The Effect of General Versus Neuraxial Anaesthesia on Bleeding and Thrombotic Outcomes in Neck of Femur Fracture Surgery: A Meta-Analysis
Abstract
1. Introduction
2. Method
2.1. Study Registration
2.2. Search Strategy
2.3. Types of Studies
2.4. Types of Participants
2.5. Types of Intervention
2.6. Type of Outcome Measures
2.7. Data Extraction
2.8. Data Analysis
2.9. Strategy for Data Synthesis
2.10. Analysis of Subgroups or Subsets
2.11. Assessment of the Certainty of Evidence
3. Results
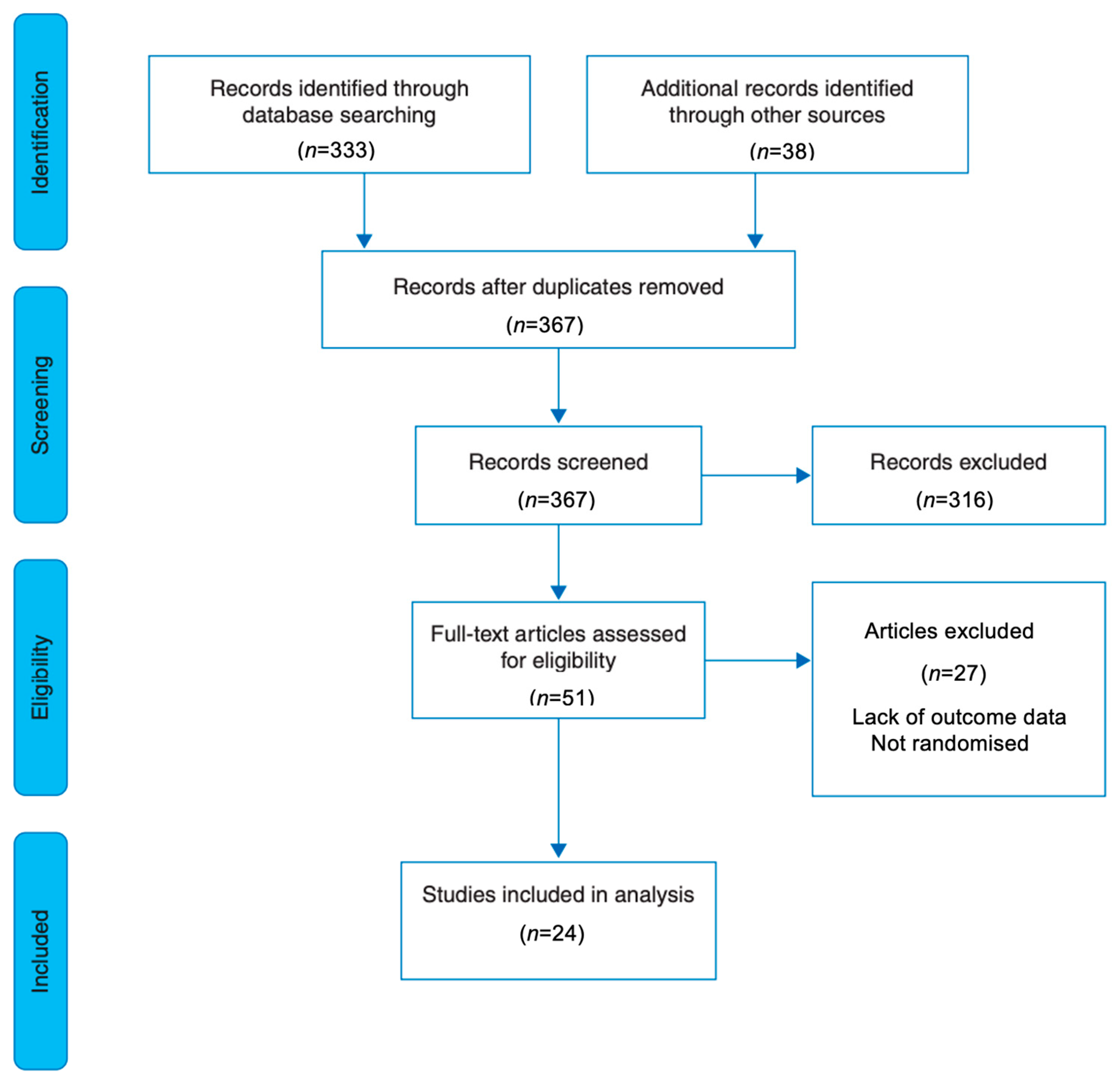
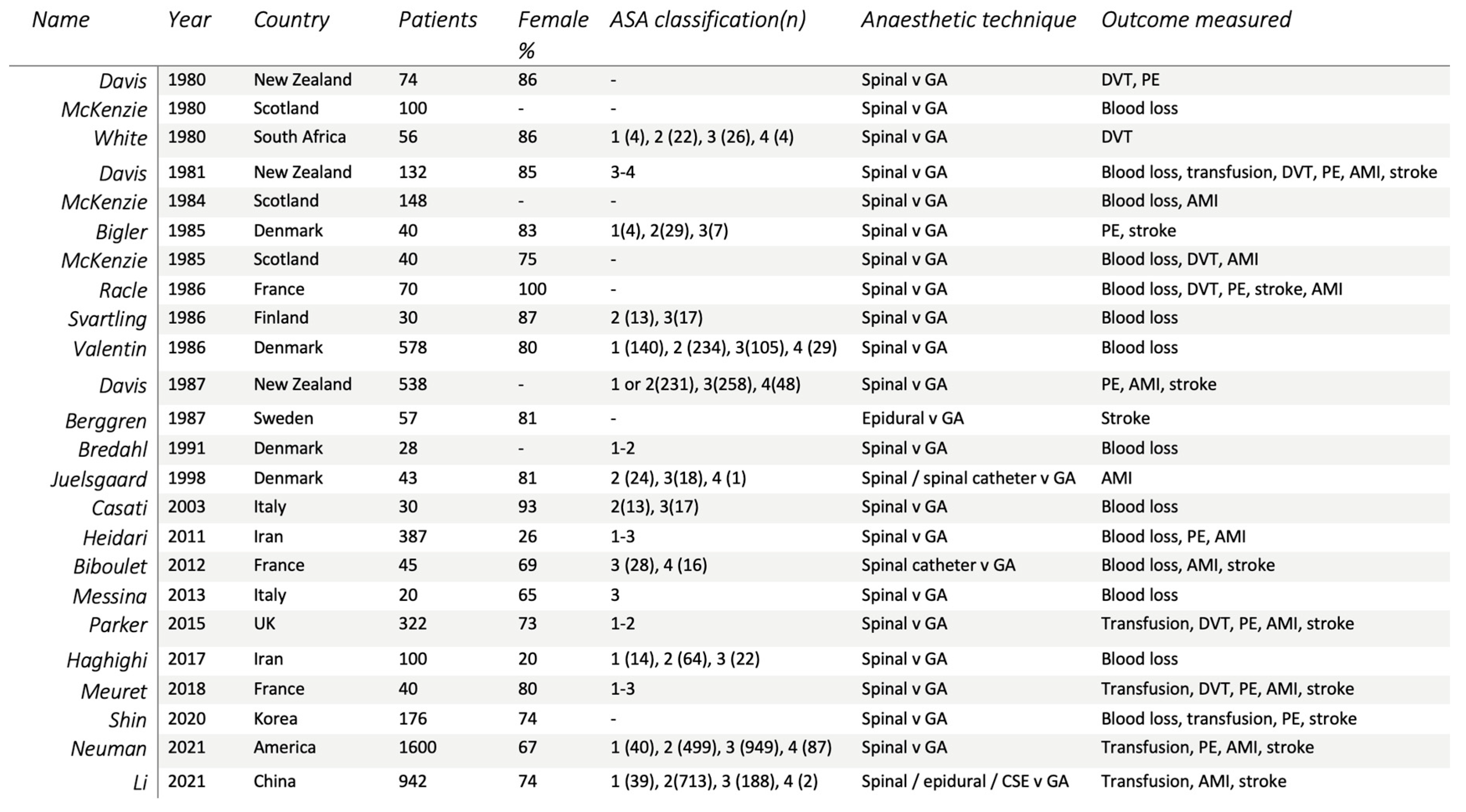
3.1. Search Results
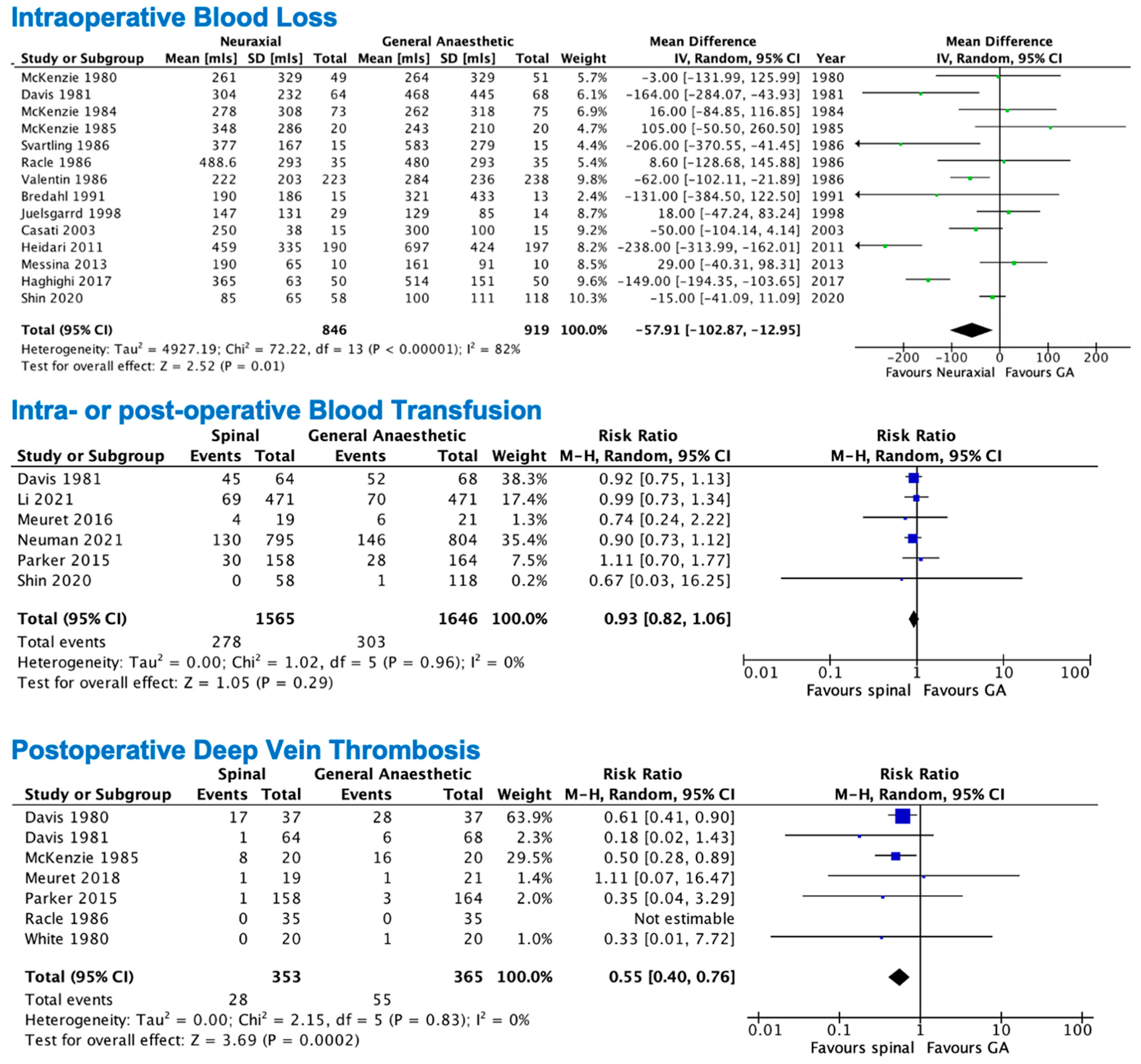
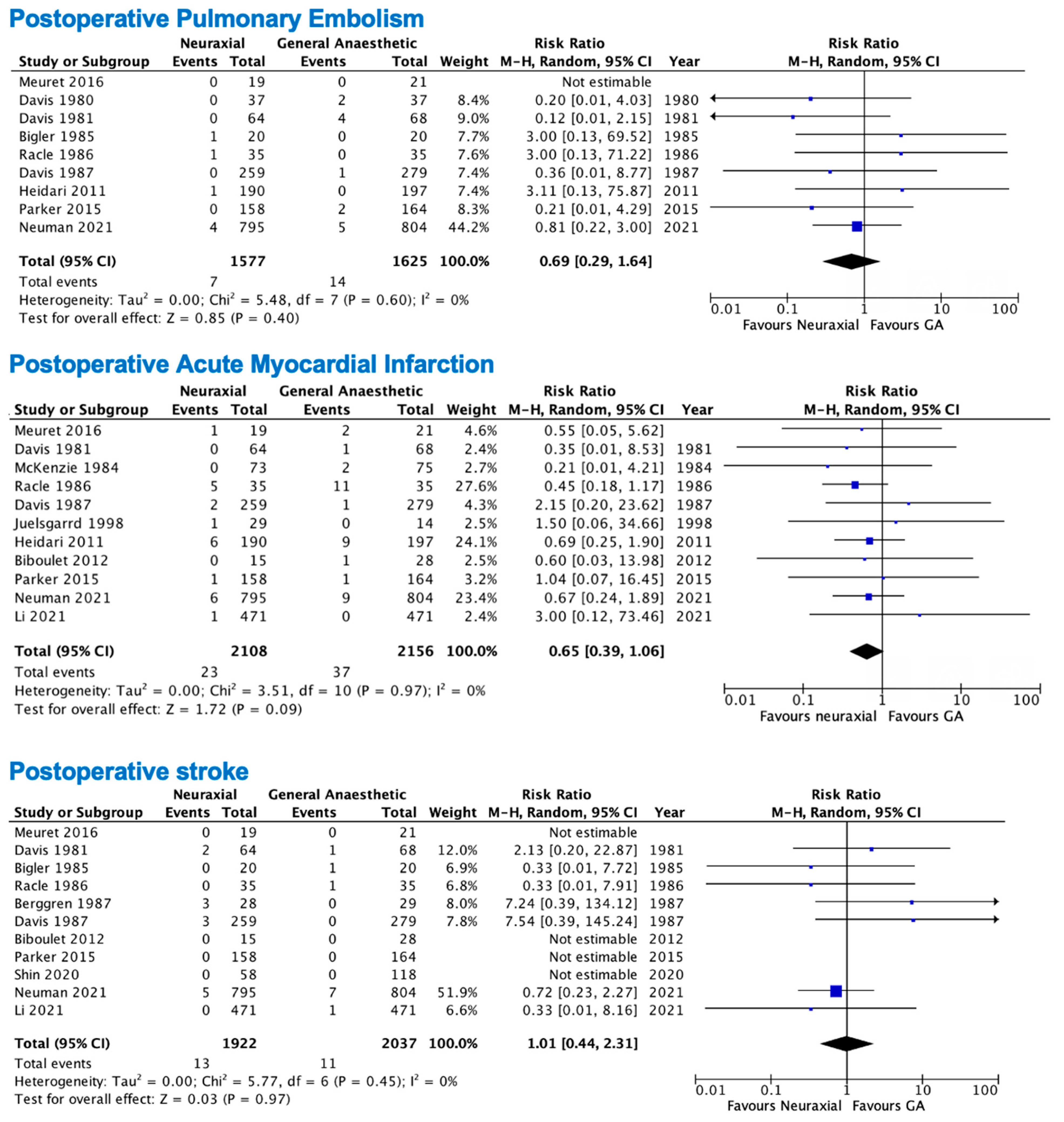
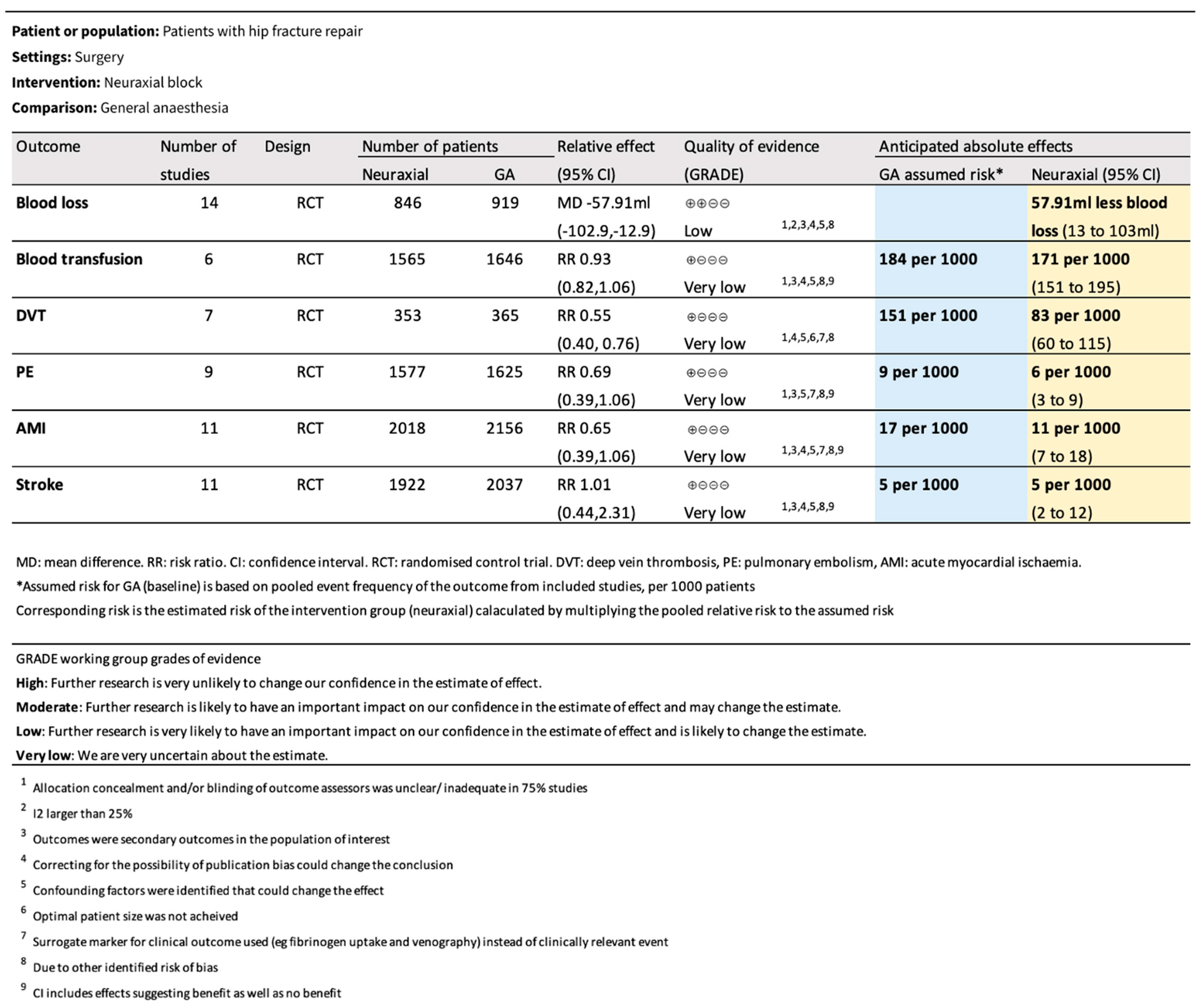
3.2. Primary Outcomes
3.3. Secondary Outcomes
3.4. Overall
3.5. Year Dependant Confounders
4. Discussion
5. Primary Outcomes
Secondary Outcomes
6. Overall
Strengths and Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Australian Institute of Health and Welfare (AIHW). The Problem of Osteoporotic Hip Fracture in Australia; Bulletin 76; Australian Government: Canberra, Australia, 2010. Available online: https://www.aihw.gov.au/getmedia/ea96bc4d-0b97-4c5a-b792-08b343752adc/10695.pdf.aspx?inline=true (accessed on 23 May 2023).
- Royal College of Physicians. National Hip Fracture Database; FFFAP: London, UK, 2024; Available online: https://www.nhfd.co.uk/2024report (accessed on 4 July 2024).
- Swenning, T.; Lieghton, J.; Nentwig, M.; Dart, B. Hip fracture care and national systems: The United States and Canada. OTA Int. 2020, 3, e000073. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare (AIHW). Hip Fracture Care Pathways in Australia; PHE 336; Australian Government: Canberra, Australia, 2023. Available online: https://www.aihw.gov.au/getmedia/dc202a83-d8b2-4477-9bbc-6890ed2e226c/aihw-phe-336.pdf?v=20240618123107&inline=true (accessed on 10 September 2025).
- American Academy of Orthopaedic Surgeons. Management of Hip Fractures in Older Adults: Evidence-Based Clinical Practice Guideline; AAOS: Rosemont, IL, USA, 2021; Available online: https://www.aaos.org/hipfxcpg (accessed on 1 April 2023).
- Guay, J.; Parker, M.; Gajendragadkar, P.; Kopp, S. Anaesthesia for hip fracture surgery in adults. Cochrane Database Syst. Rev. 2016, 2, CD000521. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council (NHMRC). Clinical Practice Guideline for the Prevention of Venous Thromboembolism in Patients Admitted to Australian Hospitals; Australian Government: Canberra, Australia, 2009. Available online: https://ranzcog.edu.au/wp-content/uploads/NHMRC-Prevention-Venous-Thromboembolism-Australia.pdf (accessed on 23 May 2023).
- Finsterwald, M.; Muster, M.; Farshad, M.; Saporito, A.; Brada, M.; Aguirre, J.A. Spinal versus general anesthesia for lumbar spine surgery in high risk patients: Perioperative hemodynamic stability, complications and costs. J. Clin. Anes. 2018, 46, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, R.; Motlagh, S.; Zaman, B.; Sehat Kashani, S.; Ghodraty, M.R. Effect of general versus spinal anesthetic on postoperative delirium and early cognitive dysfunction in elderly patients. Anesthesiol. Pain Med. 2020, 10, e101815. [Google Scholar] [CrossRef]
- Fleisher, L.A.; Beckman, J.A.; Brown, K.A.; Calkins, H.; Chaikof, E.; Fleischmann, K.E.; Freeman, W.K.; Froehlich, J.B.; Kasper, E.K.; Kersten, J.R.; et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. Circulation 2007, 116, e418–e500. [Google Scholar] [CrossRef]
- Quintero, J.; Cardenas, L.; Navas, M.; Bautista, M.P.; Bonilla, G.A.; Llinás, A.M.; Clinical Care Program in Joint Replacement Surgery. Primary joint arthroplasty surgery: Is the risk of major bleeding higher in elderly patients? A retrospective cohort study. J. Arthroplast. 2016, 31, 2264–2268. [Google Scholar] [CrossRef]
- Gu, A.; Maybee, C.; Wei, C.; Probasco, W.V.; Ast, M.P.; Sculco, P.K. Preoperative blood transfusion associated with increased length of stay and postoperative complications after revision total knee arthroplasty. J. Orthop. 2019, 16, 265–268. [Google Scholar] [CrossRef]
- Peng, S.; Haijing, M.; Jin, J.; MacCormick, A. The effectiveness of venous thromboembolism prophylaxis interventions in trauma patients: A systematic review and network meta-analysis. Injury 2023, 54, 111078. [Google Scholar] [CrossRef]
- Neuman, M.; Feng, R.; Carson, J.; Gaskins, L.J.; Dillane, D.; Sessler, D.I.; Sieber, F.; Magaziner, J.; Marcantonio, E.R.; Mehta, S.; et al. Spinal anesthesia or general anesthesia for hip surgery in older adults. N. Engl. J. Med. 2021, 385, 2025–2035. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Yuan, L.; Wu, J.; Jiang, C.; Daniels, J.; Mehta, R.L.; Wang, M.; Yeung, J.; Jackson, T.; et al. Effect of regional vs general anesthesia on incidence of postoperative delirium in older patient undergoing hip fracture surgery: The RAGA randomized trial. JAMA 2022, 327, 50–58. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Li, T.; Deeks, J.J. Chapter 6: Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.3; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2022; Available online: https://training.cochrane.org/handbook/current/chapter-06 (accessed on 1 April 2023).
- Schünemann, H.; Higgins, J.; Vist, G.; Glasziou, P.; Akl, E.; Skoetz, N.; Guyatt, G.; on behalf of the Cochrane GRADEing Methods Group (formerly Applicability and Recommendations Methods Group) and the Cochrane Statistical Methods Group. Chapter 14: Completing “Summary of findings” tables and grading the certainty of the evidence. In Cochrane Handbook for Systematic Reviews of Interventions; Version 6.3; Cochrane: London, UK, 2022; Available online: https://training.cochrane.org/handbook/current/chapter-14 (accessed on 1 April 2023).
- Davis, F.M.; Quince, M.; Laurenson, V.G. Deep vein thrombosis and anaesthetic technique in emergency hip surgery. Br. Med. J. 1980, 281, 1528–1529. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McKenzie, P.J.; Wishart, H.Y.; Smith, G. Long-term outcome after repair of fractured neck of femur: Comparison of subarachnoid and general anaesthesia. Br. J. Anaesth. 1980, 56, 581–584. [Google Scholar] [CrossRef] [PubMed]
- White, I.; Chappell, W. Anaesthesia for surgical correction of fractured femoral neck: A comparison of three techniques. Anaesthesia 1980, 35, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.M.; Laurenson, V.G. Spinal anaesthesia or general anaesthesia for emergency hip surgery in elderly patients. Anaesth. Intensive Care 1981, 9, 352–358. [Google Scholar] [CrossRef]
- McKenzie, P.; Wishart, H.; Dewar, K.; Gray, I.; Smith, G. Comparison of the effects of spinal anaesthesia and general anaesthesia on postoperative oxygenation and perioperative mortality. Br. J. Anaesth. 1984, 52, 49–54. [Google Scholar] [CrossRef]
- Bigler, D.; Adelhoj, B.; Petting, O.; Pederson, N.; Busch, P.; Kalhke, P. Mental function and morbidity after acute hip surgery during spinal and general anaesthesia. Anaesthesia 1985, 40, 672–676. [Google Scholar] [CrossRef]
- Racle, J.; Benkhadra, A.; Poy, J.; Gleizal, B.; Gaudray, A. Comparative study of general and spinal anaesthesia in the elderly female patient undergoing hip surgery. Ann. Fr. Anesth. Reanim. 1986, 5, 24–30. [Google Scholar] [CrossRef]
- McKenzie, P.; Wishart, H.; Gray, I.; Smith, G. Effects of anaesthetic technique on deep vein thrombosis: A comparison of subarachnoid and general anaesthesia. Br. J. Anaesth. 1985, 57, 853–857. [Google Scholar] [CrossRef]
- Svartling, N.; Lehtinen, A.; Tarkkanen, L. The effect of anaesthesia on changes in blood pressure and plasma cortisol levels induced by cementation with methylmethacrylate. Acta Anaesthesiol. Scand. 1986, 30, 247–252. [Google Scholar] [CrossRef]
- Valentin, N.; Lomholt, B.; Jensen, S.; Hejgaard, N.; Kreiner, S. Spinal or General Anaesthesia for Surgery of the Fractured Hip? Br. J. Anaesth. 1986, 58, 284–291. [Google Scholar] [CrossRef]
- Davis, F.; Woolner, D.; Frampton, C.; Wilkinson, A.; Grant, A.; Harrison, R.; Roberts, M.; Thadaka, R. Prospective, multi-centre trial of mortality following general or spinal anaesthesia for hip fracture surgery in the elderly. Br. J. Anaesth. 1987, 59, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Berggren, D.; Gustafson, Y.; Eriksson, B.; Bucht, G.; Hansson, L.I.; Reiz, S.; Winblad, B. Postoperative confusion after anesthesia in elderly patients with femoral neck fractures. Anesth. Analg. 1987, 66, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Bredahl, C.; Hindsholm, K.; Frandsen, P. Changes in body heat during hip fracture surgery: A comparison of spinal analgesia and general anaesthesia. Acta Anaesthesiol. Scand. 1991, 35, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Juelsgaard, P.; Sand, N.; Felsby, S.; Dalsgaard, J.; Jakobsen, K.B.; Brink, O.; Carlsson, P.S.; Thygesen, K. Perioperative myocardial ischaemia in patients undergoing surgery for fractured hip randomized to incremental spinal, single-dose spinal or general anaesthesia. Eur. J. Anaesthesiol. 1998, 15, 656–663. [Google Scholar] [CrossRef]
- Casati, A.; Aldegheri, G.; Vinciguerra, F.; Marsan, A.; Fraschini, G.; Torri, G. Randomised comparison between sevoflurane anaesthesia and unilateral spinal anaesthesia in elderly patients undergoing orthopaedic surgery. Eur. J. Anaesthesiol. 2003, 20, 640–646. [Google Scholar] [CrossRef]
- Heidari, S.; Soltani, H.; Hashemi, S.; Talakoub, R.; Soleimani, B. Comparative study of two anesthesia methods according to postoperative complications and one month mortality rate in the candidates of hip surgery. J. Res. Med. Sci. 2011, 16, 323–330. [Google Scholar]
- Biboulet, P.; Jourdan, A.; Van Haevre, V.; Morau, D.; Bernard, N.; Bringuier, S.; Capdevila, X. Hemodynamic profile of target-controlled spinal anesthesia compared with 2 target-controlled general anesthesia techniques in elderly patients with cardiac comorbidities. Reg. Anesth. Pain Med. 2012, 37, 433–440. [Google Scholar] [CrossRef]
- Messina, A.; Frassanito, L.; Colombo, D.; Vergari, A.; Draisci, G.; Della Corte, F.; Antonelli, M. Hemodynamic changes associated with spinal and general anesthesia for hip fracture surgery in severe ASA III elderly population: A pilot trial. Minerva Anestesiol. 2013, 79, 1021–1029. [Google Scholar]
- Parker, M.; Griffiths, R. General versus regional anaesthesia for hip fractures. A pilot randomised controlled trial of 322 patients. Injury 2015, 46, 1562–1566. [Google Scholar] [CrossRef]
- Haghighi, M.; Sedighinejad, A.; Nabi, B.; Mardani-Kivi, M.; Tehran, S.G.; Mirfazli, S.A.; Mirbolook, A.; Saheli, N.A. Is spinal anesthesia with low dose lidocaine better than sevoflurane anesthesia in patients undergoing hip fracture surgery. Arch. Bone Jt. Surg. 2017, 5, 226–230. [Google Scholar]
- Meuret, P.; Bouvet, L.; Villet, B.; Hafez, M.; Allaouchiche, B.; Boselli, E. Hypobaric unilateral spinal anaesthesia versus general anaesthesia in elderly patients undergoing hip fracture surgical repair: A prospective randomised open trial. Turk. J. Anaesthesiol. Reanim. 2018, 46, 121–130. [Google Scholar] [CrossRef]
- Shin, S.; Kim, S.; Park, K.; Kim, S.J.; Bae, J.C.; Choi, Y.S. Effects of anesthesia techniques on outcomes after hip fracture surgery in elderly patients: A prospective, randomized, controlled trial. J. Clin. Med. 2020, 9, 1605. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook. 2013. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 23 May 2023).
- Basques, B.; Bohl, D.; Golinvaux, N.; Samuel, A.M.; Grauer, J.G. General versus spinal anaesthesia for patients aged 70 years and older with a fracture of the hip. Bone Joint J. 2015, 97-B, 689–695. [Google Scholar] [CrossRef]
- Bouarfa, L.; Passini, E.; Sucher, J.F.; Blake, N.; Balcome, C.D.; Prokuski, L.; Dzandu, J.; Barletta, J.F.; Shirah, G.R. Consequences of anemia in geriatric hip fractures: How low is too low? Trauma Surg. Acute Care Open 2024, 9, e001175. [Google Scholar] [CrossRef] [PubMed]
- Brunskill, S.; Millette, S.; Shokoohi, A.; Pulford, E.C.; Doreee, C.; Murphy, M.; Stanworth, S. Red blood cell transfusion for people undergoing hip fracture surgery. Cochrane Database Syst. Rev. 2015, CD009699. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Quality Statement 2: Tranexamic Acid for Adults—Moderate Blood Loss (>500 mL); NICE Quality Standard QS138; NICE: London, UK, 2015; Available online: https://www.nice.org.uk/guidance/qs138/chapter/quality-statement-2-tranexamic-acid-for-adults (accessed on 23 May 2023).
- Association of Anaesthetists. Joint Guideline on the Management of Hip Fractures in Adults: Anaesthetic Management; Association of Anaesthetists: London, UK, 2020; Available online: https://anaesthetists.org/Home/Resources-publications/Guidelines (accessed on 23 May 2023).
- Carson, J.; Guyatt, G.; Heddle, N.; Grossman, B.J.; Cohn, C.S.; Fung, M.K.; Gernsheimer, T.; Holcomb, J.B.; Kaplan, L.J.; Katz, L.M.; et al. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA 2016, 316, 2025–2035. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.; Estcourt, L.; Goodnough, L. Blood transfusion strategies in elderly patients. Lancet Haematol. 2017, 4, e453–e454. [Google Scholar] [CrossRef]
- Lin, Y.; Yu, C.; Xian, G. Calculation methods for intraoperative blood loss: A literature review. BMC Surg. 2024, 24, 394. [Google Scholar] [CrossRef]
- Benson, M.; Hartmann, B.; Junger, A.; Dietrich, G.; Böttger, S.; Hempelmann, G. Causes of higher blood loss during general anaesthesia compared to spinal anesthesia in total hip replacement—A retrospective analysis of data collected online. Transfus. Med. Hemother. 2000, 27, 311–316. [Google Scholar] [CrossRef]
- Polderman, K. Hypothermia and coagulation. Crit. Care 2012, 16, 20. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, H.; Li, Y.; Ma, B.B.; Xie, T.; Wang, C.; Chen, H.; Rui, Y.F. The efficacy and safety of intravenous tranexamic acid in hip fracture surgery: A systematic review and meta-analysis. J. Orthop. Transl. 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Meibner, N.; Halder, A.; Schrednitzki, D. Cemented and hybrid total hip arthroplasty lead to lower blood loss in primary total hip arthroplasty: A retrospective study. Arch. Orthop. Trauma Surg. 2023, 143, 6447–6451. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Roychoudhury, P.; Lohia, S.; Kim, J.S.; Kim, H.T.; Ro, Y.J.; Koh, W.U. Comparison of general and spinal anaesthesia on systemic inflammatory response in patients undergoing total knee arthroplasty: A propensity score matching analysis. Medicina 2021, 57, 1250. [Google Scholar] [CrossRef] [PubMed]
- White, R.H. The epidemiology of venous thromboembolism. Circulation 2003, 107 (Suppl. S1), I4–I8. [Google Scholar] [CrossRef]
- Stein, P.D.; Henry, J.W.; Relyea, B. Untreated patients with pulmonary embolism: Outcome, clinical, and laboratory assessment. Chest 1991, 100, 780–782. [Google Scholar]
- Falck-Ytter, Y.; Francis, C.W.; Johanson, N.A.; Curley, C.; Dahl, O.E.; Schulman, S.; Ortel, T.L.; Pauker, S.G.; Colwell, C.W., Jr. Prevention of VTE in orthopedic surgery patients: Antithrombotic therapy and prevention of thrombosis, 9th ed: ACCP guidelines. Chest 2012, 141 (Suppl. S2), e278S–e325S. [Google Scholar] [CrossRef]
- Kakkos, S.K.; Gohel, M.; Baekgaard, N.; Bauersachs, R.; Bellmunt-Montoya, S.; Black, S.A.; Cate-Hoek, A.J.T.; Elalamy, I.; Enzmann, F.K.; Geroulakos, G.; et al. Editor’s Choice-European Society for Vascular Surgery (ESVS) 2021 Clinical Practice Guidelines on the Management of Venous Thrombosis. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 9–82. [Google Scholar]
- Wijeysundera, D.N.; Beattie, W.S.; Austin, P.C.; Hux, J.E.; Laupacis, A. Epidural anaesthesia and survival after intermediate-to-high risk non-cardiac surgery: A population-based cohort study. Lancet 2008, 372, 562–569. [Google Scholar] [CrossRef]
- Mashour, G.; Moore, L.; Lele, A.; Robicsek, S.A.; Gelb, A.W. Perioperative care of patients at high risk for stroke during or after non-cardiac, non-neurologic surgery: Consensus statement from the society for neuroscience in anesthesiology and critical care. J. Neurosurg. Anesthesiol. 2014, 26, 273–285. [Google Scholar] [CrossRef]
- Jeandin, T.; Albrecht, E.; Wegrzyn, J.; Cachemaille, M. Hemodynamic stability between spinal and general anaesthesia in patients undergoing primary total knee arthroplasty: A retrospective study. Reg. Anesth. Pain Med. 2021, 70, A30. [Google Scholar]
- Kadkim, A.; Al-Khikani, H.; Hamza, Q.; Habib, Y.K.; Hussein, M.M.; Muhammad, H.T. Comparison between general and spinal anesthesia in the effect on hemodynamic stability in patients undergoing hernia repair. Matthews J. Anesth. 2022, 5, 10015. [Google Scholar] [CrossRef]
- Basques, B.; Toy, J.; Bohl, D.; Golinvaux, N.S.; Grauer, J.N. General compared with spinal anesthesia for total hip arthroplasty. J. Bone Jt. Surg. Am. 2015, 97, 455–461. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyons, A.; Yii, N.; White, L.; Bright, M.; Velli, G. The Effect of General Versus Neuraxial Anaesthesia on Bleeding and Thrombotic Outcomes in Neck of Femur Fracture Surgery: A Meta-Analysis. Anesth. Res. 2025, 2, 25. https://doi.org/10.3390/anesthres2040025
Lyons A, Yii N, White L, Bright M, Velli G. The Effect of General Versus Neuraxial Anaesthesia on Bleeding and Thrombotic Outcomes in Neck of Femur Fracture Surgery: A Meta-Analysis. Anesthesia Research. 2025; 2(4):25. https://doi.org/10.3390/anesthres2040025
Chicago/Turabian StyleLyons, Alexandra, Nathan Yii, Leigh White, Matthew Bright, and Gina Velli. 2025. "The Effect of General Versus Neuraxial Anaesthesia on Bleeding and Thrombotic Outcomes in Neck of Femur Fracture Surgery: A Meta-Analysis" Anesthesia Research 2, no. 4: 25. https://doi.org/10.3390/anesthres2040025
APA StyleLyons, A., Yii, N., White, L., Bright, M., & Velli, G. (2025). The Effect of General Versus Neuraxial Anaesthesia on Bleeding and Thrombotic Outcomes in Neck of Femur Fracture Surgery: A Meta-Analysis. Anesthesia Research, 2(4), 25. https://doi.org/10.3390/anesthres2040025





