Axonal Projections of Neurons in the Brainstem Mesopontine Tegmental Anesthesia Area (MPTA) That Effect Anesthesia, Enabling Pain-Free Surgery
Abstract
1. Introduction
2. Materials and Methods
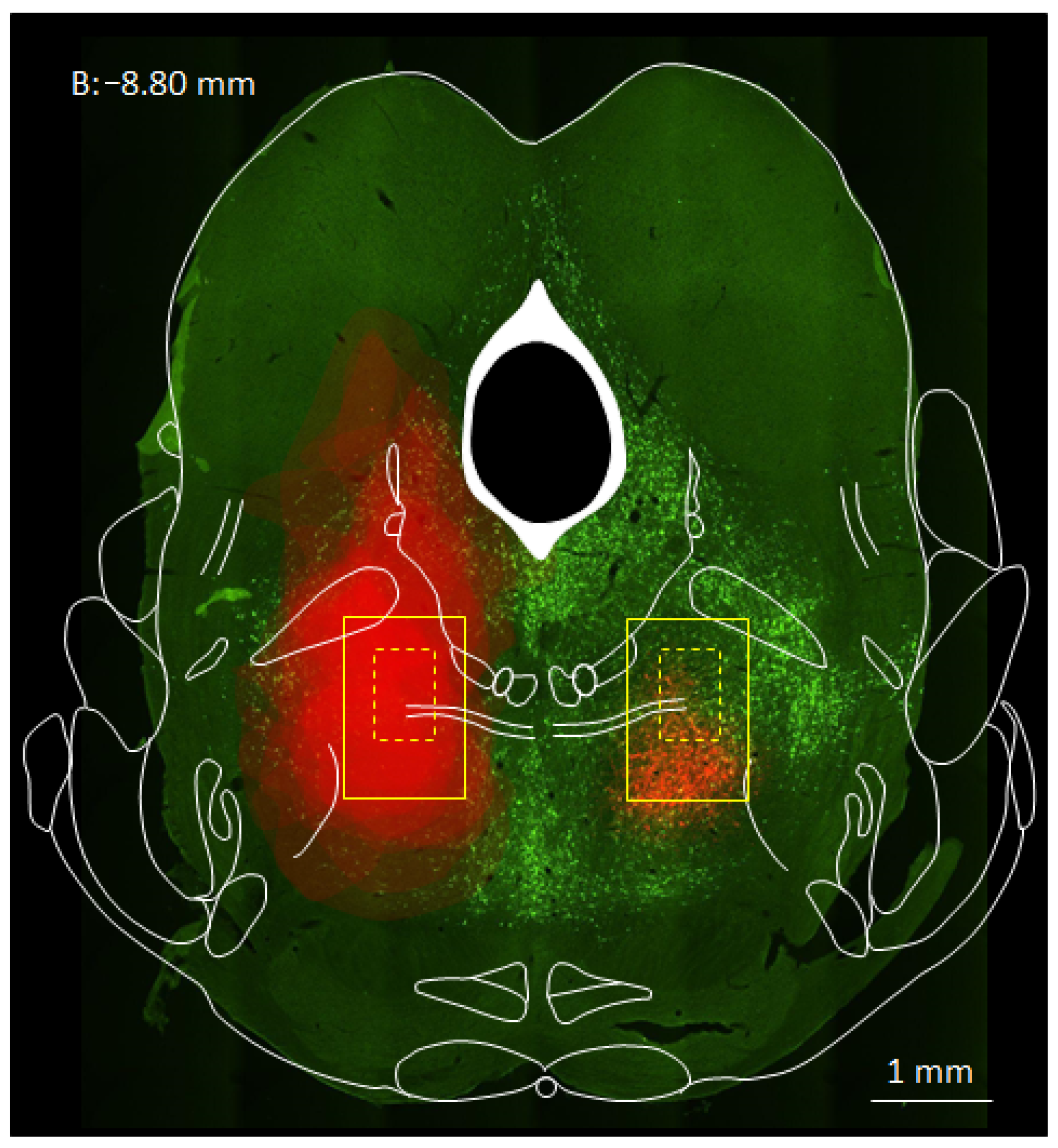
2.1. Animals and Surgery
2.2. Histology
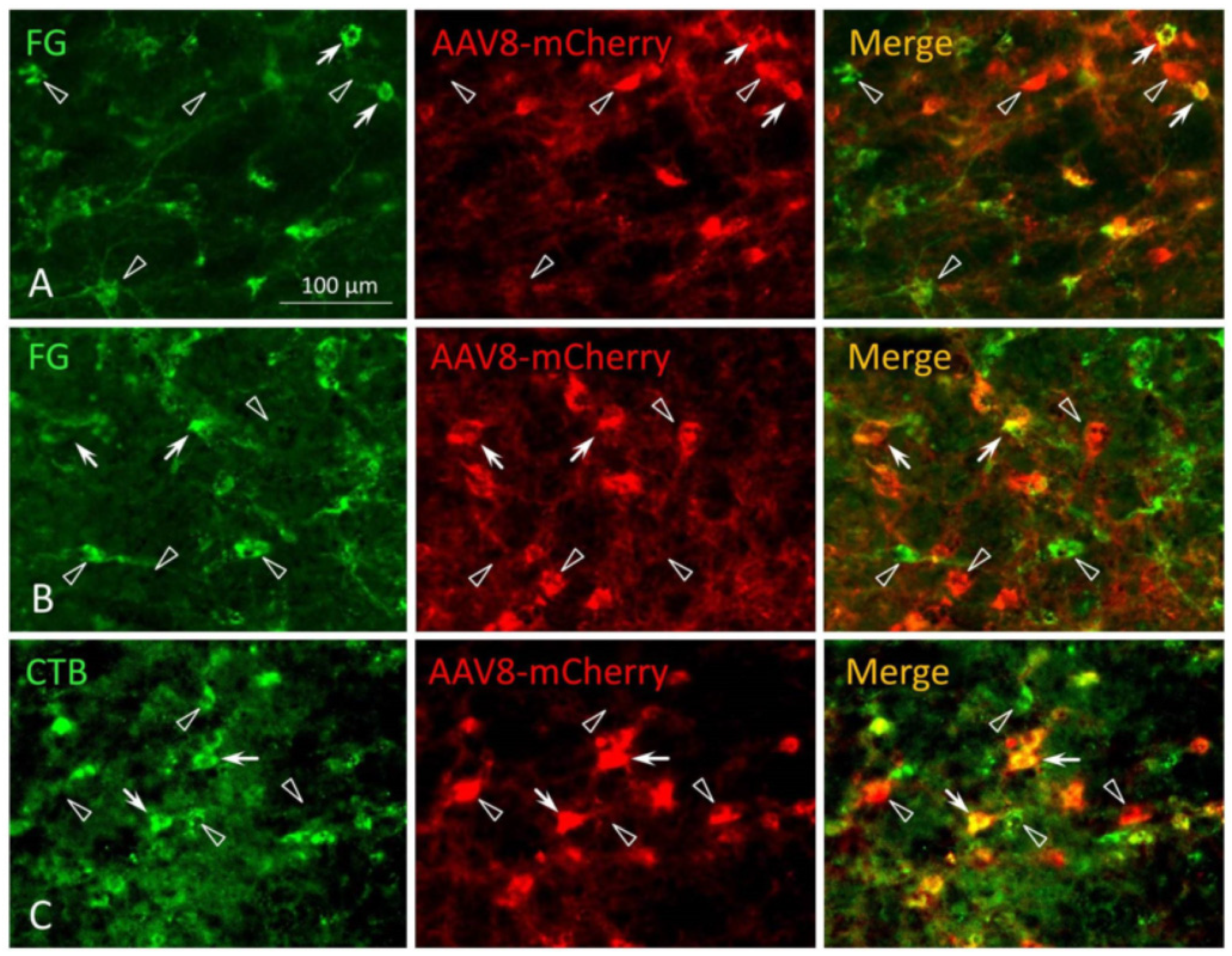
2.2.1. Immunolabelling
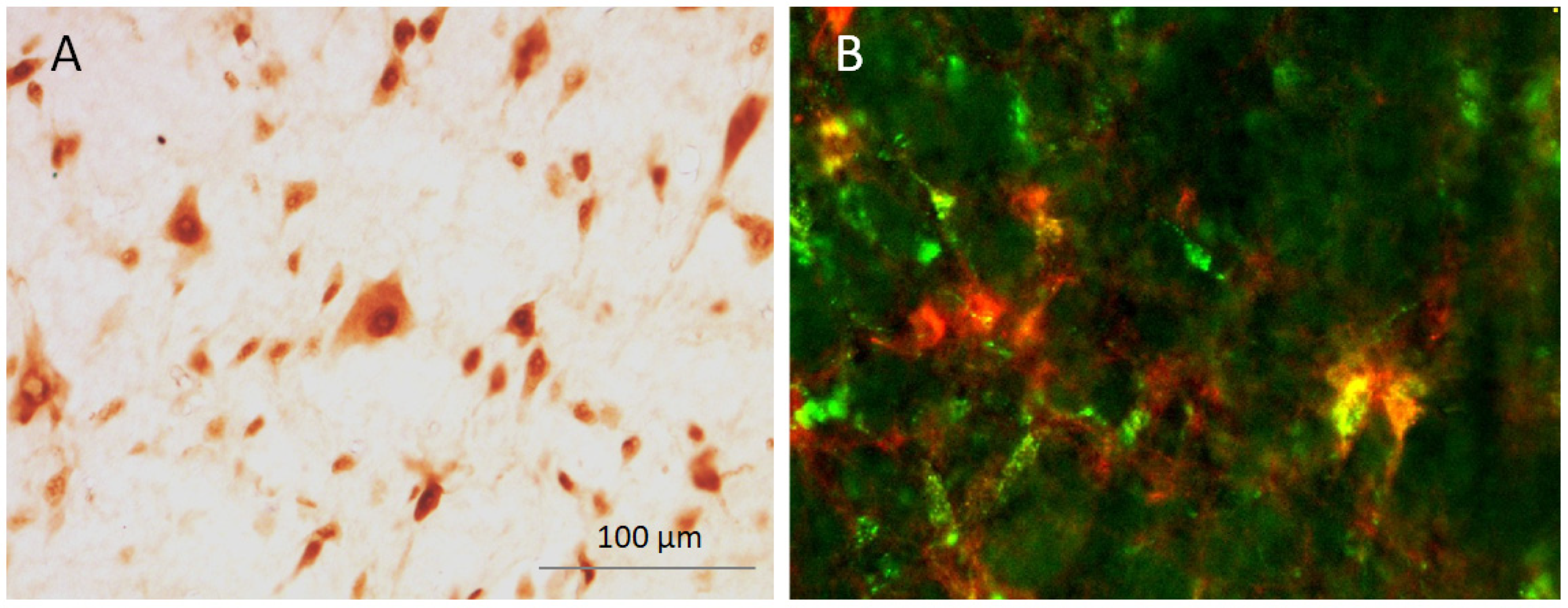
2.2.2. Imaging and Neuron Counting
2.3. Statistical Analysis
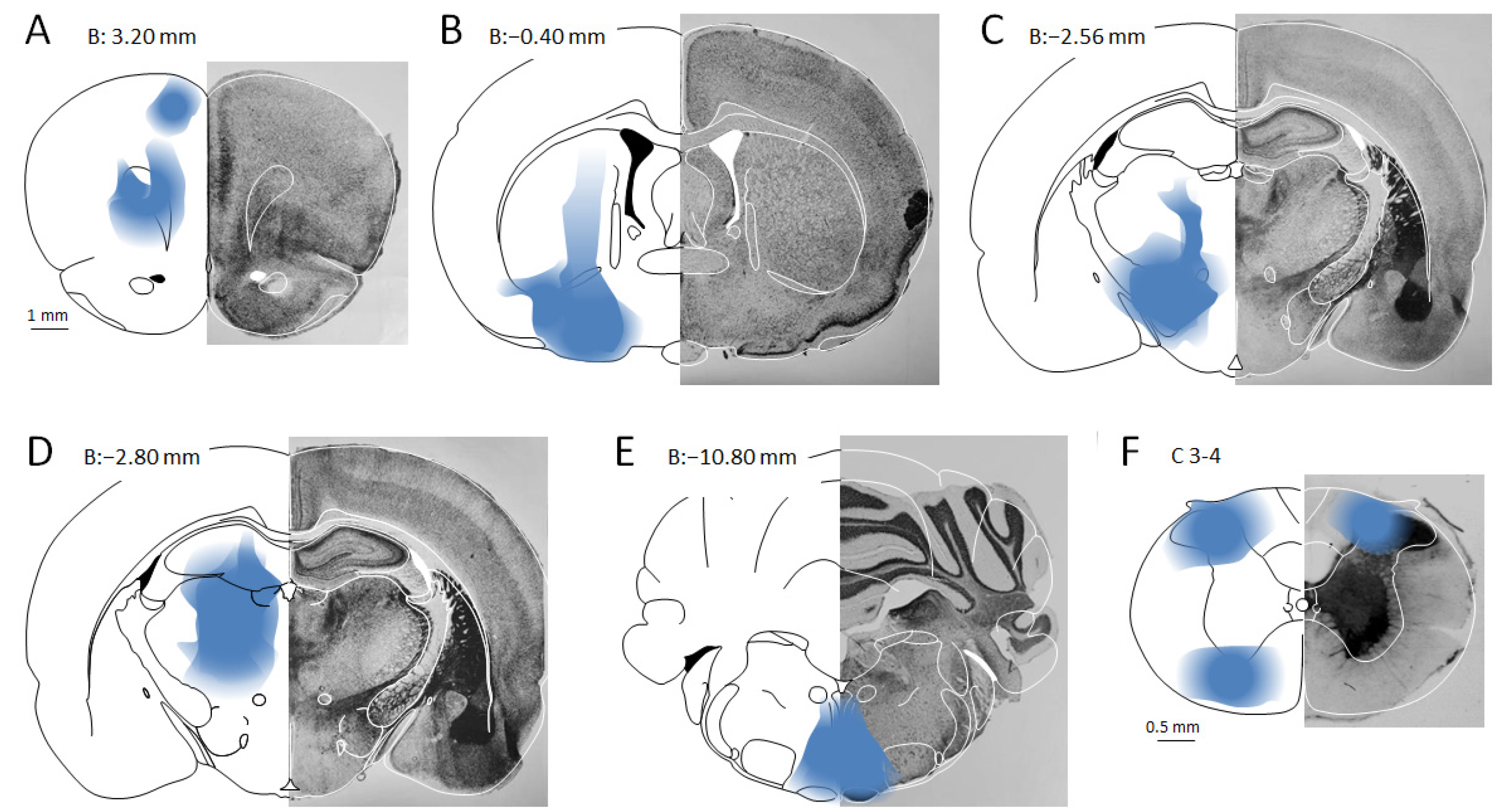
3. Results
3.1. MPTA Effector-Neurons Project to the PFC, the Major Cortical Relay Nuclei, the RVM and the SC
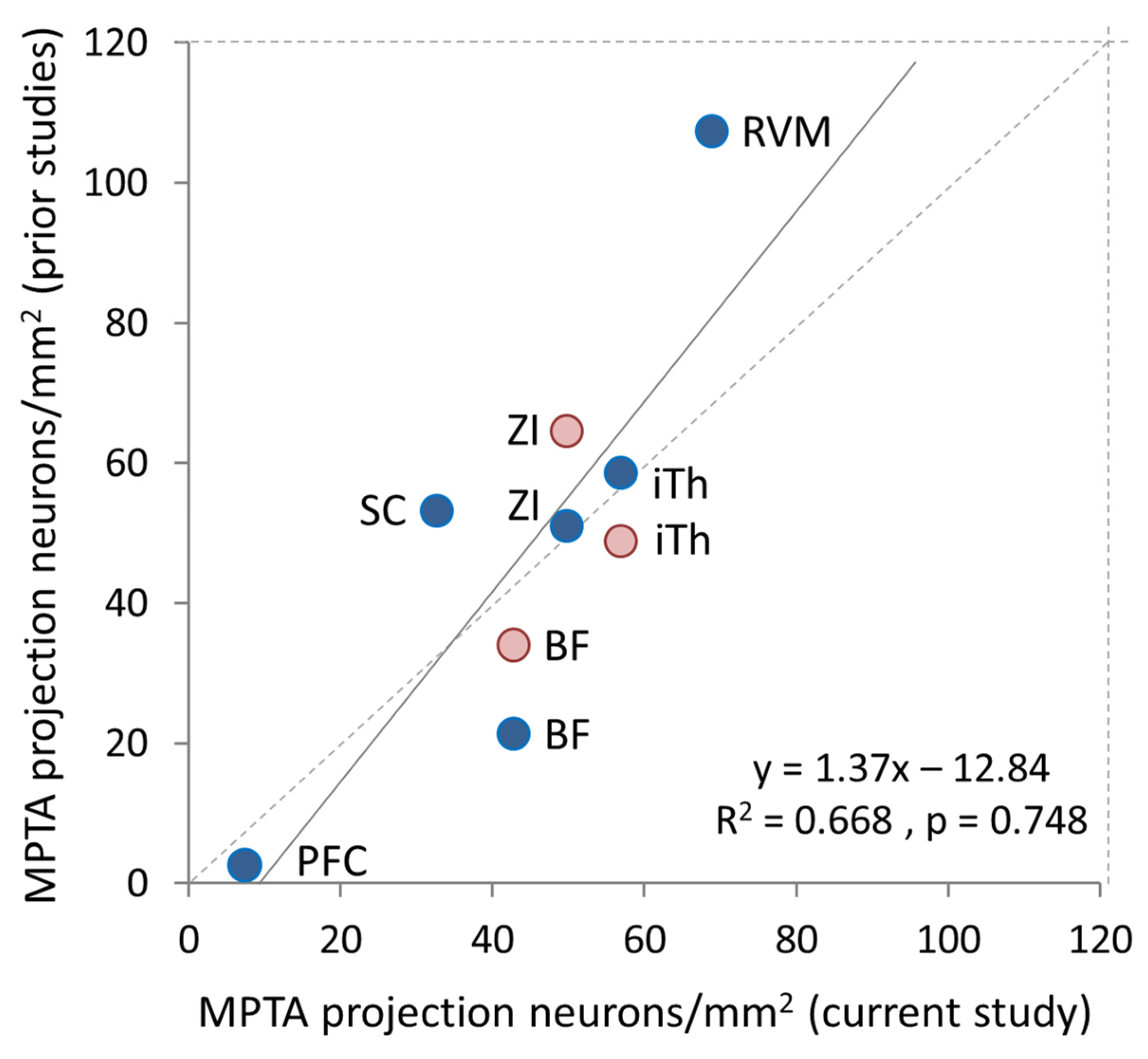
3.2. Prevalence of Effector-Neurons
3.3. MPTA Projection-Neurons That Are Not Effectors
4. Discussion
4.1. Limitations and Caveats
4.2. Additional Ascending and Descending Targets
4.3. Laterality and Collateralization
4.4. Loss-of-Consciousness Beyond General Anesthesia
4.5. Conclusions and Future Scope
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Antognini, J.F.; Carstens, E.; Tabo, E.; Buzin, V. Effect of differential delivery of isoflurane to head and torso on lumbar dorsal horn activity. Anesthesiology 1998, 88, 1055–1061. [Google Scholar] [CrossRef]
- Antognini, J.F.; Carstens, E.; Raines, D.E. (Eds.) Neural Mechanisms of Anesthesia; Human Press: Totowa, NJ, USA, 2003. [Google Scholar]
- Franks, N.P.; Lieb, W.R. Where do general anaesthetics act? Nature 1978, 274, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Alkire, M.T.; Haier, R.J.; Shah, N.K.; Anderson, C.T. Positron emission tomography study of regional cerebral metabolism in humans during isoflurane anesthesia. Anesthesiology 1997, 86, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Koblin, D. Mechanisms of action. In Anesthesia, 4th ed.; Miller, R., Ed.; Churchill Livingston: New York, NY, USA, 1994; pp. 67–99. [Google Scholar]
- Brown, E.N.; Purdon, P.L.; Van Dort, C.J. General anesthesia and altered states of arousal: A systems neuroscience analysis. Annu. Rev. Neurosci. 2011, 34, 601–628. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Sherman, D.; Devor, M.; Saper, C.B. A putative flip-flop switch for control of REM sleep. Nature 2006, 441, 589–594. [Google Scholar] [CrossRef]
- Lu, J.; Nelson, L.E.; Franks, N.; Maze, M.; Chamberlin, N.L.; Saper, C.B. Role of endogenous sleep-wake and analgesic systems in anesthesia. J. Comp. Neurol. 2008, 508, 648–662. [Google Scholar] [CrossRef]
- Baron, M.; Devor, M. From molecule to oblivion: Dedicated brain circuitry underlies anesthetic loss of consciousness permitting pain-free surgery. Front. Mol. Neurosci. 2023, 16, 1197304. [Google Scholar] [CrossRef]
- Scharf, M.T.; Kelz, M.B. Sleep and anesthesia interactions: A pharmacological appraisal. Curr. Anesth. Rep. 2013, 3, 1–9. [Google Scholar] [CrossRef]
- French, J.; Verzeano, M.; Magoun, H. A neural basis of the anesthetic state. Arch. Neurol. Psychiat. 1953, 69, 519–529. [Google Scholar] [CrossRef]
- Magni, F.; Moruzzi, G.; Rossi, G.; Zanchetti, A. EEG arousal following inactivation of the lower brain stem by selective injection of barbiturate into the vertebral circulation. Arch. Ital. Biol. 1959, 97, 33–41. [Google Scholar]
- Grady, F.S.; Boes, A.D.; Geerling, J.C. A Century Searching for the Neurons Necessary for Wakefulness. Front. Neurosci. 2022, 16, 930514. [Google Scholar] [CrossRef]
- Leung, L.S.; Luo, T.; Ma, J.; Herrick, I. Brain areas that influence general anesthesia. Prog. Neurobiol. 2014, 122, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Gelegen, C.; Miracca, G.; Ran, M.Z.; Harding, E.C.; Ye, Z.; Yu, X.; Tossell, K.; Houston, C.M.; Yustos, R.; Hawkins, E.D.; et al. Excitatory Pathways from the Lateral Habenula Enable Propofol-Induced Sedation. Curr. Biol. 2018, 28, 580–587.e5. [Google Scholar] [CrossRef] [PubMed]
- Zecharia, A.Y.; Yu, X.; Gotz, T.; Ye, Z.; Carr, D.R.; Wulff, P.; Bettler, B.; Vyssotski, A.L.; Brickley, S.G.; Franks, N.P.; et al. GABAergic inhibition of histaminergic neurons regulates active waking but not the sleep-wake switch or propofol-induced loss of consciousness. J. Neurosci. 2012, 32, 13062–13075. [Google Scholar] [CrossRef] [PubMed]
- Boly, M.; Massimini, M.; Tsuchiya, N.; Postle, B.R.; Koch, C.; Tononi, G. Are the Neural Correlates of Consciousness in the Front or in the Back of the Cerebral Cortex? Clinical and Neuroimaging Evidence. J. Neurosci. 2017, 37, 9603–9613. [Google Scholar] [CrossRef]
- Crick, F.C.; Koch, C. What is the function of the claustrum? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 1271–1279. [Google Scholar] [CrossRef]
- Nauta, W.J.H. Hypothalamic regulation of sleep in rats. An experimental study. J. Neurophysiol. 1946, 9, 285–316. [Google Scholar] [CrossRef]
- Van der Werf, Y.D.; Witter, M.P.; Groenewegen, H.J. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res. Brain Res. Rev. 2002, 39, 107–140. [Google Scholar] [CrossRef]
- Devor, M.; Zalkind, V. Reversible analgesia, atonia, and loss of consciousness on bilateral intracerebral microinjection of pentobarbital. Pain 2001, 94, 101–112. [Google Scholar] [CrossRef]
- Mao, R.; Cavelli, M.L.; Findlay, G.; Driessen, K.; Peterson, M.J.; Marshall, W.; Tononi, G.; Cirelli, C. Behavioral and cortical arousal from sleep, muscimol-induced coma, and anesthesia by direct optogenetic stimulation of cortical neurons. iScience 2024, 27, 109919. [Google Scholar] [CrossRef]
- Voss, L.J.; Young, B.J.; Barnards, J.P.; Sleigh, J. Differential anaesthetic effects following microinjection of thiopentone and propofol into the pons of adult rats: A pilot study. Anaesth. Intensive Care 2005, 33, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Namjoshi, D.R.; McErlane, S.A.; Taepavarapruk, N.; Soja, P.J. Network actions of pentobarbital in the rat mesopontine tegmentum on sensory inflow through the spinothalamic tract. J. Neurophysiol. 2009, 102, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Minert, A.; Devor, M. Brainstem node for loss of consciousness due to GABA receptor-active anesthetics. Exp. Neurol. 2016, 275, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Minert, A.; Baron, M.; Devor, M. Reduced sensitivity to anesthetic agents upon lesioning the mesopontine tegmental anesthesia area in rats depends on anesthetic type. Anesthesiology 2020, 132, 535–550. [Google Scholar] [CrossRef]
- Yatziv, S.-L.; Yudco, O.; Dickmann, S.; Devor, M. Patterns of neural activity in the mouse brain: Wakefulness vs. General anesthesia. Neurosci. Lett. 2020, 735, 135212. [Google Scholar] [CrossRef]
- Minert, A.; Yatziv, S.L.; Devor, M. Location of the mesopontine neurons responsible for maintenance of anesthetic loss of consciousness. J. Neurosci. 2017, 37, 9320–9331. [Google Scholar] [CrossRef]
- Baron, M.; Vaso, K.; Ibraheem, A.; Minert, A.; Devor, M. Molecular and cellular targets of GABAergic anesthetics in the mesopontine tegmentum that enable pain-free surgery. Pain 2025, 166, 1549–1564. [Google Scholar] [CrossRef]
- Baron, M.; Devor, M. Neurosteroids foster sedation by engaging tonic GABAA-Rs within the mesopontine tegmental anesthesia area (MPTA). Neurosci. Lett. 2024, 843, 138030. [Google Scholar] [CrossRef]
- Lellouche, Y.; Minert, A.; Schreiber, C.; Aroch, I.; Vaso, K.; Fishman, Y.; Devor, M. Individual Mesopontine Neurons Implicated in Anesthetic Loss-of-consciousness Employ Separate Ascending Pathways to the Cerebral Cortex. Neuroscience 2020, 432, 188–204. [Google Scholar] [CrossRef]
- Goldenberg, A.M.; Minert, A.; Fishman, Y.; Wolf, G.; Devor, M. Mesopontine neurons implicated in anesthetic loss-of-consciousness have either ascending or descending axonal projections, but not both. Neuroscience 2018, 369, 152–167. [Google Scholar] [CrossRef]
- Sukhotinsky, I.; Zalkind, V.; Lu, J.; Hopkins, D.A.; Saper, C.B.; Devor, M. Neural pathways associated with loss of consciousness caused by intracerebral microinjection of GABA A-active anesthetics. Eur. J. Neurosci. 2007, 25, 1417–1436. [Google Scholar] [CrossRef] [PubMed]
- Sukhotinsky, I.; Reiner, K.; Govrin-Lippmann, R.; Belenky, M.; Lu, J.; Hopkins, D.; Saper, C.; Devor, M. Projections from the mesopontine tegmental anesthesia area to regions involved in pain modulation. J. Chem. Neuroanat. 2006, 32, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Reiner, K.; Sukhotinsky, I.; Devor, M. Mesopontine tegmental anesthesia area projects independently to the rostromedial medulla and to the spinal cord. Neuroscience 2007, 146, 1355–1370. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Dong, S.; Roth, B.L. Generation of designer receptors exclusively activated by designer drugs (DREADDs) using directed molecular evolution. Curr. Protoc. Neurosci. 2010, 50, 4.33.1–4.33.25. [Google Scholar] [CrossRef]
- Baron, M.; Vaso, K.; Avigdor, T.; Charit, Y.; Minert, A.; Devor, M. Anesthetic loss of consciousness induced by chemogenetic excitation of mesopontine effector neurons. Exp. Neurol. 2022, 357, 114169. [Google Scholar] [CrossRef]
- Avigdor, T.; Minert, A.; Baron, M.; Devor, M. Paradoxical anesthesia: Sleep-like EEG during anesthesia induced by mesopontine microinjection of GABAergic agents. Exp. Neurol. 2021, 343, 113760. [Google Scholar] [CrossRef]
- Sukhotinsky, I.; Hopkins, D.A.; Lu, J.; Saper, C.B.; Devor, M. Movement suppression during anesthesia: Neural projections from the mesopontine tegmentum to areas involved in motor control. J. Comp. Neurol. 2005, 489, 425–448. [Google Scholar] [CrossRef]
- Sukhotinsky, I.; Minert, A.; Soja, P.; Devor, M. Mesopontine switch for the induction of general anesthesia by dedicated neural pathways. Anesth. Analg. 2016, 123, 1274–1285. [Google Scholar] [CrossRef]
- Namjoshi, D.; Vukicevic, S.; Sanoja, R.; Soja, P.J. Spinal Cord Targets of Relevance to the Anesthesiologist, Chapter 7. In Neuroscientific Foundations of Anesthesiology; Mashour, G.A., Lydic, R., Eds.; Oxford University Press: New York, NY, USA, 2011; pp. 91–106. [Google Scholar]
- Meiri, G.; Lanir, S.; Minert, A.; Devor, M. Transient loss of consciousness during hypercapnia and hypoxia: Involvement of pathways associated with general anesthesia. Exp. Neurol. 2016, 284, 67–78. [Google Scholar] [CrossRef]
- Hayes, R.L.; Pechura, C.M.; Katayama, Y.; Povlishock, J.T.; Giebel, M.L.; Becker, D.P. Activation of pontine cholinergic sites implicated in unconsciousness following cerebral concussion in the cat. Science 1984, 223, 301–303. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Mullen, R.J.; Buck, C.R.; Smith, A.M. NeuN, a neuronal specific nuclear protein in vertebrates. Development 1992, 116, 201–211. [Google Scholar] [CrossRef]
- Devor, M.; Zalkind, V.; Fishman, Y.; Minert, A. Model of anaesthetic induction by unilateral intracerebral microinjection of GABAergic agonists. Eur. J. Neurosci. 2016, 43, 846–858. [Google Scholar] [CrossRef]
- Reiner, K.; Sukhotinsky, I.; Devor, M. Bulbospinal neurons implicated in mesopontine-induced anesthesia are substantially collateralized. J. Comp. Neurol. 2008, 508, 418–436. [Google Scholar] [CrossRef]
- Baron, M.; Minert, A.; Devor, M. Pain and the evolutionary origins of subjective experience. Behav. Brain Sci. 2025, 48, e80. [Google Scholar] [CrossRef]
- Lanir-Azaria, S.; Meiri, G.; Avigdor, T.; Minert, A.; Devor, M. Enhanced wakefulness following lesions of a mesopontine locus essential for the induction of general anesthesia. Behav. Brain Res. 2018, 341, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, A.; Vaso, K.; Minert, A.; Yatziv, S.-L.; Baron, M.; Devor, M. Loss-of-consciousness: Sources of GABAergic input to the mesopontine tegmental anesthesia area. Front. Neurosci. 2025, 19, 1594984. [Google Scholar] [CrossRef] [PubMed]
- Carli, G.; Farabollini, F. Neurophysiological mechanisms involved in tonic immobility (TI). Prog. Brain Res. 2022, 271, 145–166. [Google Scholar] [PubMed]
- Cataldi, J.; Stephan, A.M.; Haba-Rubio, J.; Siclari, F. Shared EEG correlates between non-REM parasomnia experiences and dreams. Nat. Commun. 2024, 15, 3906. [Google Scholar] [CrossRef]
- Chicago Tribune. Sleepwalking Canadian Walks on Murder Charge. Chicago Tribune News, 17 July 1988. Available online: https://www.chicagotribune.com/1988/07/17/sleepwalking-canadian-walks-on-murder-charge/ (accessed on 3 September 2025).
| MPTA Bilateral | PFC * Unilateral | BF Unilateral | iTh * Unilateral | ZI Unilateral | RVM Midline | SC d/v Horn | |
|---|---|---|---|---|---|---|---|
| anterior–posterior mm. post bregma | −8.5 | 2.5, 3.1 | −0.5 | −3.3 | −3 | −11 | C3–4 |
| dorsal–ventral mm. below dura | −6.3 | 0.8 | −7.5 | −5, −6 | −7.5 | −10 | 0.5, 2.5 |
| medio-lateral mm. lateral to midline | ±1.3 | 1.4, 1.9 | 2.5 | 1.2 | 1.5 | 0 | ±1.5 |
| number of cases left/right | 15/15 16 rats | 3/0 | 2/2 | 3/1 | 1/3 | 6 | 2 |
| retrograde tracer FG/CTB | 2/1 | 2/2 | 3/1 | 2/2 | 3/3 | 1/1 | |
| marker of effector-neurons | AAV8-mCherry | ||||||
| volume microinjected (nL) | 50–250 | 200 × 4 | 200 | 200 × 2 | 100–200 | 100–200 | 150–200 |
| Projection Target Ipsilateral (n Rats CTB/FG) | CTB/ mm2 Singles | FG/ mm2 Singles | CTB + FG/ mm2 ± SD Singles | CTB/ mm2 Doubles | FG/ mm2 Doubles | CTB + FG/ mm2 ± SD Doubles | CTB % Doubles | FG % Doubles | CTB + FG % Doubles |
|---|---|---|---|---|---|---|---|---|---|
| MPTA Projection-Neurons | Projecting Effector-Effectors | Projecting Effector-Neurons % | |||||||
| PFC (1/2) | 6.67 | 8.83 | 8.11 ± 4.99 | 0.00 | 0.78 | 0.52 ± 0.54 | 0.00 | 8.83 | 6.41 |
| BF (1/2) | 12.67 | 61.33 | 45.11 ± 32.11 | 2.50 | 26.13 | 18.25 ± 24.15 | 19.73 | 42.61 | 40.46 |
| iTh (1/3) | 58.33 | 77.00 | 72.33 ± 23.94 | 18.04 | 31.64 | 28.24 ± 11.34 | 30.93 | 41.09 | 39.04 |
| ZI (2/2) | 23.67 | 97.83 | 60.75 ± 44.38 | 19.26 | 31.88 | 27.57 ± 12.45 | 81.37 | 32.59 | 45.38 |
| RVM * (2/3) | 32.61 | 100.72 | 68.85 ± 41.15 | 16.53 | 34.60 | 25.57 ± 12.90 | 50.69 | 34.35 | 37.14 |
| SC * (1/1) | 43.00 | 22.33 | 32.67 ± 13.70 | 29.44 | 15.33 | 22.38 ± 9.97 | 68.47 | 68.65 | 68.50 |
| total | 287.82 | 122.53 | |||||||
| p-value | 0.111 | 0.223 | 0.801 | ||||||
| Projection- Target (n Rats) | Projecting Effector-Neurons (Double-Labelled) | All MPTA Effector-Neurons Sampled | |||||
|---|---|---|---|---|---|---|---|
| Ipsi | Contra | Total (n) | Ipsi (n) | Contra (n) | Total (n) | Total (%) | |
| PFC (3) | 2 | 4 | 6 | 182 | 208 | 390 | 1.5 |
| BF (4) | 64 | 152 | 216 | 218 | 423 | 641 | 33.7 |
| iTh (4) | 204 | 90 | 294 | 389 | 323 | 712 | 41.3 |
| ZI (4) | 245 | 65 | 310 | 526 | 241 | 767 | 40.4 |
| RVM * (6) | 443 | 443 | 1021 | 1021 | 43.4 | ||
| SC * (2) | 114 | 114 | 196 | 196 | 58.2 | ||
| 6 targets | 1383 | 3727 | 37.1% | ||||
| 5 targets # | 1377 | 3337 | 41.3% | ||||
| Projection- Target Contralateral (n Rats CTB/FG) | CTB/mm2 Singles | FG/mm2 Singles | CTB + FG/ mm2 ± SD Singles | CTB/mm2 Doubles | FG/mm2 Doubles | CTB + FG/mm2 ± SD Doubles | CTB % Doubles | FG % Doubles | CTB +FG % Doubles |
|---|---|---|---|---|---|---|---|---|---|
| MPTA Projection Neurons | MPTA Projecting Effectors | Projecting Effector-Neurons % | |||||||
| PFC (1/2) | 6.67 | 6.83 | 6.78 ± 4.83 | 0.79 | 1.43 | 1.21 ± 0.83 | 11.84 | 20.94 | 17.85 |
| BF (2/2) | 9.00 | 73.17 | 41.08 ± 37.54 | 8.79 | 46.23 | 27.51 ± 25.31 | 97.67 | 63.18 | 66.97 |
| iTh (1/2) | 54.67 | 27.17 | 36.33 ± 17.16 | 20.92 | 14.51 | 16.65 ± 4.09 | 38.27 | 53.40 | 45.83 |
| ZI (2/1) | 17.17 | 71.33 | 35.22 ± 31.94 | 12.83 | 11.49 | 12.38 ± 6.71 | 74.72 | 16.11 | 35.15 |
| RVM * (3/3) | 32.61 | 100.72 | 68.85 ± 41.15 | 16.53 | 34.60 | 25.57 ± 12.90 | 50.69 | 34.35 | 37.14 |
| SC * (1/1) | 43.00 | 22.33 | 32.67 ± 13.70 | 29.44 | 15.33 | 22.38 ± 9.97 | 68.47 | 68.65 | 68.50 |
| total | 220.93 | 105.70 | |||||||
| p-value | 0.212 | 0.488 | 0.377 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, J.; Minert, A.; Koukoui, M.; Heller, S.; Morein, R.; Baron, M.; Vaso, K.; Devor, M. Axonal Projections of Neurons in the Brainstem Mesopontine Tegmental Anesthesia Area (MPTA) That Effect Anesthesia, Enabling Pain-Free Surgery. Anesth. Res. 2025, 2, 26. https://doi.org/10.3390/anesthres2040026
Miller J, Minert A, Koukoui M, Heller S, Morein R, Baron M, Vaso K, Devor M. Axonal Projections of Neurons in the Brainstem Mesopontine Tegmental Anesthesia Area (MPTA) That Effect Anesthesia, Enabling Pain-Free Surgery. Anesthesia Research. 2025; 2(4):26. https://doi.org/10.3390/anesthres2040026
Chicago/Turabian StyleMiller, Juliet, Anne Minert, Mary Koukoui, Shaked Heller, Roza Morein, Mark Baron, Kristina Vaso, and Marshall Devor. 2025. "Axonal Projections of Neurons in the Brainstem Mesopontine Tegmental Anesthesia Area (MPTA) That Effect Anesthesia, Enabling Pain-Free Surgery" Anesthesia Research 2, no. 4: 26. https://doi.org/10.3390/anesthres2040026
APA StyleMiller, J., Minert, A., Koukoui, M., Heller, S., Morein, R., Baron, M., Vaso, K., & Devor, M. (2025). Axonal Projections of Neurons in the Brainstem Mesopontine Tegmental Anesthesia Area (MPTA) That Effect Anesthesia, Enabling Pain-Free Surgery. Anesthesia Research, 2(4), 26. https://doi.org/10.3390/anesthres2040026








