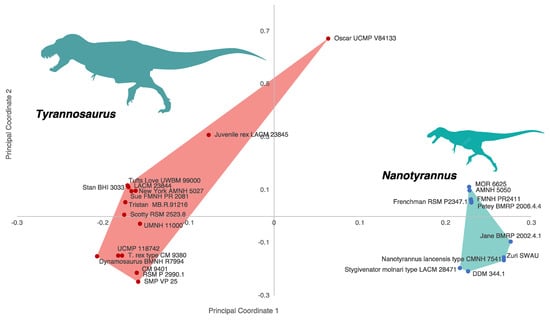
Figure 15.
Principal coordinates analysis (PCoA) showing discrete clusters of Tyrannosaurus and Nanotyrannus using characters identified during this study (Supplementary Information S2). The first principal coordinate explains 60.138% of the variation and drives most of the separation between Tyrannosaurus and Nanotyrannus; the second explains 10.921% of the variation.
The discrete clusters found here are not a result of our choice of characters but reflect the highly dissimilar anatomy of the fossils. This can be shown by repeating the same analysis using the dataset of Carr []. This character–taxon dataset is meant to capture ontogenetic changes but recovers a similar pattern to the one found with our dataset. This pattern is seen with UPGMA analysis (Figure 16) and PCoA (Figure 17). The lone exception is BMRP 2006.4.4, which clusters with Tyrannosaurus rex. This does not seem to result from a strong character signal because (i) the animal lacks cranial material, (ii) the femur was not coded, and (iii) the matrix includes a very large number of subtle characters of the pedal phalanges which (as the material has not been described) we could not verify, but which may drive this pattern. We suspect the placement of BMRP 2006.4.4 is a coding artifact, but further study of the characters and material is needed.
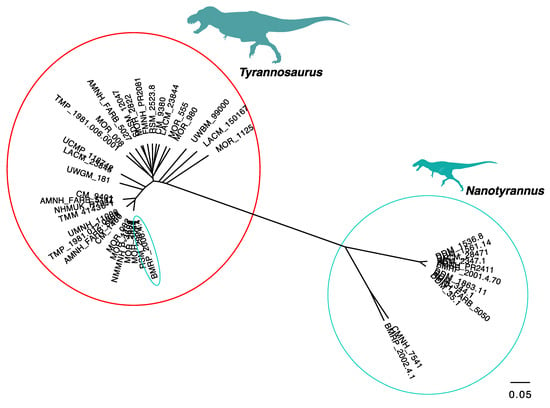
Figure 16.
Unweighted pair group with arithmetic mean (UPGMA) tree showing distinct clustering of Tyrannosaurus and Nanotyrannus, using the Carr [] dataset.
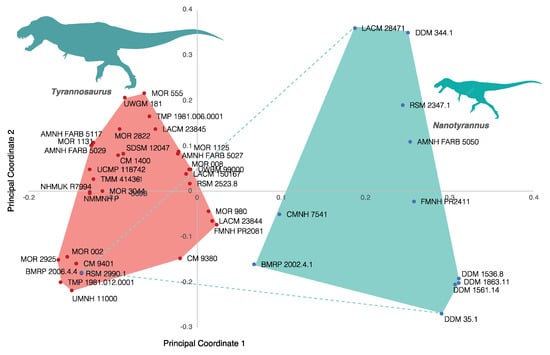
Figure 17.
Principal coordinates analysis (PCoA) showing the first two principal coordinates using the Carr [] dataset. Tyrannosaurus and Nanotyrannus form discrete clusters with the exception of Nanotyrannus BMRP 2006.4.4, which lacks cranial material. The first principal coordinate explains 29.348% of the variation; the second principal coordinate explains 6.1163%.
Again, variation is discrete; the Tyrannosaurus morphs cluster to the exclusion of the Nanotyrannus morphs without intermediates. This pattern is consistent with two distinct evolutionary lineages rather than a growth series.
Finally, we studied the variation of a discrete, multi-state character, tooth count, versus size (Figure 18), using dentary toothrow length as a size proxy []. Nanotyrannus has more teeth than Tyrannosaurus. Although tooth count has been hypothesized to change as the animals grow, when the Tyrannosaurus tooth count is plotted against toothrow length, the slope is almost horizontal, with no clear correlation between toothrow length and maxillary tooth count (R2 = 0.0123) or dentary tooth count (R2 = 0.0077). This suggests that tooth count, while variable between individuals, does not change markedly as animals grow. Nanotyrannus shows a slight increase in tooth count with size, but the sample size is small. These results suggest that the difference in tooth count between Nanotyrannus and Tyrannosaurus does not result from differences in size and age of the animals.
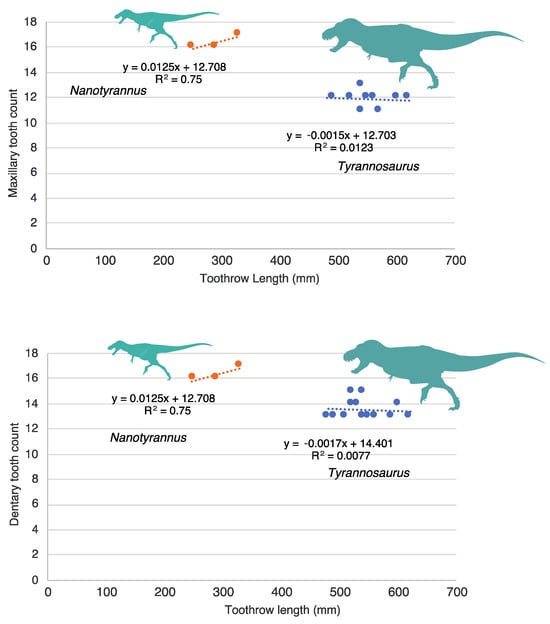
Figure 18.
Tooth count versus dentary toothrow length in Nanotyrannus and Tyrannosaurus. Top, maxillary tooth count versus dentary toothrow length; bottom, dentary tooth count versus dentary toothrow length. Tooth count shows no correlation with toothrow length in Tyrannosaurus, suggesting that tooth count does not change with size. Data from Larson [] and maxilla HRS 0438.
3.3. Nanotyrannus Morphology Inconsistent with Predicted Morphology of Juvenile Tyrannosaurinae
The hypothesized “growth series” linking Nanotyrannus to Tyrannosaurus can be tested by comparing it with the growth series of other tyrannosaurids, especially tyrannosaurines. If Nanotyrannus is a juvenile of Tyrannosaurus, and its distinctive morphology is the result of immaturity, then features of Nanotyrannus are predicted to occur in juveniles of other tyrannosaurs. If Nanotyrannus is a distinct taxon, then these features will be absent. We argue that juveniles of other tyrannosaurs do not conform to the “growth series” proposed for Tyrannosaurus [].
A young juvenile of Tarbosaurus bataar, a close relative of Tyrannosaurus, is known []. In several features—posteriorly wide nasals, a gracile postorbital, a slender dentary, and lack of the suborbital process of the orbit—the animal resembles Nanotyrannus. This means some features seen in Nanotyrannus could conceivably be juvenile characters, but these features do not necessarily mean that the animals are juvenile since they occur in adults of tyrannosauroids such as Alioramus [,].
However, the juvenile Tarbosaurus skull differs from that of Nanotyrannus in many ways while resembling adult Tarbosaurus and T. rex (Figure 19). Features shared with Tarbosaurus and T. rex (but not Nanotyrannus) include the tall and deep maxilla, the narrow rim of the antorbital fossa, an anteriorly placed maxillary fenestra, a large maxillary fenestra, limited contribution of the lacrimal to the antorbital fossa, weak curvature of the ventral ramus of the lacrimal, an anteriorly expanded jugal, and a broad base of the jugal postorbital process.
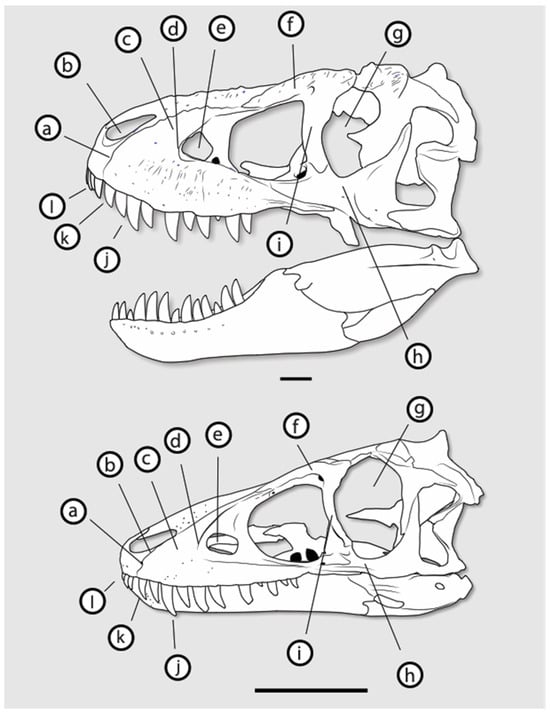
Figure 19.
Ontogenetically stable characters in juveniles and adults of Tarbosaurus (Tyrannosaurini). Characters: (a) promaxillary fenestra concealed, (b) narial process of premaxilla faces anteriorly, (c) maxilla relatively short and tall, (d) promaxillary fenestra concealed in lateral view, (e) maxillary fenestra positioned anteriorly and ventrally, (f) lacrimal horn low, (g) orbit tall, (h) jugal narrow below orbit, (i) weakly curved orbital bar of lacrimal, (j) large anterior maxillary teeth, (k) first maxillary tooth large, (l) premaxillary teeth with pointed apices and serrated. Scale = 10 cm.
These features appear early in the ontogeny of Tarbosaurus and would presumably occur early in the ontogeny of Tyrannosaurus. The absence of these features in absolutely larger Nanotyrannus specimens is difficult to explain in terms of ontogeny unless Tyrannosaurus had a pattern of development unlike that of Tarbosaurus (Figure 20).
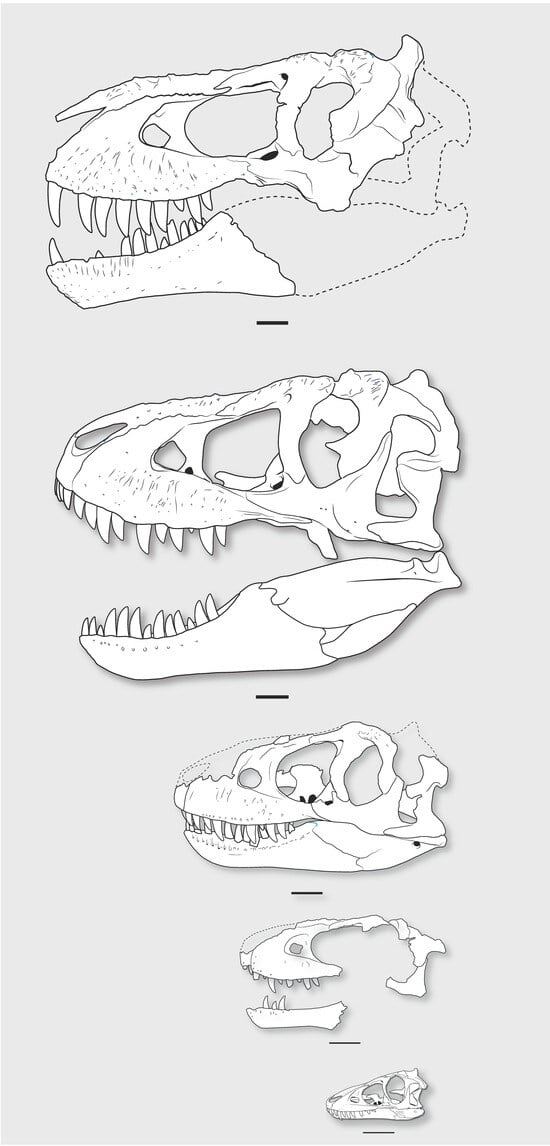
Figure 20.
Growth series of Tarbosaurus. Top to bottom: PIN 551-1 [], ZPAL MgD-I/4 [], PIN 553-1 [], PIN 552-2 []. Scale = 10 cm.
Some features of Tyrannosaurinae, especially those related to the skull ornamentation, orbits, and skull roof, appear to develop late, but others appear in even the youngest specimens (Figure 19 and Figure 20). At least some tyrannosaurine features would be expected in Nanotyrannus if it was a juvenile tyrannosaurine, but few, if any, are present.
Juveniles are also known for Gorgosaurus libratus, including skulls [,] and isolated elements []. Juveniles are remarkably similar to adult Gorgosaurus, particularly in the shape of the maxilla, antorbital fenestra, antorbital fossa, and maxillary fenestra, implying that Gorgosaurus did not undergo radical changes in skull anatomy as it grew. Neither do juvenile Gorgosaurus exhibit Nanotyrannus-like characters such as the expanded antorbital fossa, procumbent premaxillary teeth, or a pneumatized quadratojugal. Growth patterns in Gorgosaurus, therefore, argue against Nanotyrannus’ morphology being the result of immaturity.
For Nanotyrannus to be a juvenile Tyrannosaurus, Tyrannosaurus would have had to have a radically different development pattern than Tarbosaurus or Gorgosaurus. This is not impossible; ontogeny evolves. However, it is more parsimonious to treat Nanotyrannus and Tyrannosaurus as distinct species.
Finally, the proportions of the manus in the two animals are inconsistent with Nanotyrannus developing into Tyrannosaurus (Figure 21). Despite coming from much smaller animals, approximately 5–6 m in length (versus 12 m or more in Tyrannosaurus), manual phalanges of BMRP 2006.4.4 and HRS 15001 are significantly larger than those of even very large Tyrannosaurus, FMNH PR 2081. While allometric growth is possible, with the manus becoming proportionately smaller, the proportions seen in Nanotyrannus require the manus and claws to become absolutely smaller–for bone to be resorbed and elements reduced in length–as the animal matures. We are unaware of any amniote that develops in this fashion. Another problem is that the tip of the vomer is deeper in Nanotyrannus than in Tyrannosaurus; this would require the end of the vomer to shrink or be resorbed [].
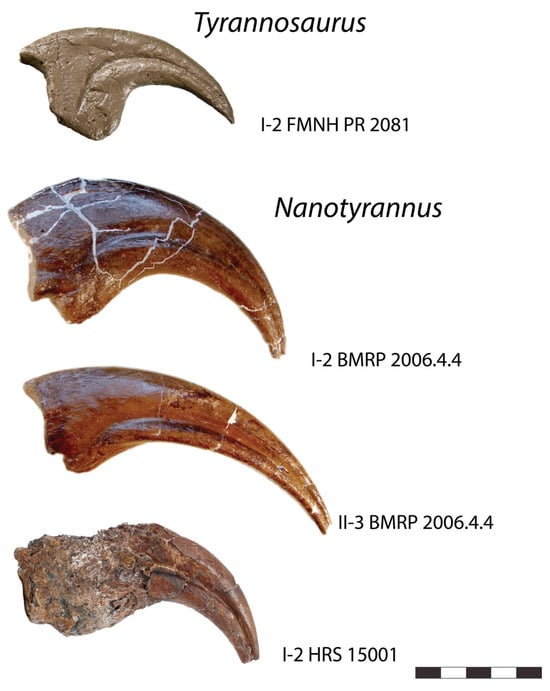
Figure 21.
Manual unguals of Tyrannosaurus and Nanotyrannus, shown to scale; small Nanotyrannus specimens have absolutely larger unguals than much larger Tyrannosaurus. Scale = 5 cm.
Larson [] also notes that patterns of pneumaticity are stable in birds as they grow, which makes differences in the presence and position of pneumatic foramina, such as the maxillary fenestra (Figure 4), difficult to explain.
3.4. Histology Supports Existence of Mature Nanotyrannus
3.4.1. Use of Histology to Test the Two Hypotheses
If Nanotyrannus is a juvenile of Tyrannosaurus, then all individuals showing the Nanotyrannus morphology must be immature relative to Tyrannosaurus. Bone histology can be used to infer the age and maturity of fossils, either to estimate absolute age (i.e., years of age) or relative maturity (e.g., young, rapidly growing juveniles and subadults, slower growing young adults versus old adults with slowed/ceased growth). The study of histology encompasses all aspects of bony tissue development, not simply thin sections and growth lines. To assess whether animals putatively identified as Nanotyrannus represent juveniles of T. rex or a distinct, small-bodied tyrannosaur taxon, maturity can be assessed in at least five distinct ways:
- (i)
- Patterns of skeletal fusion;
- (ii)
- Bone surface texture;
- (iii)
- Presence/absence of an external fundamental system (EFS);
- (iv)
- Patterns of annual growth rates, either in terms of measures of bone deposition or kilograms of mass;
- (v)
- Predicted adult mass, extrapolated from growth curves.
Nanotyrannus individuals show skeletal fusion and rugose facial bone, suggesting they were approaching maturity. Histology shows that Nanotyrannus individuals lack an external fundamental system, meaning that they are not old adults, but they show annual growth rates suggesting maturity. They also have predicted adult masses strongly suggestive of a distinct, small-bodied taxon rather than of juveniles of the giant Tyrannosaurus.
3.4.2. Skeletal Fusion
In vertebrates, composite elements such as the skull, vertebrae, shoulder girdle, sacrum, and pelvis may fuse late in development when growth slows. In crocodilians, centra and neural arches of vertebrae typically fuse late in life []. In ceratopsids, skull elements and their associated osteoderms fuse late in development []. Which elements fuse and the sequence of fusion can vary from taxon to taxon and even individual to individual []. Furthermore, some skull elements fuse early in ontogeny. The parietal bones, for example, are fused even in very young individuals in ceratopsids [] and tyrannosaurids [,], and nasals are fused even in very young tyrannosaurids [,]. Therefore, not all fusions signal maturity. Some elements, however, only fuse in large individuals, suggesting their fusion correlates with skeletal maturity.
The scapula and coracoid fuse appear to fuse late in many dinosaurs, including Herrerasaurus [], Abelisauridae [], and at least some dromaeosaurs, including Velociraptor mongoliensis [] and Achillobator giganticus []. Fusion of the scapulocoracoid also occurs in tyrannosaurs. Partial scapulocoracoid fusion is seen in Albertosaurus sarcophagus []; complete fusion is seen in a large T. rex [] and a Tyrannosaurus from the Naashoibito member of the Kirtland Formation [].
In some theropods, the pelvis shows partial or complete fusion in large individuals. The pubis and ilium fuse in the microraptorine Hesperonychus elizabethae []; the ilium, ischium, and pubis fuse in Coelophysoidea [], Abelisauridae [,,], and Ornithomimidae [,,,]. Fusion of the pubis and ischium also occurs in a large individual of T. rex []; the pubes and ischia are fused in Daspletosaurus UALVP 52981.
While not all skeletal fusions are correlated with maturity, fusion of the vertebrae, pectoral girdle, and pelvic girdle do seem to correlate with maturity. Strikingly, a number of fusions occur in Nanotyrannus BMRP 2002.4.1 []. These include fusion or partial fusion of neural arches to centra, fusion of the scapulocoracoid, and fusion of the ilium, pubis, and ischium []. This degree of skeletal fusion is consistent with the animal being a nearly full-sized subadult or early adult []. Further study of skeletal fusion patterns is needed for tyrannosaurs (and dinosaurs more generally), but evidence from skeletal fusions suggests that Nanotyrannus are not juveniles of Tyrannosaurus.
3.4.3. Surface Texture
In many dinosaurs, the adult skull bones take on a rugose to gnarled surface texture and may develop sculpturing. In chasmosaurine ceratopsians, for example, juveniles and subadults have smooth, striated skull bones. In adults, the bone takes on a gnarled texture, resembling tree bark, often with extensive, high-relief rugosity [,,] and grooves for blood vessels. The appearance of rugose bone texture can be used as a rough proxy for maturity in Ceratopsidae. Striated bone is not seen in the very oldest individuals but is seen in very large individuals of Torosaurus [], showing that it persists relatively late in subadults and young adults.
A similar pattern is seen in tyrannosaurids. In Gorgosaurus, nasals [], maxillae [], and postorbitals [] are relatively smooth in juveniles, and become more rugose in subadults and adults. A similar pattern occurs in postorbitals of Daspletosaurus []. Young Tarbosaurus show weak sculpturing of the maxilla, while nasals and lacrimals are almost smooth []; smooth facial bones are seen in another juvenile tyrannosaurine, the holotype of “Raptorex kriegsteini” [], likely a juvenile Tarbosaurus []. Adults have highly rugose facial bones [].
These patterns are hard to quantify or characterize objectively, but overall, it appears that rugosity of facial elements increases as animals mature, providing a rough proxy for maturity. As in Ceratopsidae, striated bone persists relatively late in ontogeny, being seen in subadult Alioramus [], young adult Gorgosaurus [], and in the types of the tyrannosaurines Bistahieversor [] and Thanatotheristes []. Although the presence of striated bone may show that an animal has not ceased growing entirely, its presence in relatively large young adults suggests that it cannot be used to identify animals as juveniles.
In the smallest Nanotyrannus specimen, LACM 28471, the surface of the maxillae and nasals is smooth, with little sculpture. However, in the larger N. lancensis holotype, CMNH 7541 (Figure 22), much of the nasals, maxillae, and the anteroventral surface of the dentary are rugose, as are the lateral surface of the lacrimal, the descending process of the postorbital, and the jugal ventral surface. Striated bone occurs inside the antorbital fossa, on the dentary’s dorsolateral surface, and the dorsal part of the jugal. The specimen, therefore, shows a mixture of textures, as expected for a subadult or young adult.

Figure 22.
Facial bone rugosity in the Nanotyrannus lancensis holotype CMNH 7541. (A), nasal in left lateral view, (B), right maxilla, (C), right dentary. Scale = 5 cm.
In Jane (BMRP 2002.4.1), the maxillae, lacrimals, postorbitals, nasals, and the tip of the dentary are highly rugose and covered with grooves, sculpturing, and gnarled bone (Figure 23); striated bone is found on the antorbital fossa of the maxilla and lacrimal, the posterior end of the nasal, and the posterior end of the dentary. These bone textures suggest a subadult or young adult. The Zuri specimens show highly rugose sculpturing on the maxilla, nasals, lacrimals, and dentary tip. The maxilla of KU 155809 is also highly rugose. Meanwhile, the nasals of LACM 23845, the smallest definitive Tyrannosaurus skull, show weak sculpturing.
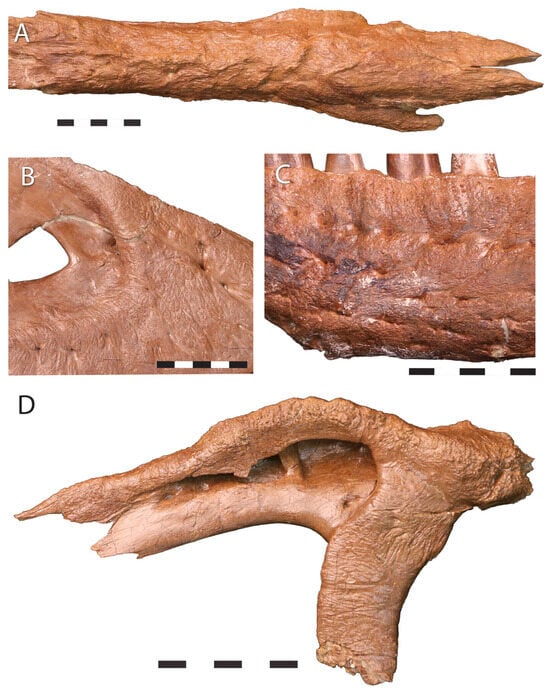
Figure 23.
Facial bone rugosity in Jane BMRP 2002.4.1. (A), nasals, (B) right maxilla, (C), left dentary, (D), left lacrimal. The facial bones are extensively sculptured, similar to the condition seen in large Tyrannosaurus rex. Scale = 5 cm.
Striated surface textures associated with growth occur in the holotype of Nanotyrannus lancensis CMNH 7541 and in BMRP 2002.41. However, striated bone is seen in subadults or yong adults of other tyrannosaurs [,,,]. Overall, bone textures suggest a degree of maturity in these animals, suggesting they are subadults or young adults of a distinct taxon, not juveniles of Tyrannosaurus.
3.4.4. External Fundamental System
The external fundamental system, or EFS, is an outermost band of very slow-growing bone with multiple, closely spaced lines of arrested growth (LAGs). It is deposited as growth rates slow and plateau late in life. An EFS can be used as an indicator of the cessation of significant growth and the attainment of maximum body size in a highly mature animal.
The existence of an EFS would be strong evidence that an animal was old and had effectively stopped growing. The absence of an external fundamental system would suggest that the animal had yet to achieve full adult size. It would mean the animal was not an old adult; however, given that the EFS appears late in life, as the animal attains maximum size [,,], it would not preclude the possibility that an animal was a young adult just short of full size.
Three putative Nanotyrannus, BMRP 2002.4.1, BMRP 2006.4.4, and HRS 081514, have been sectioned and lack an EFS [,]. This shows these animals are not old adults but does not preclude the possibility that these animals are young adults. In T. rex, individuals are nearly full size before establishing an external fundamental system. Sue, FMNH PR 2081, grew to an estimated 7930 kg before establishing an EFS [,], then died at 8223 kg [], adding only around 300 kg (i.e., <4% increase) after the appearance of the EFS. BMRP 2002.4.1, BMRP 2006.4.4, and HRS 081514 may represent young adults.
3.4.5. Growth Rates
Lines of arrested growth (LAGs) record changes in bone circumference and diameter over time (Figure 24). Assuming that such lines develop annually, as is commonly done in paleohistology, it becomes possible to reconstruct growth rates by using measured and estimated circumferences either reported in or calculated from published data [,,] to estimate mass [] at various points in the individual’s lifespan. By converting femoral circumferences into body mass estimates [], one can estimate changes in mass in terms of kilograms per year (Table 5). Note that Jane’s femur is incomplete, so the circumference was approximated as a circle using LAG spacing from the endosteum and using femur width (Supplemental Material).
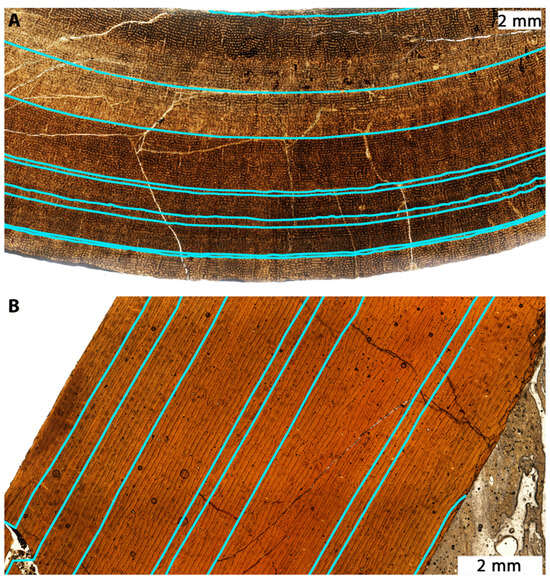
Figure 24.
Thin sections of the femur of (A), BMRP 2002.4.1 (“Jane”) and (B), BMRP 2006.4.4 (“Petey”). An external fundamental system (EFS) is absent, but lines of arrested growth or LAGs (blue) become closer toward the outer edge of the bone, showing decelerating growth. Modified from []. Note that some LAGs are likely split (multi-LAGs) and do not represent a full year of growth.

Table 5.
LAG data, body mass, and growth rate estimates rounded to the nearest kg for Sue (FMNH PR 2081), Petey (BMRP 2006.4.4), and Jane (BMRP 2002.4.4) based on femoral circumferences, including corrected values for Jane based on a split multi-LAG interpretation []. Campione et al. [] estimate Sue’s mass at death (i.e., after LAG #23) as 7377 kg after applying a correction for non-circular femoral cross-section. Hutchinson et al. [] estimate Jane’s mass at death (i.e., after LAG #9) as 954 kg based on 3D modeling. See Supplemental File for equations used in calculations. Note that LAG #1 and the periosteum do not record a full year of growth. Periosteum is assigned a LAG pseudo-number of +0.5 after the final LAG, indicating that death could have occurred at any point after the last yearly marker was deposited while assigning an age at the midpoint of that final year. Jane required estimation of LAG/periosteum circumferences from LAG spacing and femoral width due to the incomplete cross-section of the femur midshaft. (1) LAG (or periosteum) distance from endosteum is measured at the time of death, (2) femoral width at midshaft (including medullary cavity) is measured at time of death and back estimated using LAG spacing, (3) femoral circumference derived from femoral width at a given year is approximated as a circular cross-section. Data sources: Campione et al. []; Cullen et al. []; Woodward et al. [].
Juvenile tyrannosaurs have high maximal growth rates, approaching [] or exceeding 800 kg/y in Tyrannosaurus. Immature Tyrannosaurus, particularly juveniles weighing ~1000–2000 kg, are predicted to have high growth rates as they enter their exponential growth phase. If Nanotyrannus is a distinct, small-bodied tyrannosaur, then it will have much lower growth rates at this size, comparable to growth curves modeled for small-bodied tyrannosaurids such as Gorgosaurus or Albertosaurus []. However, if the two taxa are synonymous, then specimens the size of BMRP 2002.4.1 and BMRP 2006.4.4 should be in their rapid, exponential growth phase—especially if one assumes that they have entered their teenage years [].
Narrow spacing of LAGs (Figure 24), especially towards the periosteum, shows low growth rates in putative Nanotyrannus specimens (Figure 25, Table 5). Growth rates do not exceed 150 kg/year for the last few years of life and can be less than 50 kg/year. This rejects the hypothesis that these are young, rapidly growing T. rex, which achieved peak growth rates exceeding 800 kg/y based on the estimates from FMNH PR2081 (Table 5).
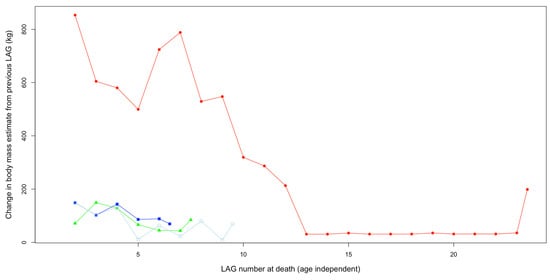
Figure 25.
Changes in estimated body mass from previous LAG as preserved in the femurs of Sue (red, circles), Petey (green, triangles), and Jane BMRP 2002.4.1, corrected for possible split multi-LAGs as in Cullen et al. [] (dark blue, solid squares) and uncorrected as in Woodward et al. [] (light blue, open squares), at the time of death. Periosteum included here as the final half LAG. LAG 1 excluded here because (1) it is a partial record of growth due to medullary cavity remodeling and bone resorption and (2) because Jane’s incomplete femur and Sue’s core sampling (rather than a complete transverse section) exclude easy measurement/estimation of endosteal circumference. An EFS is readily apparent in the last decade or so of Sue’s life. See the Supplemental Material for plots that exclude the periosteum.
3.4.6. Growth Trends
Growth rates change over time. Growth accelerates until roughly the middle of life in symmetric logistic and asymmetric Gompertz growth models as commonly applied to dinosaurs [,,,] then decelerates. Growth finally slows and almost stops in the last few years of life, reaching asymptotic growth. Based on their size (and even their approximated age from prior studies []), if the animals assigned to Nanotyrannus are juvenile T. rex, then they should show increasing growth rates (i.e., exponential growth) as they approach the rapid, protracted growth spurt at the middle of the tyrannosaur life cycle at around 1000–4000 kg []. If they are subadults or adults of a distinct, small-bodied species, they would be expected to show decelerating or ceased growth.
Growth rates in Nanotyrannus show a general trend of deceleration in their final years of life. These trends resemble those seen in mature T. rex as growth begins to plateau just before it establishes an EFS. These patterns are strongly suggestive of relatively mature animals, either late-stage subadults or early adults, not rapidly growing juveniles.
Estimated maximum size. It is possible to fit various kinds of growth curves to estimated masses [], which can be extrapolated and used to predict the mass that a given individual would have achieved at full size. This approach can be used to test whether Nanotyrannus specimens would have grown to the enormous sizes (~8000 kg) seen in T. rex. If the putative Nanotyrannus were juveniles of T. rex, then their predicted adult masses should be on the order of 5000–10,000 kg, as in T. rex. If they are subadults or young adults of small-bodied tyrannosaurs, then their predicted adult masses should be much lower.
A caveat is that, when fitting a growth model to a year-by-year growth record from a single individual rather than to mass-age data from separate individuals, the assumption of independence of data is violated, making these pseudo-regression analyses (and making the calculation of confidence/prediction intervals moot). These models are nevertheless useful in extrapolating adult masses when individuals die prior to reaching full size. This is because, although extrapolation is always highly uncertain in science, we are limited to the growth record preserved in the femora; speculation that growth rates could have exponentially increased had these putative Nanotyrannus specimens lived longer is, therefore, a weaker argument than the use of adult size estimates from these pseudo-regressions. We prefer the admittedly high uncertainty of extrapolation modeled on empirical evidence to speculation (i.e., one could speculate that any number of changes in growth rate or morphology might have occurred post-mortem since such speculation is unbounded by fossil evidence).
Growth curves using asymptotic logistic, Gompertz, and von Bertalanffy models predict fully adult masses (Figure 26) on the order of perhaps ~700–1100 kg for BMRP 2006.4.4 and ~1200–2100 kg for BMRP 2002.4.1 (when corrected for split multi-LAGs []). Non-asymptotic logarithmic models can achieve higher masses since they have no upper limit, but these predicted masses still fall far short of T. rex (Figure 27) and are closer to that of Albertosaurus. These estimates are, in the context of comparison with T. rex, also roughly consistent with mass estimates at the time of death for BMRP 2002.4.1 derived from 3D modeling []. Mass estimates are not available for the Zuri specimen (HRS 081514) because the pubis was sectioned rather than the femur. However, plotting the growth of this specimen using data from Griffin [] shows slow growth and growth deceleration rather than rapid, accelerating growth (Figure 28); it was apparently near full size when it died.
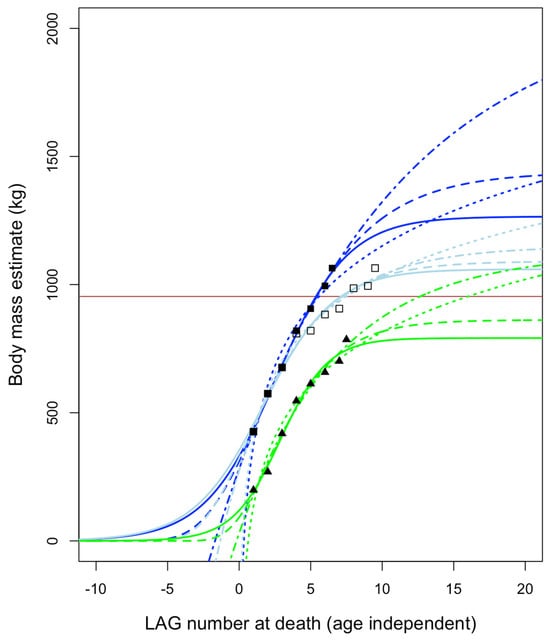
Figure 26.
Age-independent growth curves for Nanotyrannus BMRP 2006.4.4 Petey (green, triangles) and Jane, the latter both corrected (dark blue, solid squares) for split multi-LAGs as in Cullen et al. [] and uncorrected (light blue, open squares) as in Woodward et al. []. LAG #1 and the periosteum (arbitrarily assigned a half-year value) are included in the regressions. Four models are fit to each specimen: logistic (solid), Gompertz (dashed), logarithmic (dotted), and von Bertalanffy (dot–dash). Horizontal line is the mass estimate at the time of death for Jane from Hutchinson et al. [] using 3D modeling. Results are similar to those deriving from regressions that exclude LAG #1 and the periosteum (Supplemental Material).
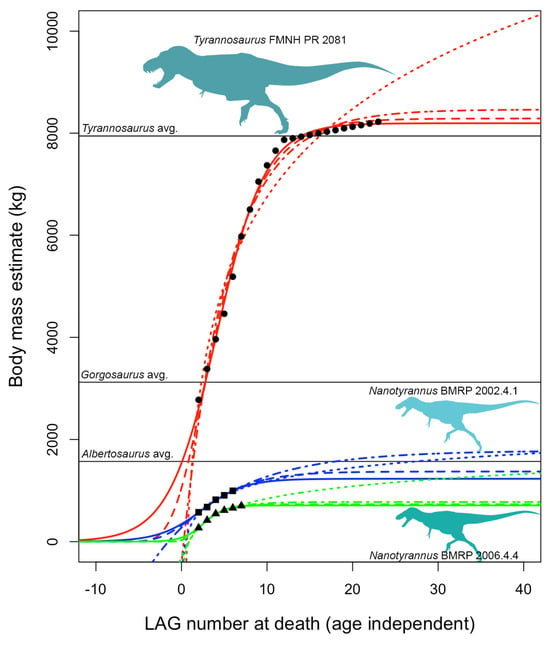
Figure 27.
Age-independent growth curves for a large, old Tyrannosaurus, Sue FMNH PR 2081 (red, circles), and two Nanotyrannus, Petey BMRP 2006.4.4 (green, triangles) and Jane BMRP 2002.4.1 (dark blue, squares), corrected for split multi-LAGs as in Cullen et al. []. LAG #1 and the periosteum are not included in the regressions shown here. Four different growth models are fit to each specimen: logistic (solid), Gompertz (dashed), logarithmic (dotted), and von Bertalanffy (dot—dash). Horizontal black lines are asymptotic masses derived from logistic regressions of multiple individuals (i.e., multiple specimens used in the regression, with each specimen assigned a single mass and age at death) from Longrich et al. (in review) and are presented in decreasing order as follows: Tyrannosaurus, Gorgosaurus, Albertosaurus. Results are similar to those deriving from regressions that include LAG #1 and the periosteum (Supplemental Material).
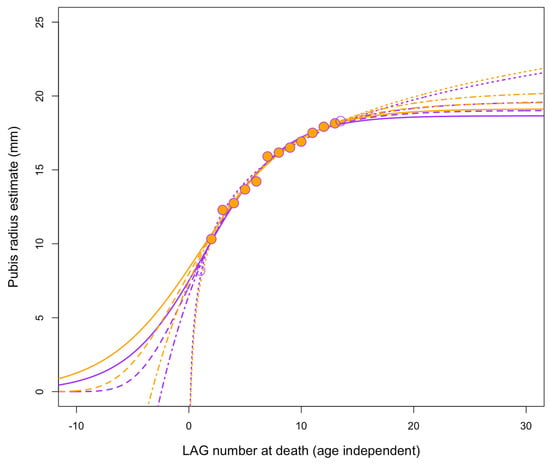
Figure 28.
Growth record from Zuri (HRS 081514) pubis, with radius back-estimated from LAG spacing []. Models including the periosteum and LAG 1 are in purple; excluding the periosteum and LAG 1 are in orange. Four different growth models are fit under both conditions: logistic (solid), Gompertz (dashed), logarithmic (dotted), and von Bertalanffy (dot–dash).
All mass estimates for adult Nanotyrannus are far below those expected for T. rex (Figure 27), which is predicted to hit ~8000 kg or more depending upon the model and mass estimates used. Growth trajectories of BMRP 2002.4.1 and BMRP 2006.4.4 are, therefore, inconsistent with their identification as juvenile Tyrannosaurus rex, even under a variety of growth models and initial conditions during curve fitting (Supplemental Material). Our estimates instead suggest that they represent a distinct, small-bodied taxon. Although it is conceivable that young Tyrannosaurus sometimes showed slow growth rates due to sickness, lack of food, or other stresses, it is unlikely that all three individuals sectioned would exhibit similar growth anomalies; it is more likely that they exhibit typical growth rates for their taxon.
Another alternative hypothesis for this variation in growth trajectories, while assuming taxonomic synonymy, would be that the putative Nanotyrannus specimens are members of the smaller sex in T. rex. While it is reasonable to assume that Sue is fairly representative of average adult T. rex size for its sex (i.e., as far as fossil discovery approximates random sampling of the population of T. rex), the magnitude of hypothetical body mass dimorphism between Sue and the Nanotyrannus specimens from at least the asymptotic models (Supplemental Material) would be implausible. This hypothetical dimorphism would exceed those estimated or observed in other non-avian [] and avian [] dinosaurs, highly sexually dimorphic mammals such as sperm whales [], and would only be comparable to the most extreme examples of sexual dimorphism in extant tetrapods (e.g., southern elephant seals [,]).
3.5. Existence of Juvenile Tyrannosaurus Refutes Identification of Nanotyrannus as Juvenile Tyrannosaurus
The hypothesis that Nanotyrannus is a juvenile Tyrannosaurus predicts that the two forms should not overlap in size; that is, all Nanotyrannus will be small, and all Tyrannosaurus will be big. No small Tyrannosaurus should exist. Conversely, if Nanotyrannus is a distinct species, then small juveniles of Tyrannosaurus—approaching the size of Nanotyrannus or smaller—must exist. Juvenile dinosaurs tend to be extremely rare; however, potential juveniles of Tyrannosaurus are known, including a partial skull.
The smallest unambiguous Tyrannosaurus skeleton known is LACM 28345 []. This specimen exhibits diagnostic features of T. rex, including broad, posteriorly tapering nasals, short nasal processes of the frontals, loss of the cornual process of the lacrimal, a reduced antorbital fossa of the lacrimal, and reduced exposure of the antorbital fossa on the maxilla [].
The skull of LACM 28345 is an estimated 800 mm long. This is 40% longer than the holotype of Nanotyrannus lancensis (CMNH 7541) [], which measures 570 mm [] but only about 12% larger than the estimated skull length for Jane BMRP 2002.4.1, which measures ~710 mm. LACM 28345 is unfortunately incomplete but exhibits the Tyrannosaurus morphology in almost all characters for which it can be coded [].
Although it is conceivable that the differences in morphology seen could rapidly develop as the animals mature at this size, it seems unlikely. The apparent absence of smaller Tyrannosaurus has been considered evidence that Nanotyrannus represents a juvenile T. rex. However, isolated specimens document individuals comparable to or smaller than Nanotyrannus in size.
One such specimen is UCMP V84133 from the Hell Creek Formation (Figure 29). The specimen is a small right frontal. It differs from the frontals of the Nanotyrannus lancensis holotype CMNH 7541 and DDM 334.1 [,,] in several respects (Figure 30). First, the nasal process is narrow, half the width of the frontal or less; the nasal processes are more than half the width of the frontal in Nanotyrannus. Second, the lacrimal is broadly extended inward to constrict the skull table and inserts into deep depressions on the lateral surface of the frontal, approaching the condition seen in T. rex [,]. Third, the orbital margin is not visible, with the postorbital and the lacrimal contacts approaching one another so they would have contacted, excluding the frontal from the orbit.
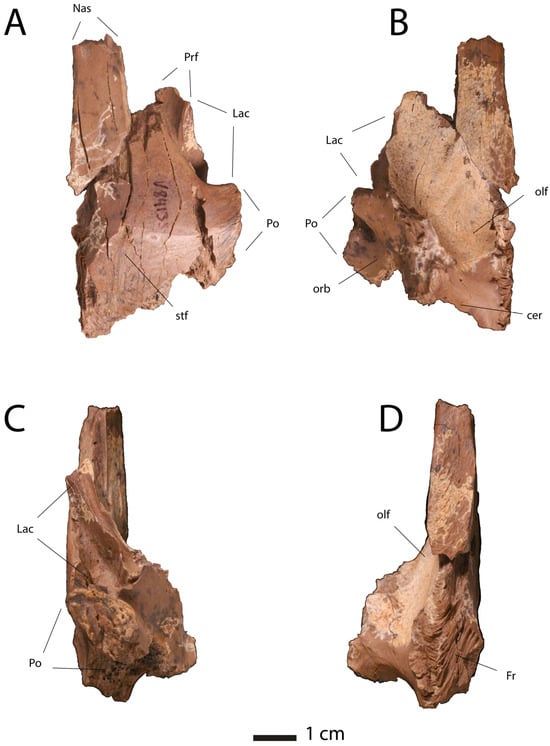
Figure 29.
Frontal of juvenile Tyrannosaurus UCMP V84133 in (A), dorsal, (B), ventral, (C), lateral, and (D), medial view. Abbreviations: cer, cerebral fossa; Fr, frontal facet; Lac, lacrimal, Nas, nasal; olf, olfactory tract; orb, orbital fossa; Po, postorbital facet; Prf, prefrontal facet, stf, supratemporal fossa.
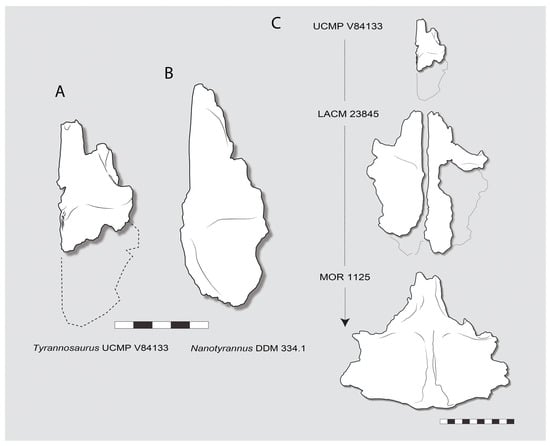
Figure 30.
(A,B), frontals of juvenile Tyrannosaurus compared with Nanotyrannus DDM 334.1 (Scale bar = 5 cm); (C), growth series connecting UCMP V84133 Tyrannosaurus juvenile to the larger juvenile LACM 23845 and, finally, adult MOR 1125. Scales = 5 cm (A,B); 10 cm (C).
Furthermore, the postorbital process is dorsoventrally extended, again resembling T. rex; this feature is absent in Tarbosaurus and is, therefore, an autapomorphy of Tyrannosaurus []. The posterior end of the frontal is deflected downward relative to the skull table, another feature characteristic of T. rex. The bone is dorsoventrally thickened, a feature of tyrannosaurids. The frontals also become thinner where the sagittal crust approaches the midline, showing the development of the double-ridge condition characterizing T. rex []. None of these features are seen in Nanotyrannus [].
Although the frontal differs from adult specimens of Tyrannosaurus rex in its shape and proportions, it can be connected with other specimens to form a growth series (Figure 30), suggesting it represents a young T. rex.
The paired frontals would have been about 80 mm across, suggesting an animal about 60% larger than the smallest known Tarbosaurus [], implying a skull length of around 465 mm—significantly smaller than the type specimen of Nanotyrannus. Assuming skull length was around 12% of body length, this would imply a total length of ~4 m.
Phylogenetic analysis of this specimen (Figure 31) recovers it as a derived tyrannosaurine, but unresolved with respect to Tyrannosaurus, Tarbosaurus, and Zhuchengtyrannus. This is consistent with its referral to Tyrannosaurus rex. This result is recovered in the dataset derived from Dalman et al. [,,] and also the dataset of Brusatte and Carr [,].
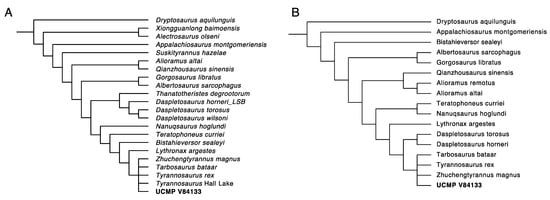
Figure 31.
Phylogenetic analysis of the isolated frontal UCMP V84133. (A), analyzed using the Dalman et al. dataset [], strict consensus of 14 trees (; Tree length = 1787; Consistency index (CI) = 0.3788; Retention index (RI) = 0.7484) and (B) the dataset based on Brusatte and Carr [,], strict consensus of 105 trees (Tree length = 760; Consistency index (CI) = 0.5592; Retention index (RI) = 0.8149).
3.6. Phylogenetic Analysis Suggests Nanotyrannus Is Not a Member of Tyrannosauridae
If Nanotyrannus is not Tyrannosaurus, then what is it? Gilmore [] concluded that Nanotyrannus lancensis was a species of Gorgosaurus. This would place it in the family Tyrannosauridae, in the subfamily Albertosaurinae.
Bakker et al. [], however, argued Nanotyrannus lay outside of the split between Tyrannosaurinae and Albertosaurinae, and represented a primitive side-branch of tyrannosaurs. This puts Nanotyrannus outside of Tyrannosauridae (depending on the precise definition used). This hypothesis was not tested using a morphological phylogenetic analysis. Surprisingly, little attempt has been made to test the phylogenetic position of Nanotyrannus since the work of Bakker et al.
We added Nanotyrannus to a previously published character–taxon matrix by Loewen et al. [], updated by Wolfe et al. [] and Dalman et al. [], and ran a phylogenetic analysis using equal weights parsimony under PAUP* 4.10 b10 []. The analysis recovered the two most parsimonious trees (Figure 32).
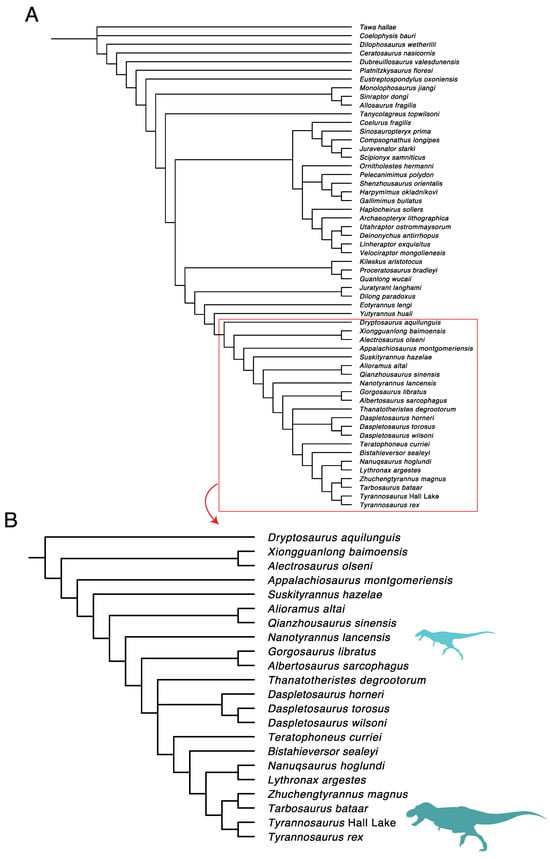
Figure 32.
(A) Phylogenetic placement of Nanotyrannus as a non-tyrannosaurid member of Tyrannosauroidea based on equal-weight parsimony Nanotyrannus based on the Dalman et al. matrix [] (Tree length = 1808; Consistency index (CI) = 0.3744; Retention index (RI) = 0.7492); (B) closeup focused on derived tyrannosauroids.
These results corroborate Bakker et al. in recovering Nanotyrannus just outside the Tyrannosaurinae–Albertosaurinae split, although our analysis differs from theirs in placing Alioraminae in a more basal position, below Nanotyrannus. We found almost no character support for the placement of Nanotyrannus in Tyrannosaurinae or Tyrannosauridae. Although characters can change through ontogeny, the near-total absence of any T. rex-like features in animals exceeding 1000 kg is striking.
We repeated our phylogenetic analysis using another dataset, the Brusatte and Carr matrix [], updated by Wolfe et al. []. This matrix produces similar results (Figure 33). Nanotyrannus emerges below Alioraminae but with Albertosaurinae further down the tree. This would make Nanotyrannus a basal member of the Tyrannosaurinae. We note, however, numerous discrepancies between specimens and codings in this matrix, particularly miscodes that appear to force Bistahieversor outside of Tyrannosauridae. This raises issues of whether the backbone of the tree is properly reconstructed; we suspect that the first topology better reflects tyrannosauroid phylogeny.
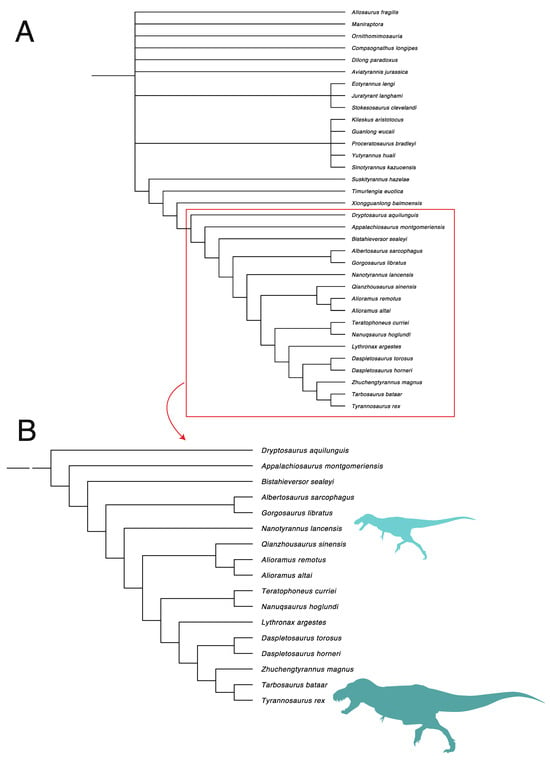
Figure 33.
(A) Phylogenetic placement of Nanotyrannus based on the Brusatte and Carr matrix [], updated by Wolfe et al. []. Strict consensus of 21 trees (Tree length = 778; Consistency index (CI) = 0.5463; Retention index (RI) = 0.8084) (B) closeup focused on derived tyrannosauroids.
Because derived features appear late in ontogeny, immature animals may be artificially pushed down the tree, as seen by the inclusion of “Raptorex kriegsteini”, a juvenile tyrannosaurine [] in phylogenetic analysis []. We, therefore, undertook a second series of analyses, with Nanotyrannus only coded for characters that are ontogenetically stable in tyrannosaurines, i.e., present both in young juveniles and adults. As discussed above, we assess ontogenetically stable characters as characters that are coded identically in juveniles [] and adults [] of Tarbosaurus baatar.
When this is done, Nanotyrannus still emerges as a non-tyrannosaurid (Figure 34), with an identical position as found in the previous pair of analyses. This is because those characters of Tyrannosaurus, Tyrannosaurinae, and even Tyrannosauridae that are predicted to be present even in very young Tyrannosaurus (based on their occurrence in Tarbosaurus) are conspicuously absent in Nanotyrannus, causing it to fall outside of Tyrannosauridae. Unless Tyrannosaurus developed in a way completely unlike Tarbosaurus (or any tyrannosaurid), these results refute the idea that Nanotyrannus is a juvenile Tyrannosaurus.
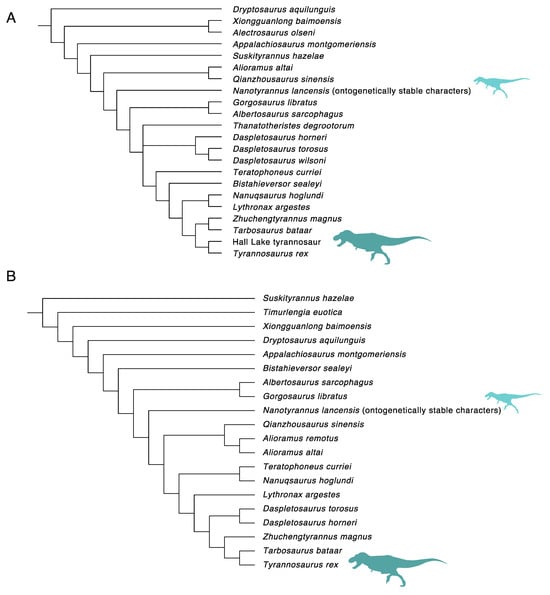
Figure 34.
Phylogenetic analysis using only ontogenetically stable characters using (A) the Dalman et al. matrix [], strict consensus of 2 most-parsimonious trees (Tree length = 1793, Consistency index (CI) = 0.3776, Retention index (RI) = 0.7484) and (B) the Brusatte and Carr matrix [,], strict consensus of 21 trees (Tree length = 767; Consistency index (CI) = 0.5541; Retention index (RI) = 0.8119).
4. Discussion
4.1. Summary of Evidence for the Validity of Nanotyrannus
All available evidence is consistent with the identification of Nanotyrannus as a distinct species of small-bodied tyrannosaur rather than a juvenile T. rex.
First, the high diversity of predators in many dinosaur-dominated ecosystems, and terrestrial and marine ecosystems in general, suggests that more than one tyrannosaur should exist in the late Maastrichtian of the Western Interior.
Second, extensive morphological differences separate Nanotyrannus and Tyrannosaurus (158 characters identified here), but no clear intermediate specimens exist. A discrete pattern of variation exists; all specimens conform either to the Nanotyrannus morphology or the Tyrannosaurus morphology. Further sampling could reveal intermediates, but since the ‘growth series’ hypothesis was originally proposed [], new fossils have reinforced this pattern, with Nanotyrannus and Tyrannosaurus forming discrete clusters rather than a continuum as predicted by the ‘growth series’ hypothesis. We found no characters supporting the referral of Nanotyrannus to Tyrannosaurus or even Tyrannosaurinae.
Third, morphological changes seen during ontogeny in other tyrannosaurs [,,,] are inconsistent with the ‘growth series’ hypothesis [,]. The “growth series” requires changes in tooth morphology, an increase in tooth number, changes in the shape of the antorbital fossa and accessory antorbital fenestra, and elongation of the rostra. Such changes are not seen in Tarbosaurus. Instead, tyrannosaurine features are evident in small Tarbosaurus [] and would be expected in much larger Nanotyrannus specimens (Figure 19). Subadult Tarbosaurus is largely identical to the adult []. For Nanotyrannus to represent a juvenile T. rex would require that Tyrannosaurus have to have a radically different ontogeny than other tyrannosaurs. Although ontogeny can evolve, it is more parsimonious to assume that Nanotyrannus is not part of the T. rex ontogenetic series. Several changes required—including loss of the ventral flange of the vomer, and the reduction of the manual phalanges—seem to defy known patterns of amniote skeletal development.
Fourth, histology suggests that specimens of Nanotyrannus are subadults or young adults of a small-bodied tyrannosaur. Large Nanotyrannus individuals show skeletal fusions, adult bone texture on facial bones, relatively slow growth, and decelerating growth, and their growth curves predict adult body masses of ~1200–2100 kg (BMRP 2002.4.1) and ~700–1100 kg (BMRP 2006.4.4), below the mass of adult T. rex. The higher mass estimates are biologically unrealistic because they depend on models (logarithmic and von Bertalanffy) in which growth is at a maximum at hatching and then decelerates. Histological studies show that dinosaur growth follows an S-curve, with absolute growth rates being low early on, accelerating, then decelerating []. The most realistic models are, therefore, logistic and Gompertz curves, which suggest adult masses of <1500 and <900 kg for these animals. Regardless of the model employed, none of the specimens sectioned show growth patterns consistent with growing to the large sizes achieved by Tyrannosaurus. Although it is not impossible that juvenile T. rex might show unusual patterns such as a growth deceleration, perhaps due to poor food availability, all Nanotyrannus specimens that have been thin-sectioned show these growth patterns. This suggests it is a real phenomenon, not an artifact.
Fifth, a small frontal, UCMP V84133, shows diagnostic features of T. rex in an animal smaller than the Nanotyrannus lancensis type. The identification of a young juvenile of T. rex—even a single bone of a single individual—definitively rejects the hypothesis that the two are the same species.
Last, phylogenetic analysis recovers Nanotyrannus as a non-tyrannosaurid. Character evidence supports a basal position in the tree, with little evidence for affinities with Tyrannosaurinae or even Tyrannosauridae. This result is robust, being recovered even when restricted to a limited subset of ontogenetically invariant characters.
Although any single line of evidence can potentially be contested, the consilience between all lines of evidence, each supporting the distinctiveness of Nanotyrannus, is striking. The absence of evidence either that Nanotyrannus represents a juvenile or of features allowing referral to T. rex is equally striking. The simplest explanation that fits the facts— what we know about dinosaur diversity, the morphology of the fossils, the development of tyrannosaurs, the histology of the individuals, and the existence of small T. rex—is that Nanotyrannus is a distinct taxon. Based on the characters identified above, we suggest that at least 11 specimens can either be referred to as Nanotyrannus or represent close relatives of Nanotyrannus (Table 6) although just how many species they represent remains unclear.

Table 6.
Specimens of Nanotyrannus.
4.2. Critique of Previous Work Synonymizing Nanotyrannus and Tyrannosaurus
The seminal work identifying Nanotyrannus as a juvenile Tyrannosaurus is that of Carr 1999 [], although it to a degree builds on arguments made by Carpenter []. Carr’s conclusion rests on two claims: first, that the holotype of Nanotyrannus lancensis CMNH 7541 is a juvenile, and second, that it shows diagnostic features of Tyrannosaurus. We argue that neither claim is supported by the evidence.
First, Carr [] argues that striated bone can be used to infer relative age in tyrannosaurs and that it is correlated with rapidly growing juveniles. As discussed, striated bone persists until late in ontogeny in tyrannosaurids; for example, young adults of Gorgosaurus libratus have striated bone []. Striated bone indicates that an animal has not ceased growing, not that it is a young juvenile. Carr also argues that features of Nanotyrannus are consistent with those seen in juvenile tyrannosaurs []; this may be true but as discussed above similar features are seen in primitive tyrannosaurs such as Alioramus [] and could represent the adult morphology of a primitive tyrannosaur.
Second, Carr [] lists thirteen characters supposedly shared by Nanotyrannus and Tyrannosaurus and supporting synonymy. We had difficulty verifying these observations. Of these characters, most, if not all, are either shared by a broader range of tyrannosaurs (Table 7) (and are, therefore, not diagnostic of Tyrannosaurus) or else do not appear to be shared by Tyrannosaurus and Nanotyrannus.

Table 7.
Characters reported by Carr [] as supporting referral of Nanotyrannus to Tyrannosaurus and assessment made here.
For example, the closely appressed nasal processes of the premaxilla and narrow jugal exposure in the antorbital fenestra are interpreted as a diagnostic feature of Tyrannosaurus, but these features occur in other tyrannosaurids. The skull shapes are not similar, either. The broad, T. rex-like muzzle illustrated for the holotype of Nanotyrannus lancensis by Carr (1999) is not present; the snout as preserved is very narrow as in Gorgosaurus and alioramins [], not like the broad rostrum of T. rex and Tarbosaurus. This does not appear to be an artifact of crushing; other Nanotyrannus specimens have narrow nasals and lack the broad palatal shelves and vomers associated with the broad rostrum of Tyrannosaurus. Neither is the temporal region of the skull in Nanotyrannus unusually broad relative to skull length []. We suggest the evidence presented by Carr is insufficient to support the referral of Nanotyrannus to Tyrannosaurus or even Tyrannosaurinae. We concede that more detailed character analysis is required, but we were unable to confidently identify characters in Nanotyrannus that are shared with Tyrannosaurus but not with other tyrannosaurids. We argue that Carr (1999) fails to show either that the type is a juvenile, or that it exhibits features supporting referral to Tyrannosaurus.
In a subsequent paper, Carr and Williamson [] assemble Nanotyrannus and Tyrannosaurus skulls into a “growth series”. However, simply because large and small animals can be assembled into a series does not demonstrate it is a growth series; if different species are arranged by size, what emerges is a size series, which assumes (rather than proves) that they are a single species. Furthermore, the very large number of characters identified as separating small T. rex from Nanotyrannus in this series [] is inconsistent with a gradual change over the course of development in a single species.
Finally, Carr [] attempted to reconstruct growth in Tyrannosaurus and argued that 21 distinct growth stages could be identified, identified by ‘synontomorphies’. Carr’s [] analysis of T. rex growth begins by taking for granted the assumption that Nanotyrannus is synonymous with T. rex. By forcing all these specimens into a single growth trajectory, Carr [] is forced to conclude that T. rex showed extremely unusual growth.
As shown above, a large number of differences separate Nanotyrannus and Tyrannosaurus without clear intermediates, such that this requires a massive, extremely rapid gain in novel character states between the Nanotyrannus form and the Tyrannosaurus morphotype. Carr notes that in this model, “sharp boundaries between categories are seen at the subadult and adult categories” and concludes that most of these changes occurred within two years, a pattern not seen in other tyrannosaurs or other dinosaurs. To account for this unusual observation, Carr argues, “The extreme number of changes at the transition between juveniles and subadults shows that the ontogeny of T. rex exhibits secondary metamorphosis, analogous to the abrupt ontogenetic changes that are seen at sexual maturity among teleosts”; that is, that Tyrannosaurus underwent a sudden change similar to that seen among certain fish, e.g., Pacific Salmon, Onchorhynchus spp., which change their skull and body shape on entering freshwater to breed. We are unaware of any amniote that develops in this way. It seems far simpler to assume that the two are distinct species.
Another problem is the use of cladistics as a model for interpreting development. Carr’s “ontogram” model may be inappropriate for studying patterns of ontogenetic variation because the assumptions of cladistic analysis do not match the way animals grow. Cladistic analysis assumes that there is a single, correct tree—there is a single true evolutionary history of the clade analyzed described by a branching diagram, with gains and losses of characters along its branches.
The problem is that ontogeny is not a single coordinated hierarchy; there are many sources of variation with respect to size and morphology other than age. Carr suggests that size decouples from maturity among adults [], but ontogeny is even more complex than this. Individual variation, developmental plasticity because of environmental factors, and sex are all common factors that can drastically lower the nearly perfect correlation between size, age, and morphology that Carr concludes. A cladistic analysis that assumes a single growth trajectory may simply be an inappropriate way to model growth, which can show considerable variation. It might serve to roughly order individuals in an objective and repeatable way [] but not to identify a large number of distinct growth stages.
The attempt to force the data to conform to a model—rather than to let the patterns emerge from the data—is perhaps best seen in Figure 12 of Carr []. The plot of age versus mass for 31 specimens results from forcing specimens onto the best-fit regression calculated by Erickson et al. []. These specimens do not all have estimated ages and masses measured, and those used in the regression analysis of Erickson et al. [] have their estimates presented incorrectly in order to place them precisely onto the regression line. Few empirical datasets will look like this, as no organisms grow identically from individual to individual due to variations in sex, genetics, and life history. The resulting curve and “growth series” are an attempt to force the data to a model, not to test whether the data fit the model; it is a hypothesis, not a result.
4.3. Affinities of Nanotyrannus-like Dinosaurs
A question raised by the removal of Nanotyrannus from Tyrannosaurus is whether all of the small tyrannosaurs from Hell Creek represent the same species or even the same lineage. Overall, the morphology of the animals is very similar, as shown by their codings in the character–taxon matrix (Supplementary Information S2); however, almost all of the characters shared by the Nanotyrannus type and referred specimens are plesiomorphies, and so do not necessarily support the monophyly of these individuals.
Several characters appear to be derived, however. These include (i) the upturned premaxilla, (ii) the procumbent premaxillary teeth (perhaps correlated/redundant with the first character, (iii) unserrated premaxillary teeth, and (iv) the pneumatic foramen of the quadratojugal. These characters suggest that Nanotyrannus and specimens referred to it form a clade to the exclusion of other tyrannosaurs. Whether these animals all represent a single species or even a single genus remains unclear.
A high degree of variability exists within both Tyrannosaurus and Nanotyrannus. Within T. rex, differences exist in the arrangement of the dentary teeth and the structure of the lacrimal, which is more L-shaped in the holotype and certain specimens and more hooked in others. The holotype of T. rex also has a very straight squamosal, a feature shared with a large Tyrannosaurus from the Frenchman Formation [] but not a number of other specimens [,]. Insofar as the morphological differences tend to be associated, that would support the idea that more than one species of Tyrannosaurus exists [,], especially given the existence of distinct species of Triceratops [,]. However, further work is needed to support or reject the existence of distinct species.
Similarly, a high degree of variation is seen in specimens referred to Nanotyrannus []. The type of Stygivenator molnari differs from the type of Nanotyrannus lancensis in several ways. The anterior end of the antorbital fossa is narrow [], the maxilla is long and low, and the tip of the mandible hooks upwards. These same features are seen in the “Dueling Dinosaurs” tyrannosaur found in association with a Triceratops [], although they are approached in Jane (BMRP 2002.4.1) and Zuri. This raises the possibility that specimens referred to as Nanotyrannus represent two distinct lineages, Nanotyrannus lancensis and Stygivenator (or Nanotyrannus) molnari. Again, further work and more fossils are needed to corroborate or reject this possibility.
The existence of Nanotyrannus as a distinct tyrannosaur lineage implies that other members of the lineage might exist in North America. A potential relative of Nanotyrannus is NMMNH P-25049 from the Kirtland Formation of New Mexico [] (NRL pers. obs.; J. Sertich pers. comm. 2023). NMMNH P-25049 lacks features allowing referral to Bistahieversor but resembles Nanotyrannus in having a longirostrine skull, low and rounded lacrimal horns, a dentary groove, and a low nasal weakly interlocking with the maxilla. NMMNH P-25049 also resembles juvenile Gorgosaurus [], but the broad dorsal and ventral margins of the antorbital fossa, the shape of the orbit, and the dentary groove are overall more similar to Nanotyrannus.
Other potential Nanotyrannus-like theropods are represented by premaxillary teeth identified as “Aublysodon”. Aublysodon is a tooth taxon originally described by Joseph Leidy [] from the Campanian-aged Judith River Formation of Montana. Aublysodon teeth are distinctive in being unserrated with blunt, chisel-shaped tips. Similar teeth occur in the Dinosaur Park Formation of Alberta []. These teeth could represent Nanotyrannus-like animals, but juvenile Gorgosaurus [] are described as having unserrated premaxillary teeth [], while those of adults are serrated []. Furthermore, Currie et al. [] note that Aublysodon in Dinosaur Park are invariably small individuals, suggesting these teeth may be juvenile albertosaurines.
4.4. Weaknesses in the Hypothesis
We argue that the weight of the evidence favors Nanotyrannus as distinct, but some evidence appears inconsistent with this hypothesis. The absence of Nanotyrannus individuals with an external fundamental system is curious. We suggest this may be a sampling effect since many large dinosaurs are actually still-growing subadults rather than old adults with growth cessation [,]. If so, a specimen of Nanotyrannus will eventually be discovered with an EFS.
It may be that few individuals live long enough to form an EFS. Strikingly, none of the Gorgosaurus sampled by Erickson [] had an EFS, and only one individual among each of the T. rex, Albertosaurus, and Daspletosaurus samples sectioned had an EFS. A study of life history and mortality in Albertosaurus suggests that perhaps 5% of all individuals lived beyond 20 years [], with the EFS forming at 22 years in one Albertosaurus []. A rarity of old Nanotyrannus might be expected if most tyrannosaurs died young.
Another issue concerns the rarity of small T. rex. We maintain that LACM 23845 represents such a juvenile, however. The estimated skull length of this animal (80 cm) is barely larger than that of BMRP 2002.4.1 (est. 71 cm), about 113% the length of this animal. The minor difference in size seems inconsistent with the radically different morphology seen in BMRP 2002.4.1 and LACM 23845. Furthermore, the frontal described here (UCMP V84133) appears to come from a very small T. rex, with a skull around 45–50 cm in length. The rarity of young T. rex may reflect the rarity of juvenile dinosaurs in general; juveniles are rare even for common dinosaurs like Triceratops [], and small dinosaurs in general tend to be rare []. This may be due to taphonomic biases; in particular, small animals are more likely to be consumed by predators and scavengers. Young T. rex, less than 3–4 m in length, may have been swallowed in a few bites, or whole, by adults, and would not become fossils.
4.5. Systematics and The Evolution of Development
Evolution often proceeds by terminal addition or subtraction of characters, i.e., new features tend to be added or subtracted late in development rather than early. This may be because mutations appearing early are more likely to be harmful or lethal; sexually selected features also tend to appear late [].
Evolution via the terminal addition of characters is known as peramorphosis. In peramorphosis, new stages or features are added to the end of the ancestral development sequence, often by elaborating and extending existing development trajectories. For example, in Triceratops, derived features, including orbital horns, elongation of the frill, and elongation of the rostrum, are absent in young individuals [,], as are fusions between skull elements [], but develop later in life. The result of the late development of these features is that juvenile Triceratops resemble primitive ceratopsids such as Protoceratops []. Peramorphosis seems to explain the evolution of a number of features in Tyrannosaurinae. The keyhole-shaped orbit, massive lacrimal boss and cornual boss, and modifications of the skull roof, such as the narrow frontal–nasal contact, are absent in the youngest tyrannosaurines [] but develop in older juveniles and subadults []. However, primitive tyrannosauroids such as Alioramus [] retain the primitive condition even as adults and, therefore, resemble the juveniles of Tyrannosaurinae. Because of this, it is illogical to assume that Nanotyrannus must be a juvenile because it lacks features associated with adulthood in Tyrannosaurinae.
Terminal addition can force juveniles down the tree when included in a phylogenetic analysis because they have not yet developed the late-appearing, derived features to place them with their clade. An example is a small tyrannosaurid described as “Raptorex kriegsteini” []. “Raptorex” is an immature [,] tyrannosaurid from the Nemegt of Mongolia []. It shows features seen in adults of primitive tyrannosaurs, including a large orbit, slender lacrimal and postorbitals, and a frontal participating in the orbit. As a result, it emerges basally in phylogenetic analyses [,,]. However, several features—the short and tall maxilla, pointed premaxillary teeth, the large and anteroventrally located maxillary fenestra, and anterior expansion of the jugal—suggest affinities with Tyrannosaurinae, specifically Tarbosaurus.
We concede that the terminal addition of characters could cause Nanotyrannus to slip down the tree if it were a young juvenile. However, there is no evidence that the morphology of the specimens here is, in fact, the result of immaturity. Nanotyrannus specimens show no trend toward the acquisition of Tyrannosaurus-like features as they become larger and older. Crucially, features associated with immaturity in derived Tyrannosaurinae such as Tarbosaurus cannot be assumed to be juvenile; that is, features associated with juveniles in one lineage cannot be assumed to be associated with immaturity in another lineage because developmental patterns evolve. Finally, when we exclude ontogenetically variable features, Nanotyrannus still emerges as a basal tyrannosauroid, not a tyrannosaurid.
Another developmental mechanism complicating systematics is the terminal deletion of characters. Variously termed paedomorphosis, or neoteny, here juvenile traits are retained in adults. In chimpanzees and Neanderthals, for example, a rounded cranium and small orbital ridges are juvenile characters, but these features are typical of adult Homo sapiens due to neoteny []. Birds retain characteristics of juvenile theropods []. Paedomorphosis is a common means by which development evolves new morphologies. It is conceivable that Nanotyrannus is a paedomorphic tyrannosaurid. In at least one feature, it appears to be paedomorphic: whereas basal tyrannosauroids have serrated premaxillary teeth [], Nanotyrannus lacks serrations on the premaxillary teeth, as in embryonic tyrannosaurs []. Assuming Nanotyrannus is a juvenile because it resembles juvenile tyrannosaurids is like assuming that a Homo sapiens skull is immature because it resembles a young Neanderthal child. One species “juvenile features” can be the adult morphology of another species.
4.6. Implications
Systematics can be seen as a battle between ‘splitters’, who tend to recognize a large number of species, and ‘lumpers’, who tend to recognize few. Both are important because sometimes scientists err in failing to recognize distinct species and other times, they mistake differences between individuals, males and females, or adults and juveniles as species-level differences. Ideally, through lumping and splitting, we arrive at a better understanding of life’s diversity.
Different systematic philosophies have prevailed at different times. The history of Nanotyrannus is interesting as part of a broader history of conflicting taxonomic practices in paleontology. Cope and Marsh, who helped found vertebrate paleontology in North America, were famous, if not notorious, for naming species based on incomplete fossils, as with “Ornithomimus grandis” [] and “Manospondylus gigas” []. Their approach could charitably be described as entrepreneurial and exuberant—with many new fossils and new taxa to name, their strategy seems to have been to stake a claim on as many species as possible and let others clean up problems that emerged later. Alternatively, one could view it as a competition driven by personal vanity.
Unlike Marsh and Cope, Gilmore was a more cautious worker. Gilmore would describe a number of dinosaur species, almost all of which—Thescelosaurus neglectus [], Chirostenotes pergracilis [], Bactrosaurus johnsoni [], Alectrosaurus olsoni [], and Alamosaurus sanjuanensis []—are considered valid. One, Brachyceratops montanensis [], is an indeterminate juvenile of a horned dinosaur, possibly Achelousaurus or Einiosaurus []. Gilmore’s approach reflects a more conservative, thorough approach to paleontological taxonomy that prevailed after the more exuberant days of Marsh and Cope, probably in part because of the problems that came from trying to navigate the maze of names left behind by those two. In this light it is striking that Gilmore chose to recognize Nanotyrannus lancensis as distinct from Tyrannosaurus; the species results not from the early excesses of the Bone Wars, but was named as paleontology entered a more cautious, conservative phase. The recognition of Nanotyrannus as a distinct genus by Bakker et al. [], in turn, represents a swing of the pendulum back towards recognizing more species and genera. Several things drive this trend toward recognizing new species.
First, we simply have more fossils as time progresses, which gives us a better understanding of patterns of variation. The large number Tyrannosaurus specimens [,], for example, including highly complete skulls and skeletons, provide context for diagnosing other species and provide a better understanding of patterns of intraspecific variation (or at least intrageneric variation []). Bonebeds of dinosaurs [,,] are particularly useful in understanding growth and variation since they preserve large numbers of individuals from a small area and a brief period of time, recording population-level variation without the confounding factors of evolution across space or time.
Juveniles and growth series of closely related animals like Tarbosaurus [,,,,,] and Gorgosaurus [,,] also shed light on patterns of development in tyrannosaurs. Similarly, the discovery of juveniles of other kinds of dinosaurs, such as Protoceratops [] and Triceratops [,], is critical to understanding which features are labile and subject to change over ontogeny and which are stable—that is, which features are diagnostic.
Furthermore, cladistic methodologies, although not without problems, make it possible to interpret fossils in an evolutionary framework [,,]. Even where fossils are incomplete or subtly different, if they can be shown to lie on a distinct branch of the evolutionary tree, justification may exist for the recognition of new species.
We have also come to a better understanding of dinosaur evolution, recognizing that dinosaurs show high levels of species turnover through time [,] and high levels of endemism [,]. Few dinosaur species were widespread or long-lived. These patterns are not unique to dinosaurs; mammals show endemism [], lizards [,] and plants show marked turnover [,]. Turnover and endemism seem to be the rule for dinosaurs and other extinct species.
And arguably, paleontologists have simply gotten better at recognizing distinct species—certainly, one would hope that two centuries of experience have taught us something. This is not to say that we should be incautious in naming or recognizing species, but we should recognize that countless dinosaurs and other animals existed over the millions of years that these lineages roamed Earth, that they can be hard to tell apart, and are often distinguished by subtle features.
Recognizing these species is complicated by the biases of the fossil record. Vertebrates are relatively rare in the fossil record; complete remains are especially rare. Focusing on skulls and skeletons gives us a large amount of information per specimen but at the cost of having few specimens to work with. Focusing on incomplete remains, such as isolated teeth, jaws, and bones, provides a larger sample size, but incomplete remains have fewer informative characters.
Furthermore, telling fossil species apart is simply difficult. Modern species are often diagnosed by characters that are not, or are rarely, preserved in the fossil record. Consider a pair of extant birds—the raven, Corvus corax, and the American crow, Corvus brachyrhynchos. Ravens can be distinguished from crows by their larger size, bigger bill, long throat feathers, gray neck feathers (in the juvenile), long and tapered wings, pointed tail, a hoarse call, soaring flight, and a tendency to associate in small groups []. Of these characters, only the larger size and longer beak would commonly preserve as fossils. Given this, the differences between C. corax and C. brachyrhynchos might easily be mistaken for intraspecific variation, with only fossils to work with. Features diagnosing species are often subtle. Species evolve not to be identifiable by human beings but to be identifiable by each other, using clues like color, songs, and scents. Biologists often struggle to discriminate species on the basis of morphology and undercount species. In recent years, DNA has revealed the existence of cryptic species. For example, the Sacred Crocodile, Crocodilus suchus, was long thought to be synonymous with the Nile Crocodile, C. niloticus, by scientists (although ancient Egyptians and 19th-century taxonomists recognized the two as separate) until DNA showed the species was distinct []. Similarly, African elephants were put into a single species, Loxodonta africana, until genomics resurrected the species L. cyclotus []. Genomics has revealed new species of baleen whale [,] and giraffe []. Even when working with extant animals, discriminating species is difficult.
We argue that the trend toward splitting in paleontology represents a broad pattern in science towards the recognition of more species, notwithstanding a number of recent attempts to synonymize genera. Some of these synonymies seem plausible, e.g., the synonymy of the flat-headed pachycephalosaurs Ornathotholus and Homalocephale as juveniles (or females) of the dome-headed pachycephalosaurs Stegoceras [] and Prenocephale [], respectively. Others, such as the synonymization of Torosaurus and Triceratops [], sinking Stygimoloch into Dracorex [], or (as we argue here) the synonymization of Nanotyrannus and Tyrannosaurus, are not supported by the available evidence. Overall, the number of dinosaur species continues to increase, driven by collecting, but also by splitting of known fossils into new species and genera [,,,].
The debate between splitting and lumping is not a mere philosophical issue, an arbitrary human decision in the way Osborn arbitrarily chose the name Tyrannosaurus over Dynamosaurus. The recognition of a species is an evolutionary hypothesis; it suggests that a period of evolution and reproductive isolation separates the populations that the fossils in question are drawn from. The recognition of a species is fundamentally a hypothesis about the course of evolution; names merely reflect these hypotheses [], and so how we choose to name species affects how we reconstruct past diversity.
Logically, at any given point we may accurately estimate a group’s diversity, overestimate diversity, or underestimate it. It seems likely that we are significantly underestimating fossil diversity. For many reasons—because species are often distinguished by subtle differences and are hard to tell apart, because paleontologists tend to work with small sample sizes, and because the development, variation, and sexual dimorphism of fossil groups are poorly understood—we may be underestimating fossil diversity as a whole.
If there is one thing the modern world and the fossil record tell us, it is that evolution tends to increase diversity over time, slowly increasing the total number of lineages. Such increases are temporarily interrupted by turnover events or mass extinctions [], which depress diversity, at which point it tends to increase again []. Evolution also causes anagenesis [], such that lineages that fail to go extinct slowly turn into new species over time. Species speciate, splitting into multiple descendant lineages—it is a fundamental pattern in evolution []. We should expect to find large numbers of species in the fossil record, and given the limits of the fossil record, we almost certainly underestimate fossil diversity. This matters because many of the problems we are interested in as paleontologists and evolutionary biologists—changes in diversity, mass extinction, diversity gradients, endemism, and the drivers of these patterns—require an accurate understanding of the number of species.
It is remarkable that the systematics of an animal as famous, as well-known, and as intensively studied as Tyrannosaurus have remained so incompletely understood and controversial. This emphasizes how little we really know about past diversity. If we still do not understand T. rex, what else do we not understand? There is a great deal we do not know, and may never know, about the life of the past.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/fossils2010001/s1: Supplementary Information S1, Character-Taxon matrix for clustering analysis; Supplementary Information S2, Growth Curves and Asymptote Estimates; Supplementary Information S3, Character-taxon Matrix for Phylogenetic Analysis 1; Supplementary Information S4, Character-Taxon Matrix for Phylogenetic Analysis 2. See also Supplementary Data, data for histology analysis, and Supplementary Code, code for histological analysis.
Author Contributions
Conceptualization, N.R.L.; methodology, N.R.L. and E.T.S.; writing—original draft preparation, N.R.L. and E.T.S.; writing—review and editing, N.R.L. and E.T.S.; visualization, N.R.L. and E.T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data are available in the paper or the supporting data.
Acknowledgments
Thanks for discussions to Bhart-Anjan Bhullar, Art Chadwick, Tom Cullen, Phil Currie, Brian Curtice, Jacques Gauthier, Pete Larson, Mark Loewen, Pete Makovicky, Greg Paul, Joe Sertich, Sam Shepard, Eric Snively, Jared Voris, Larry Witmer, Jared Wood and Darla Zelenitzsky. We thank H. Woodward Ballard for sharing high-resolution files for the supplemental figures in Woodward et al. [], Larry Witmer for photos of the Nanotyrannus lancensis holotype, Matt Lamanna for photos of the Tyrannosaurus rex holotype, Scott Persons for photos of RSM 2523.8, Pete Larson for photos of FMNH PR2411 and Tyrannosaurus, and Andrey Atuchin for images of the Tarbosaurus holotype. Thanks to Tetsuto Miyashita, Phil Currie, Matt Lamanna, and Larry Witmer for the photographs. Thanks to T. Cullen for helpful discussions of histology. Thanks to the Black Hills Institute and the Burpee Museum for specimen access. Finally, thanks to the three reviewers for their constructive reviews of the manuscript. Andrey Atuchin skilfully drew the line drawings for Nanotyrannus and Tyrannosaurus skulls.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brusatte, S.L.; Carr, T.D. The phylogeny and evolutionary history of tyrannosauroid dinosaurs. Sci. Rep. 2016, 6, 20252. [Google Scholar] [CrossRef]
- Zanno, L.E.; Makovicky, P.J. Neovenatorid theropods are apex predators in the Late Cretaceous of North America. Nat. Commun. 2013, 4, 2827. [Google Scholar] [CrossRef]
- Brusatte, S.L.; Benson, R.B.; Hutt, S. The osteology of Neovenator salerii (Dinosauria: Theropoda) from the Wealden group (Barremian) of the Isle of Wight. Paleontogr. Soc. Monogr. 2008, 162, 1–75. [Google Scholar]
- Holtz, T.R. Tyrannosauroidea. In The Dinosauria, 2nd ed.; Weishampel, D.B., Dodson, P., Osmolska, H., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 111–136. [Google Scholar]
- Brusatte, S.L.; Norell, M.A.; Carr, T.D.; Erickson, G.M.; Hutchinson, J.R.; Balanoff, A.M.; Bever, G.S.; Choiniere, J.N.; Makovicky, P.J.; Xu, X. Tyrannosaur paleobiology: New research on ancient exemplar organisms. Science 2010, 329, 1481–1485. [Google Scholar] [CrossRef]
- Osborn, H.F. Tyrannosaurus and other Cretaceous carnivorous dinosaurs. Bull. Am. Mus. Nat. Hist. 1905, 35, 733–771. [Google Scholar]
- Brochu, C.A. Osteology of Tyrannosaurus rex: Insights from a nearly complete skeleton and high-resolution computed tomographic analysis of the skull. J. Vertebr. Paleontol. Mem. 2003, 7, 1–138. [Google Scholar] [CrossRef]
- Erickson, G.M.; Makovicky, P.J.; Currie, P.J.; Norell, M.A.; Yerby, S.A.; Brochu, C.A. Gigantism and comparative life-history parameters of tyrannosaurid dinosaurs. Nature 2004, 430, 772–775. [Google Scholar] [CrossRef]
- Carr, T.D. Craniofacial ontogeny in Tyrannosauridae (Dinosauria, Coelurosauria). J. Vertebr. Paleontol. 1999, 19, 497–520. [Google Scholar] [CrossRef]
- Carr, T.D. A high-resolution growth series of Tyrannosaurus rex obtained from multiple lines of evidence. PeerJ 2020, 8, e9192. [Google Scholar] [CrossRef]
- Hutchinson, J.R.; Garcia, M. Tyrannosaurus was not a fast runner. Nature 2002, 415, 1018–1021. [Google Scholar] [CrossRef]
- DePalma, R.A.; Burnham, D.A.; Martin, L.D.; Rothschild, B.M.; Larson, P.L. Physical evidence of predatory behavior in Tyrannosaurus rex. Proc. Natl. Acad. Sci. USA 2013, 110, 12560–12564. [Google Scholar] [CrossRef]
- Longrich, N.R.; Horner, J.R.; Erickson, G.M.; Currie, P.J. Cannibalism in Tyrannosaurus rex. PLoS ONE 2010, 5, e13419. [Google Scholar] [CrossRef]
- Erickson, G.M.; Van Kirk, S.D.; Su, J.; Levenston, M.E.; Caler, W.E.; Carter, D.R. Bite-force estimation for Tyrannosaurus rex from tooth-marked bones. Nature 1996, 382, 706–708. [Google Scholar] [CrossRef]
- Chin, K.; Tokaryk, T.T.; Erickson, G.M.; Calk, L.C. A king-sized theropod coprolite. Nature 1998, 393, 680–682. [Google Scholar] [CrossRef]
- Larson, P.L. Variation and Sexual Dimorphism in Tyrannosaurus rex. In Tyrannosaurus rex, the Tyrant King; Larson, P.L., Carpenter, K., Eds.; Indiana University Press: Bloomington, IN, USA, 2008; pp. 103–128. [Google Scholar]
- Carr, T.D.; Williamson, T.E. Diversity of late Maastrichtian Tyrannosauridae (Dinosauria: Theropoda) from western North America. Zool. J. Linn. Soc. 2004, 142, 479–523. [Google Scholar] [CrossRef]
- Paul, G.S.; Persons, W.S.; Van Raalte, J. The Tyrant Lizard King, Queen and Emperor: Multiple Lines of Morphological and Stratigraphic Evidence Support Subtle Evolution and Probable Speciation Within the North American Genus Tyrannosaurus. Evol. Biol. 2022, 49, 156–179. [Google Scholar] [CrossRef]
- Carr, T.D.; Napoli, J.G.; Brusatte, S.L.; Holtz, T.R.; Hone, D.W.; Williamson, T.E.; Zanno, L.E. Insufficient Evidence for Multiple Species of Tyrannosaurus in the Latest Cretaceous of North America: A Comment on “The Tyrant Lizard King, Queen and Emperor: Multiple Lines of Morphological and Stratigraphic Evidence Support Subtle Evolution and Probable Speciation Within the North American Genus Tyrannosaurus”. Evol. Biol. 2022, 39, 327–341. [Google Scholar]
- Breithaup, B.H.; Southwell, E.H.; Matthews, N.A. Dynamosaurus imperiosus and the earliest discoveries of Tyrannosaurus rex in Wyoming and the West. New Mex. Mus. Nat. Hist. Sci. Bull. 2006, 35, 257–258. [Google Scholar]
- Marsh, O.C. Description of new dinosaurian reptiles. Am. J. Sci. 1890, 39, 81–86. [Google Scholar] [CrossRef]
- Cope, E.D. Fourth note on the Dinosauria of the Laramie. Am. Nat. 1892, 26, 756–758. [Google Scholar]
- Paul, G.S. Predatory Dinosaurs of the World; Simon and Schuster: New York, NY, USA, 1988; p. 464. [Google Scholar]
- Olshevsky, G. The origin and evolution of the tyrannosaurids. Kyoryugaku Saizensen 1995, 9, 92–119. [Google Scholar]
- Osborn, H.F. Tyrannosaurus, Upper Cretaceous carnivorous dinosaur (second communication). Bull. Am. Mus. Nat. Hist. 1906, 22, 281–296. [Google Scholar]
- Scannella, J.B. Anagenesis in Triceratops: Evidence from a newly resolved stratigraphic framework for the Hell Creek Formation. Cincinnati. Mus. Cent. Sci. Contrib. 2009, 3, 148–149. [Google Scholar]
- Gilmore, C.W. A new carnivorous dinosaur from the Lance Formation. Smithson. Misc. Collect. 1946, 106, 1–19. [Google Scholar]
- Rozhdestvensky, A.K. Growth changes in Asian dinosaurs and some problems of their taxonomy. Paleontol. Žurnal 1965, 3, 95–109. [Google Scholar]
- Russell, D.A. Tyrannosaurs from the Late Cretaceous of Western Canada. Natl. Mus. Nat. Sci. Publ. Palaeontol. 1970, 1, 1–34. [Google Scholar]
- Bakker, R.; Williams, M.; Currie, P.J. Nanotyrannus, a new genus of pygmy tyrannosaur, from the latest Cretaceous of Montana. Hunteria 1988, 1, 1–30. [Google Scholar]
- Molnar, R. A new theropod dinosaur from the Upper Cretaceous of central Montana. J. Paleontol. 1978, 52, 73–82. [Google Scholar]
- Molnar, R.E.; Carpenter, K. The Jordan theropod (Maastrichtian, Montana, USA) referred to the genus Aublysodon. Geobios 1989, 22, 445–454. [Google Scholar] [CrossRef]
- Leidy, J.F. Remarks on a jaw fragment of Megalosaurus. Proc. Phila. Acad. Nat. Sci. 1868, 20, 197–200. [Google Scholar]
- Carpenter, K. Tyrannosaurids (Dinosauria) of Asia and North America. In Aspects of Nonmarine Cretaceous Geology; Mateer, N.J., Chen, P.-J., Eds.; China Ocean Press: Beijing, China, 1992; pp. 250–268. [Google Scholar]
- Tumarkin-Deratzian, A.R. Evaluation of long bone surface textures as ontogenetic in centrosaurine ceratopsids. Anat. Rec. 2009, 292, 1485–1500. [Google Scholar] [CrossRef] [PubMed]
- Tumarkin-Deratzian, A.R.; Vann, D.R.; Dodson, P. Bone surface texture as an ontogenetic indicator in long bones of the Canada goose Branta canadensis (Anseriformes: Anatidae). Zool. J. Linn. Soc. 2006, 148, 133–168. [Google Scholar] [CrossRef]
- Holtz, T.R., Jr. The phylogeny and taxonomy of the Tyrannosauridae. In Mesozoic Vertebrate Life; Tanke, D.H., Carpenter, K., Eds.; Indiana University Press: Bloomington, IN, USA, 2001; pp. 64–83. [Google Scholar]
- Brusatte, S.L.; Carr, T.D.; Williamson, T.E.; Holtz, T.R., Jr.; Hone, D.W.; Williams, S.A. Dentary groove morphology does not distinguish ‘Nanotyrannus’ as a valid taxon of tyrannosauroid dinosaur. Comment on:“Distribution of the dentary groove of theropod dinosaurs: Implications for theropod phylogeny and the validity of the genus Nanotyrannus Bakker et al. 1988”. Cretac. Res. 2016, 65, 232–237. [Google Scholar]
- Currie, P.J. Cranial anatomy of tyrannosaurid dinosaurs from the Late Cretaceous of Alberta, Canada. Acta Palaeontol. Pol. 2003, 48, 191–226. [Google Scholar]
- Larson, P. The case for Nanotyrannus. In Tyrannosaurid Paleobiology; Parrish, J.M., Molnar, R.E., Currie, P.J., Koppelhus, E.B., Eds.; Indiana University Press: Bloomington, IN, USA, 2013; pp. 15–53. [Google Scholar]
- Henderson, M.D.; Harrison, W.H. Taphonomy and environment of deposition of juvenile tyrannosaurid skeleton from the Hell Creek Formation (latest Maastrichtian) of southeastern Montana. In Tyrannosaurus rex, the Tyrant King; Indiana University Press: Bloomington, IN, USA, 2008; pp. 82–90. [Google Scholar]
- Larson, P. The Validity of Nanotyrannus lancensis (Theropoda, Lancian—Upper Maastrichtian of North America). In Proceedings of the Society of Vertebrate Paleontology, Los Angeles, CA, USA, 30 October–2 November 2013. [Google Scholar]
- Witmer, L.M.; Ridgely, R.C. New insights into the brain, braincase, and ear region of tyrannosaurs (Dinosauria, Theropoda), with implications for sensory organization and behavior. Anat. Rec. 2009, 292, 1266–1296. [Google Scholar] [CrossRef]
- Witmer, L.M.; Ridgely, R.C. The Cleveland tyrannosaur skull (Nanotyrannus or Tyrannosaurus): New findings based on CT scanning, with special reference to the braincase. Kirtlandia 2010, 57, 61–81. [Google Scholar]
- Schmerge, J.D.; Rothschild, B.M. Distribution of the dentary groove of theropod dinosaurs: Implications for theropod phylogeny and the validity of the genus Nanotyrannus Bakker et al., 1988. Cretac. Res. 2016, 61, 26–33. [Google Scholar] [CrossRef]
- Woodward, H.N.; Tremaine, K.; Williams, S.A.; Zanno, L.E.; Horner, J.R.; Myhrvold, N. Growing up Tyrannosaurus rex: Osteohistology refutes the pygmy “Nanotyrannus” and supports ontogenetic niche partitioning in juvenile Tyrannosaurus. Sci. Adv. 2020, 6, eaax6250. [Google Scholar] [CrossRef]
- Jevnikar, E.; Zanno, L. Bimodal trajectories and unresolved early growth stages in Tyrannosaurus rex growth. In Proceedings of the Society of Vertebrate Paleontology 2021 Annual Meeting, Minneapolis, MN, USA, 3–7 November 2021; p. 151. [Google Scholar]
- Swofford, D.L. PAUP *. Phylogenetic Analysis Using Parsimony (* and Other Methods); 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Cullen, T.M.; Canale, J.I.; Apesteguía, S.; Smith, N.D.; Hu, D.; Makovicky, P.J. Osteohistological analyses reveal diverse strategies of theropod dinosaur body-size evolution. Proc. R. Soc. B 2020, 287, 20202258. [Google Scholar] [CrossRef]
- Campione, N.E.; Evans, D.C.; Brown, C.M.; Carrano, M.T. Body mass estimation in non-avian bipeds using a theoretical conversion to quadruped stylopodial proportions. Methods Ecol. Evol. 2014, 5, 913–923. [Google Scholar] [CrossRef]
- Griffin, C. Using osteohistology to determine the taxonomic validity of the Late Cretaceous dinosaur Nanotyrannus lancensis Bakker et al, 1988 (Theropoda: Tyrannosauridae). In Proceedings of the The Research and Scholarship Symposium, Cedarville, OH, USA, 16 April 2014. [Google Scholar]
- Dalman, S.G.; Loewen, M.A.; Pyron, R.A.; Jasinski, S.E.; Malinzak, D.E.; Lucas, S.G.; Fiorillo, A.R.; Currie, P.J.; Longrich, N.R. A giant tyrannosaur from the Campanian–Maastrichtian of southern North America and the evolution of tyrannosaurid gigantism. Sci. Rep. 2024, in press. [Google Scholar]
- Hurum, J.H.; Sabath, K. Giant theropod dinosaurs from Asia and North America: Skulls of Tarbosaurus bataar and Tyrannosaurus rex compared. Acta Palaeontol. Pol. 2003, 48, 161–190. [Google Scholar]
- Tsuihiji, T.; Watabe, M.; Tsogtbaatar, K.; Tsubamoto, T.; Barsbold, R.; Suzuki, S.; Lee, A.H.; Ridgely, R.C.; Kawahara, Y.; Witmer, L.M. Cranial osteology of a juvenile specimen of Tarbosaurus bataar (Theropoda, Tyrannosauridae) from the Nemegt Formation (Upper Cretaceous) of Bugin Tsav, Mongolia. J. Vertebr. Paleontol. 2011, 31, 497–517. [Google Scholar] [CrossRef]
- Fowler, D.W.; Woodward, H.N.; Freedman, E.A.; Larson, P.L.; Horner, J.R. Reanalysis of “Raptorex kriegsteini”: A juvenile tyrannosaurid dinosaur from Mongolia. PLoS ONE 2011, 6, e21376. [Google Scholar] [CrossRef]
- Loewen, M.A.; Irmis, R.B.; Sertich, J.J.; Currie, P.J.; Sampson, S.D. Tyrant dinosaur evolution tracks the rise and fall of Late Cretaceous oceans. PLoS ONE 2013, 8, e79420. [Google Scholar] [CrossRef] [PubMed]
- Voris, J.T.; Therrien, F.; Zelenitsky, D.K.; Brown, C.M. A new tyrannosaurine (Theropoda: Tyrannosauridae) from the Campanian Foremost Formation of Alberta, Canada, provides insight into the evolution and biogeography of tyrannosaurids. Cretac. Res. 2020, 110, 104388. [Google Scholar] [CrossRef]
- Carr, T.D.; Williamson, T.E.; Britt, B.B.; Stadtman, K. Evidence for high taxonomic and morphologic tyrannosauroid diversity in the Late Cretaceous (Late Campanian) of the American Southwest and a new short-skulled tyrannosaurid from the Kaiparowits Formation of Utah. Naturwissenschaften 2011, 98, 241–246. [Google Scholar] [CrossRef]
- Carr, T.D.; Williamson, T.E. Bistahieversor sealeyi, gen. et sp. nov., a new tyrannosauroid from New Mexico and the origin of deep snouts in Tyrannosauroidea. J. Vertebr. Paleontol. 2010, 30, 1–16. [Google Scholar] [CrossRef]
- Warshaw, E.A.; Fowler, D.W. A transitional species of Daspletosaurus Russell, 1970 from the Judith River Formation of eastern Montana. PeerJ 2022, 10, e14461. [Google Scholar] [CrossRef]
- Carr, T.D.; Varricchio, D.J.; Sedlmayr, J.C.; Roberts, E.M.; Moore, J.R. A new tyrannosaur with evidence for anagenesis and crocodile-like facial sensory system. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Molnar, R.E. A distinctive theropod dinosaur from the Upper Cretaceous of Baja California (Mexico). J. Paleontol. 1974, 48, 1009–1017. [Google Scholar]
- Zanno, L.E.; Tucker, R.T.; Canoville, A.; Avrahami, H.M.; Gates, T.A.; Makovicky, P.J. Diminutive fleet-footed tyrannosauroid narrows the 70-million-year gap in the North American fossil record. Commun. Biol. 2019, 2, 64. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, S.J.; Denton Jr, R.K.; Loewen, M.A.; Brusatte, S.L.; Smith, N.D.; Turner, A.H.; Kirkland, J.I.; McDonald, A.T.; Wolfe, D.G. A mid-Cretaceous tyrannosauroid and the origin of North American end-Cretaceous dinosaur assemblages. Nat. Ecol. Evol. 2019, 3, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Brusatte, S.L.; Averianov, A.; Sues, H.-D.; Muir, A.; Butler, I.B. New tyrannosaur from the mid-Cretaceous of Uzbekistan clarifies evolution of giant body sizes and advanced senses in tyrant dinosaurs. Proc. Natl. Acad. Sci. USA 2016, 113, 3447–3452. [Google Scholar] [CrossRef] [PubMed]
- Lambe, L.M. On a new genus and species of carnivorous dinosaur from the Belly River Formation of Alberta, with a description of the skull of Stephanosaurus marginatus from the same horizon. Ott. Nat. 1914, 28, 13–20. [Google Scholar]
- Currie, P.J. Theropods, including birds. In Dinosaur Provincial Park: A Spectacular Ancient Ecosystem Revealed; Currie, P.J., Koppelhus, E.B., Farlow, J.O., Eds.; Indiana University Press: Bloomington, IN, USA, 2005; pp. 367–397. [Google Scholar]
- Fiorillo, A.R.; Tykoski, R.S. A diminutive new tyrannosaur from the top of the world. PLoS ONE 2014, 9, e91287. [Google Scholar] [CrossRef] [PubMed]
- Carr, T.D. Significant Geographic Range Extension for the Sympatric Tyrannosaurids Albertosaurus Libratus and Daspletosaurus Torosus from the Judith River Formation (Late Campanian) of Northern Montana. In Proceedings of The Society of Vertebrate Paleontology 2018 Annual Meeting, Albuquerque, NM, USA, 17–20 October 2018. [Google Scholar]
- Brusatte, S.L.; Carr, T.D.; Norell, M.A. The osteology of Alioramus, a gracile and long-snouted tyrannosaurid (Dinosauria: Theropoda) from the Late Cretaceous of Mongolia. Bull. Am. Mus. Nat. Hist. 2012, 2012, 1–197. [Google Scholar] [CrossRef]
- Mallon, J.C.; Bura, J.R.; Schumann, D.; Currie, P.J. A problematic tyrannosaurid (Dinosauria: Theropoda) skeleton and its implications for tyrannosaurid diversity in the Horseshoe Canyon Formation (Upper Cretaceous) of Alberta. Anat. Rec. 2020, 303, 673–690. [Google Scholar] [CrossRef]
- Longrich, N.R.; Isasmendi, E.; Pereda-Suberbiola, X.; Jalil, N.-E. New fossils of Abelisauridae (Dinosauria: Theropoda) from the upper Maastrichtian of Morocco, North Africa. Cretac. Res. 2023, 152, 105677. [Google Scholar] [CrossRef]
- Gasparini, Z.; Sterli, J.; Parras, A.; O’Gorman, J.P.; Salgado, L.; Varela, J.; Pol, D. Late Cretaceous reptilian biota of the La Colonia Formation, central Patagonia, Argentina: Occurrences, preservation and paleoenvironments. Cretac. Res. 2015, 54, 154–168. [Google Scholar] [CrossRef]
- Novas, F.E.; Chatterjee, S.; Rudra, D.K.; Datta, P. Rahiolisaurus gujaratensis, n. gen. n. sp., a New Abelisaurid Theropod from the Late Cretaceous of India. In New Aspects of Mesozoic Biodiversity; Bandyopadhyay, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 45–62. [Google Scholar]
- Wilson, J.A.; Sereno, P.C.; Srivastava, S.; Bhatt, D.K.; Khosla, A.; Sahni, A. A new abelisaurid (Dinosauria, Theropoda) from the Lameta Formation (Cretaceous, Maastrichtian) of India. Contrib. Mus. Paleontol. Univ. Mich. 2003, 31, 1–42. [Google Scholar]
- Chatterjee, S. Indosuchus and Indosaurus, Cretaceous carnosaurs from India. J. Paleontol. 1978, 52, 570–580. [Google Scholar]
- Huene, F.R.; Matley, C.A. The Cretaceous Saurischia and Ornithischia of the Central provinces. Palaeontol. Indica (New Ser.) Mem. Geol. Surv. India 1933, 21, 1–74. [Google Scholar]
- Cau, A.; Dalla Vecchia, F.M.; Fabbri, M. A thick-skulled theropod (Dinosauria, Saurischia) from the Upper Cretaceous of Morocco with implications for carcharodontosaurid cranial evolution. Cretac. Res. 2013, 40, 251–260. [Google Scholar] [CrossRef]
- Sereno, P.C.; Dutheil, D.B.; Iarochene, M.; Larsson, H.C. Predatory dinosaurs from the Sahara and Late Cretaceous faunal differentiation. Science 1996, 272, 986. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.O.; Rubilar-Rogers, D.; Moreno, K. A new Abelisauridae (Dinosauria: Theropoda) from northwest Patagonia. Ameghiniana 2004, 41, 555–563. [Google Scholar]
- Coria, R.A.; Salgado, L. A new giant carnivorous dinosaur from the Cretaceous of Patagonia. Nature 1995, 377, 224–226. [Google Scholar] [CrossRef]
- Mateus, O. Late Jurassic dinosaurs from the Morrison Formation (USA), the Lourinha and Alcobaça formations (Portugal), and the Tendaguru Beds (Tanzania): A comparison. New Mex. Mus. Nat. Hist. Sci. Bull. 2006, 36, 223–231. [Google Scholar]
- Van Valkenburgh, B.; Hayward, M.W.; Ripple, W.J.; Meloro, C.; Roth, V.L. The impact of large terrestrial carnivores on Pleistocene ecosystems. Proc. Natl. Acad. Sci. USA 2016, 113, 862–867. [Google Scholar] [CrossRef]
- Barnett, R.; Barnes, I.; Phillips, M.J.; Martin, L.D.; Harington, C.R.; Leonard, J.A.; Cooper, A. Evolution of the extinct Sabretooths and the American cheetah-like cat. Curr. Biol. 2005, 15, R589–R590. [Google Scholar] [CrossRef]
- Nowak, R.M. Walker’s Mammals of the World, 6th ed.; JHU Press: Baltimore, MD, USA, 1999. [Google Scholar]
- Bibi, F.; Kiessling, W. Continuous evolutionary change in Plio-Pleistocene mammals of eastern Africa. Proc. Natl. Acad. Sci. USA 2015, 112, 10623–10628. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J. The Third Chimpanzee: The Evolution and Future of the Human Animal; Harper Perennial: New York, NY, USA, 1992. [Google Scholar]
- Bardet, N.; Falconnet, J.; Fischer, V.; Houssaye, A.; Jouve, S.; Pereda-Suberbiola, X.; Perez-García, A.; Rage, J.-C.; Vincent, P. Mesozoic marine reptile palaeobiogeography in response to drifting plates. Gondwana Res. 2014, 26, 869–887. [Google Scholar] [CrossRef]
- Longrich, N.R.; Jalil, N.-E.; Khaldoune, F.; Yazami, O.K.; Pereda-Suberbiola, X.; Bardet, N. Thalassotitan atrox, a giant predatory mosasaurid (Squamata) from the Upper Maastrichtian Phosphates of Morocco. Cretac. Res. 2022, 140, 105315. [Google Scholar] [CrossRef]
- Lambert, O.; Bianucci, G.; Post, K.; De Muizon, C.; Salas-Gismondi, R.; Urbina, M.; Reumer, J. The giant bite of a new raptorial sperm whale from the Miocene epoch of Peru. Nature 2010, 466, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.L. Pacific Fishes of Canada; Fisheries Research Board of Canada Bulletin: Ottowa, IL, USA, 1973; Volume 180, p. 741. [Google Scholar]
- Doughty, P.; Kealley, L.; Fitch, A.; Donnellan, S.C. A new diminutive species of Varanus from the Dampier Peninsula, western Kimberley region, Western Australia. Rec. West. Aust. Mus. 2014, 29, 128–140. [Google Scholar] [CrossRef]
- Dunning, J.B. Handbook of Avian Body Masses, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 672. [Google Scholar]
- Cope, E.D. Discovery of a gigantic dinosaur in the Cretaceous of New Jersey. Proc. Acad. Nat. Sci. Phila. 1866, 18, 275–279. [Google Scholar]
- Carr, T.D.; Williamson, T.E.; Schwimmer, D.R. A new genus and species of tyrannosauroid from the Late Cretaceous (middle Campanian) Demopolis Formation of Alabama. J. Vertebr. Paleontol. 2005, 25, 119–143. [Google Scholar] [CrossRef]
- Brusatte, S.L.; Carr, T.D.; Erickson, G.M.; Bever, G.S.; Norell, M. A long-snouted, multihorned tyrannosaurid from the Late Cretaceous of Mongolia. Proc. Natl. Acad. Sci. USA 2009, 106, 17251–17266. [Google Scholar] [CrossRef]
- Kurzanov, S. New carnosaur from the Late Cretaceous of Nogon− Tsav, Mongolia. Sovmest. Sov. Mong. Paleon Tologičeskaâ Eksped. Tr. 1976, 3, 93–104. [Google Scholar]
- Lü, J.; Yi, L.; Brusatte, S.L.; Yang, L.; Li, H.; Chen, L. A new clade of Asian Late Cretaceous long-snouted tyrannosaurids. Nat. Commun. 2014, 5, 3788. [Google Scholar] [CrossRef]
- McDonald, A.T.; Wolfe, D.G.; Dooley, A.C., Jr. A new tyrannosaurid (Dinosauria: Theropoda) from the Upper Cretaceous Menefee Formation of New Mexico. PeerJ 2018, 6, e5749. [Google Scholar] [CrossRef]
- Currie, P.J.; Zhiming, D. New information on Shanshanosaurus huoyanshanensis, a juvenile tyrannosaurid (Theropoda, Dinosauria) from the Late Cretaceous of China. Can. J. Earth Sci. 2001, 38, 1729–1737. [Google Scholar] [CrossRef]
- Maleev, E.A. New carnivorous dinosaurs from the Upper Cretaceous of Mongolia. Dokl. Akad. Nauk. SSSR 1955, 104, 779–782. [Google Scholar]
- Hone, D.W.; Wang, K.; Sullivan, C.; Zhao, X.; Chen, S.; Li, D.; Ji, S.; Ji, Q.; Xu, X. A new, large tyrannosaurine theropod from the Upper Cretaceous of China. Cretac. Res. 2011, 32, 495–503. [Google Scholar] [CrossRef]
- Persons IV, W.S.; Currie, P.J.; Erickson, G.M. An older and exceptionally large adult specimen of Tyrannosaurus rex. Anat. Rec. 2020, 303, 656–672. [Google Scholar] [CrossRef]
- Hutchinson, J.R.; Bates, K.T.; Molnar, J.; Allen, V.; Makovicky, P.J. A computational analysis of limb and body dimensions in Tyrannosaurus rex with implications for locomotion, ontogeny, and growth. PLoS ONE 2011, 6, e26037. [Google Scholar] [CrossRef]
- DePalma, R.A.; Burnham, D.A.; Martin, L.D.; Larson, P.L.; Bakker, R.T. The first giant raptor (Theropoda: Dromaeosauridae) from the Hell Creek Formation. Paleontol. Contrib. 2015, 14, 1–16. [Google Scholar]
- Evans, D.C.; Larson, D.W.; Currie, P.J. A new dromaeosaurid (Dinosauria: Theropoda) with Asian affinities from the latest Cretaceous of North America. Naturwissenschaften 2013, 100, 1041–1049. [Google Scholar] [CrossRef]
- Longrich, N.R.; Field, D. Torosaurus is not Triceratops: Ontogeny in Chasmosaurine Ceratopsids as a Case Study in Dinosaur Taxonomy. PLoS ONE 2012, 7, e32623. [Google Scholar] [CrossRef]
- Maleev, E.A. Giant carnosaurs of the family Tyrannosauridae. Jt. Sov. Mong. Paleontol. Exped. 1974, 1, 132–191. [Google Scholar]
- Voris, J.T.; Zelenitsky, D.K.; Therrien, F.; Ridgely, R.C.; Currie, P.J.; Witmer, L.M. Two exceptionally preserved juvenile specimens of Gorgosaurus libratus (Tyrannosauridae, Albertosaurinae) provide new insight into the timing of ontogenetic changes in tyrannosaurids. J. Vertebr. Paleontol. 2021, 41, e2041651. [Google Scholar] [CrossRef]
- Voris, J.T.; Zelenitsky, D.K.; Therrien, F.; Currie, P.J. Reassessment of a juvenile Daspletosaurus from the Late Cretaceous of Alberta, Canada with implications for the identification of immature tyrannosaurids. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brochu, C.A. Closure of neurocentral sutures during crocodylian ontogeny: Implication for maturity assessment in fossil archosaurs. J. Vertebr. Paleontol. 1996, 16, 49–62. [Google Scholar] [CrossRef]
- Goodwin, M.B.; Clemens, W.A.; Horner, J.R.; Padian, K. The smallest known Triceratops skull: New observations on ceratopsid cranial anatomy and ontogeny. J. Vertebr. Paleontol. 2006, 26, 103–112. [Google Scholar] [CrossRef]
- Sereno, P.C.; Tan, L.; Brusatte, S.L.; Kriegstein, H.J.; Zhao, X.; Cloward, K. Tyrannosaurid skeletal design first evolved at small body size. Science 2009, 326, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Sereno, P.C. The pectoral girdle and forelimb of the basal theropod Herrerasaurus ischigualastensis. J. Vertebr. Paleontol. 1994, 13, 425–450. [Google Scholar] [CrossRef]
- Burch, S.H.; Carrano, M.T. An articulated pectoral girdle and forelimb of the abelisaurid theropod Majungasaurus crenatissimus from the Late Cretaceous of Madagascar. J. Vertebr. Paleontol. 2012, 32, 1–16. [Google Scholar] [CrossRef]
- Norell, M.A.; Makovicky, P.J. Important features of the dromaeosaurid skeleton II: Information from newly collected specimens of Velociraptor mongoliensis. Am. Mus. Novit. 1999, 3282, 1–45. [Google Scholar]
- Perle, A.; Norell, M.A.; Clark, J.M. A new maniraptoran theropod Achillobator giganticus (Dromaeosauridae) from the Upper Cretaceous of Burkhant, Mongolia. Dep. Geol. Natl. Univ. Mong. Ulaanbaatar 1999, 101, 1–105. [Google Scholar]
- Jasinski, S.E.; Sullivan, R.M.; Lucas, S.G. Taxonomic composition of the Alamo Wash local fauna from the Upper Cretaceous Ojo Alamo Formation (Naashoibito Member), San Juan Basin, New Mexico. New Mex. Mus. Nat. Hist. Sci. Bull. 2011, 53, 216–265. [Google Scholar]
- Longrich, N.R.; Currie, P.J. A microraptorine (Dinosauria-Dromaeosauridae) from the Late Cretaceous of North America. PNAS 2009, 106, 5002–5008. [Google Scholar] [CrossRef] [PubMed]
- Raath, M.A.; Carpenter, K.; Currie, P.J. Morphological variation in small theropods and its meaning in systematics: Evidence from Syntarsus. In Dinosaur Systematics: Perspectives and Approaches; Currie, P.J., Carpenter, K., Eds.; Cambridge University Press: Cambridge, UK, 1990; pp. 91–105. [Google Scholar]
- Iori, F.V.; de Araújo-Júnior, H.I.; Tavares, S.A.S.; da Silva Marinho, T.; Martinelli, A.G. New theropod dinosaur from the Late Cretaceous of Brazil improves abelisaurid diversity. J. South Am. Earth Sci. 2021, 112, 103551. [Google Scholar] [CrossRef]
- Pol, D.; Rauhut, O.W. A Middle Jurassic abelisaurid from Patagonia and the early diversification of theropod dinosaurs. Proc. R. Soc. B: Biol. Sci. 2012, 279, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Cerroni, M.A.; Baiano, M.A.; Canale, J.I.; Agnolín, F.L.; Otero, A.; Novas, F.E. Appendicular osteology of Skorpiovenator bustingorryi (Theropoda, Abelisauridae) with comments on phylogenetic features of abelisaurids. J. Syst. Palaeontol. 2022, 20, 1–32. [Google Scholar] [CrossRef]
- Macdonald, I.; Currie, P.J. Description of a partial Dromiceiomimus (Dinosauria: Theropoda) skeleton with comments on the validity of the genus. Can. J. Earth Sci. 2019, 56, 129–157. [Google Scholar] [CrossRef]
- Osborn, H.F. Skeletal adaptations of Ornitholestes, Struthiomimus, Tyrannosaurus. Bull. Am. Mus. Nat. Hist. 1917, 35, 733–771. [Google Scholar]
- Yao, X.; Sullivan, C.; Tan, Q.; Xu, X. New ornithomimosaurian (Dinosauria: Theropoda) pelvis from the Upper Cretaceous Erlian Formation of Nei Mongol, North China. Cretac. Res. 2022, 137, 105234. [Google Scholar] [CrossRef]
- Xu, L.; Kobayashi, Y.; Lü, J.; Lee, Y.-N.; Liu, Y.; Tanaka, K.; Zhang, X.; Jia, S.; Zhang, J. A new ornithomimid dinosaur with North American affinities from the Late Cretaceous Qiupa Formation in Henan Province of China. Cretac. Res. 2011, 32, 213–222. [Google Scholar] [CrossRef]
- Brown, C.M.; Russell, A.P.; Ryan, M.J. Pattern and transition of surficial bone texture of the centrosaurine frill and their ontogenetic and taxonomic implications. J. Vertebr. Paleontol. 2009, 29, 132–141. [Google Scholar] [CrossRef]
- Sampson, S.D.; Ryan, M.J.; Tanke, D.H. Craniofacial ontogeny in centrosaurine dinosaurs (Ornithischia: Ceratopsidae): Taxonomic and behavioral implications. Zooogical J. Linn. Soc. 1997, 121, 293–337. [Google Scholar] [CrossRef]
- Horner, J.R.; de Ricqlès, A.; Padian, K. Variation in dinosaur skeletochronology indicators: Implications for age assessment and physiology. Paleobiology 1999, 25, 295–304. [Google Scholar] [CrossRef]
- Erickson, G.M.; Tumanova, T.A. Growth curve of Psittacosaurus mongoliensis Osborn (Ceratopsia: Psittacosauridae) inferred from long bone histology. Zool. J. Linn. Soc. 2000, 130, 551–566. [Google Scholar] [CrossRef]
- Griebeler, E.M.; Klein, N.; Sander, P.M. Aging, maturation and growth of sauropodomorph dinosaurs as deduced from growth curves using long bone histological data: An assessment of methodological constraints and solutions. PLoS ONE 2013, 8, e67012. [Google Scholar] [CrossRef][Green Version]
- Erickson, G.M. On dinosaur growth. Annu. Rev. Earth Planet. Sci. 2014, 42, 675–697. [Google Scholar] [CrossRef]
- Saitta, E.T.; Stockdale, M.T.; Longrich, N.R.; Bonhomme, V.; Benton, M.J.; Cuthill, I.C.; Makovicky, P.J. An effect size statistical framework for investigating sexual dimorphism in non-avian dinosaurs and other extinct taxa. Biol. J. Linn. Soc. 2020, 131, 231–273. [Google Scholar] [CrossRef]
- Ralls, K.; Mesnick, S. Sexual Dimorphism. In Encyclopedia of Marine Mammals; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1005–1011. [Google Scholar]
- Laws, R.M. The elephant seal (Mirounga leonina, Linn.): I. Growth and age; HMSO: London, UK, 1953; p. 62. [Google Scholar]
- McCann, T.S.; Fedak, M.A.; Harwood, J. Parental investment in southern elephant seals, Mirounga leonina. Behav. Ecol. Sociobiol. 1989, 25, 81–87. [Google Scholar] [CrossRef]
- Molnar, R.E. The cranial morpholgy of Tyrannosaurus rex Palaeontographica. Abt. A Paläozoologie Stratigr. 1991, 217, 137–176. [Google Scholar]
- Yun, C.-G.; Peters, G.F.; Currie, P.J. Allometric growth in the frontals of the Mongolian theropod dinosaur Tarbosaurus bataar. Acta Palaeontol. Pol. 2022, 67, 601–615. [Google Scholar] [CrossRef]
- Forster, C.A. Species resolution in Triceratops: Cladistic and morphometric approaches. J. Vertebr. Paleontol. 1996, 16, 259–270. [Google Scholar] [CrossRef]
- Scannella, J.B.; Fowler, D.W.; Goodwin, M.B.; Horner, J.R. Evolutionary trends in Triceratops from the Hell Creek Formation, Montana. Proc. Natl. Acad. Sci. USA 2014, 111, 10245–10250. [Google Scholar] [CrossRef]
- Pringle, H. Selling America’s fossil record. Science 2014, 343, 364–367. [Google Scholar] [CrossRef]
- Currie, P.J.; Rigby, K.J.; Sloan, R.E. Theropod teeth from the Judith River Formation of southern Alberta, Canada. In Dinosaur Systematics: Perspectives and Approaches; Currie, P.J., Carpenter, K., Eds.; Cambridge University Press: Cambridge, UK, 1990; pp. 107–125. [Google Scholar]
- Hone, D.W.; Farke, A.A.; Wedel, M.J. Ontogeny and the fossil record: What, if anything, is an adult dinosaur? Biol. Lett. 2016, 12, 20150947. [Google Scholar] [CrossRef]
- Erickson, G.M.; Currie, P.J.; Inouye, B.D.; Winn, A.A. Tyrannosaur life tables: An example of nonavian dinosaur population biology. Science 2006, 313, 213–217. [Google Scholar] [CrossRef]
- Darwin, C. The Descent of Man and Selection in Relation to Sex; Murray: London, UK, 1871. [Google Scholar]
- Horner, J.R.; Goodwin, M.B. Major cranial changes during Triceratops ontogeny. Proc. R. Soc. B 2006, 273, 2757–2761. [Google Scholar]
- Brown, B.; Schlaikjer, E.M. The structure and relationships of Protoceratops. Ann. N. Y. Acad. Sci. 1940, 40, 133–266. [Google Scholar] [CrossRef]
- Carr, T.D. A reappraisal of tyrannosauroid fossils from the Iren Dabasu Formation (Coniacian–Campanian), Inner Mongolia, People’s Republic of China. J. Vertebr. Paleontol. 2022, 42, e2199817. [Google Scholar] [CrossRef]
- De Beer, G.R. Embryos and Ancestors, 2nd ed.; Clarendon Press: Oxford, UK, 1951; p. 159. [Google Scholar]
- Bhullar, B.-A.S.; Marugán-Lobón, J.; Racimo, F.; Bever, G.S.; Rowe, T.B.; Norell, M.A.; Abzhanov, A. Birds have paedomorphic dinosaur skulls. Nature 2012, 487, 223–226. [Google Scholar]
- Naish, D.; Cau, A. The osteology and affinities of Eotyrannus lengi, a tyrannosauroid theropod from the Wealden Supergroup of southern England. PeerJ 2022, 10, e12727. [Google Scholar] [CrossRef]
- Funston, G.F.; Powers, M.J.; Whitebone, S.A.; Brusatte, S.L.; Scannella, J.B.; Horner, J.R.; Currie, P.J. Baby tyrannosaurid bones and teeth from the Late Cretaceous of western North America. Can. J. Earth Sci. 2021, 58, 756–777. [Google Scholar]
- Gilmore, C.W. A new dinosaur from the Lance Formation of Wyoming. Smithson. Misc. Collect. 1913, 61, 1–5. [Google Scholar]
- Gilmore, C.W. A new coelurid dinosaur from the Belly River Cretaceous of Alberta. Can. Geol. Surv. Bull. 1924, 38, 210639511. [Google Scholar]
- Gilmore, C.W. On the dinosaurian fauna of the Iren Dabasu Formation. Bull. Am. Mus. Nat. Hist. 1933, 67, 23–78. [Google Scholar]
- Gilmore, C.W. A new sauropod dinosaur from the Ojo Alamo Formation of New Mexico. Smithson. Misc. Collect. 1922, 72, 1–9. [Google Scholar]
- Gilmore, C.W. A new ceratopsian dinosaur from the Upper Cretaceous of Montana, with note on Hypacrosaurus. Smithson. Misc. Collect. 1914, 63, 1–10. [Google Scholar]
- Larson, N.L.; Larson, P.L.; Carpenter, K. One hundred years of Tyrannosaurus rex: The skeletons. Tyrannosaurus Rex Tyrant King 2008, 1, 1–56. [Google Scholar]
- Mallon, J.C.; Evans, D.C.; Ryan, M.J.; Anderson, J.S. Megaherbivorous dinosaur turnover in the Dinosaur Park Formation (upper Campanian) of Alberta, Canada. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 350-352, 124–138. [Google Scholar] [CrossRef]
- Sampson, S.D.; Loewen, M.A.; Farke, A.A.; Roberts, E.M.; Forster, C.A.; Smith, J.A.; Titus, A.L. New horned dinosaurs from Utah provide evidence for intracontinental dinosaur endemism. PLoS ONE 2010, 5, e12292. [Google Scholar] [CrossRef]
- Longrich, N.R. Titanoceratops ouranos, a giant horned dinosaur from the Late Campanian of New Mexico. Cretac. Res. 2011, 32, 264–276. [Google Scholar] [CrossRef]
- Hanai, T.; Tsuihiji, T. Description of tooth ontogeny and replacement patterns in a juvenile Tarbosaurus bataar (Dinosauria: Theropoda) using CT-scan data. Anat. Rec. 2019, 302, 1210–1225. [Google Scholar] [CrossRef]
- Longrich, N.R.; Scriberas, J.; Wills, M.A. Severe extinction and rapid recovery of mammals across the Cretaceous-Paleogene boundary, and the effects of rarity on patterns of extinction and recovery. J. Evol. Biol. 2016, 29, 1495–1512. [Google Scholar] [CrossRef]
- Gao, K.-Q.; Fox, R.C. Taxonomy and evoluton of Late Cretaceous lizards (Reptilia: Squamata) from Western Canada. Bull. Carnegie Mus. Nat. Hist. 1996, 33, 1–107. [Google Scholar] [CrossRef]
- Longrich, N.R.; Bhullar, B.-A.S.; Gauthier, J. Mass extinction of lizards and snakes at the Cretaceous-Paleogene boundary. Proc. Natl. Acad. Sci. USA 2012, 109, 21396–21401. [Google Scholar] [CrossRef]
- Sweet, A.R.; Braman, D.R. Cretaceous–Tertiary palynofloral perturbations and extinctions within the Aquilapollenites Phytogeographic Province. Can. J. Earth Sci. 2001, 38, 249–269. [Google Scholar] [CrossRef]
- Johnson, K.R. Megaflora of the Hell Creek and lower Fort Union Formations in the western Dakotas: Vegetational response to climate change, the Cretaceous-Tertiary boundary event, and rapid marine transgression. Geol. Soc. Am. Spec. Pap. 2002, 361, 329–391. [Google Scholar]
- Sibley, D.A. The Sibley Guide to Birds; Knopf: New York, NY, USA, 2000; p. 545. [Google Scholar]
- Hekkala, E.; Shirley, M.H.; Amato, G.; Austin, J.D.; Charter, S.; Thorbjarnarson, J.; Vliet, K.A.; Houck, M.L.; Desalle, R.; Blum, M.J. An ancient icon reveals new mysteries: Mummy DNA resurrects a cryptic species within the Nile crocodile. Mol. Ecol. 2011, 20, 4199–4215. [Google Scholar] [CrossRef] [PubMed]
- Rohland, N.; Reich, D.; Mallick, S.; Meyer, M.; Green, R.E.; Georgiadis, N.J.; Roca, A.L.; Hofreiter, M. Genomic DNA sequences from mastodon and woolly mammoth reveal deep speciation of forest and savanna elephants. PLoS Biol. 2010, 8, e1000564. [Google Scholar] [CrossRef] [PubMed]
- Rosel, P.E.; Wilcox, L.A.; Yamada, T.K.; Mullin, K.D. A new species of baleen whale (Balaenoptera) from the Gulf of Mexico, with a review of its geographic distribution. Mar. Mammal Sci. 2021, 37, 577–610. [Google Scholar] [CrossRef]
- Wada, S.; Oishi, M.; Yamada, T.K. A newly discovered species of living baleen whale. Nature 2003, 426, 278–281. [Google Scholar] [CrossRef]
- Fennessy, J.; Bidon, T.; Reuss, F.; Kumar, V.; Elkan, P.; Nilsson, M.A.; Vamberger, M.; Fritz, U.; Janke, A. Multi-locus analyses reveal four giraffe species instead of one. Curr. Biol. 2016, 26, 2543–2549. [Google Scholar] [CrossRef]
- Schott, R.K.; Evans, D.C.; Goodwin, M.B.; Horner, J.R.; Brown, C.M.; Longrich, N.R. Cranial Ontogeny in Stegoceras validum (Dinosauria: Pachycephalosauria): A Quantitative Model of Pachycephalosaur Dome Growth and Variation. PLoS ONE 2011, 6, e21092. [Google Scholar] [CrossRef]
- Longrich, N.R.; Sankey, J.T.; Tanke, D.H. Texacephale langstoni, a new genus of pachycephalosaurid (Dinosauria: Ornithischia) from the upper Campanian Aguja Formation, southern Texas, USA. Cretac. Res. 2010, 31, 274–284. [Google Scholar] [CrossRef]
- Scannella, J.; Horner, J.R. Torosaurus Marsh, 1891 is Triceratops, Marsh, 1889 (Ceratopsidae: Chasmosaurinae) synonymy through ontogeny. J. Vertebr. Paleontol. 2010, 30, 1157–1168. [Google Scholar] [CrossRef]
- Horner, J.R.; Goodwin, M.B. Extreme cranial ontogeny in the Upper Cretaceous dinosaur Pachycephalosaurus. PLoS ONE 2009, 4, 27626. [Google Scholar] [CrossRef] [PubMed]
- Longrich, N.R. Mojoceratops perifania, a new chasmosaurine ceratopsid from the Late Campanian of Western Canada. J. Paleontol. 2010, 84, 681–694. [Google Scholar] [CrossRef]
- van der Reest, A.J.; Currie, P.J. Troodontids (Theropoda) from the Dinosaur Park Formation, Alberta, with a description of a unique new taxon: Implications for deinonychosaur diversity in North America. Can. J. Earth Sci. 2017, 54, 919–935. [Google Scholar] [CrossRef]
- Darwin, C.R. The Origin of Species; John Murray: London, UK, 1859. [Google Scholar]
- Raup, D.M.; Sepkoski, J.J., Jr. Mass extinctions in the marine fossil record. Science 1982, 215, 1501–1503. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).