Effects of Propofol in the Cardiac Conduction System in Electrophysiologic Study: Systematic Review and Meta-Analysis †
Abstract
1. Introduction
2. Methods
2.1. Search Strategy, Inclusion, and Exclusion Criteria
2.2. Search Strategy
2.3. Eligibility and Exclusion Criteria
2.3.1. Eligibility Criteria
- Type of study: RCTs, observational, retrospective, and prospective cohort studies were included. Case reports, case series, and review articles were not included.
- Type of participants: adults and children with or without heart disease who underwent EPS and were sedated with propofol. All the RCTs or clinical control trials comparing propofol with other anesthetic agents were included in the study. There was no age, gender, or type of heart disease restriction.
- Type of intervention: EPS with or without radiofrequency ablation with propofol sedation.
- Types of comparison group: patients who were sedated with propofol and patients who were sedated with other anesthetic agents such as fentanyl, midazolam, ketamine, and dexmedetomidine, among others.
- Types of results:Primary outcomes: whether propofol interfered in the CCS and the inducibility of arrhythmias.Secondary outcomes: whether propofol interferes or not with cycle length (CL), atrial-His (AH), His-ventricular (HV), corrected sinus node recovery time (CSNRT), atrial effective refractory factor (AERP/FT), ventricular effective refractory period (VERP) and atrial ventricular node effective refractory period (AVNERP). And whether propofol is or is not an ideal anesthetic agent for sedation in EPS procedures compared to other anesthetics.
2.3.2. Exclusion Criteria
- Animal studies, case reports, case series studies, protocols, letters, surveys, and review articles.
- Sedation with anesthetics other than propofol.
- Studies evaluating outcomes unrelated to cardiac conduction or EPS were excluded.
2.3.3. Selection of Studies
2.3.4. Data Extraction
2.4. Statistical Analysis and Data Synthesis
2.5. Quality Assessment
3. Results
3.1. Primary Results
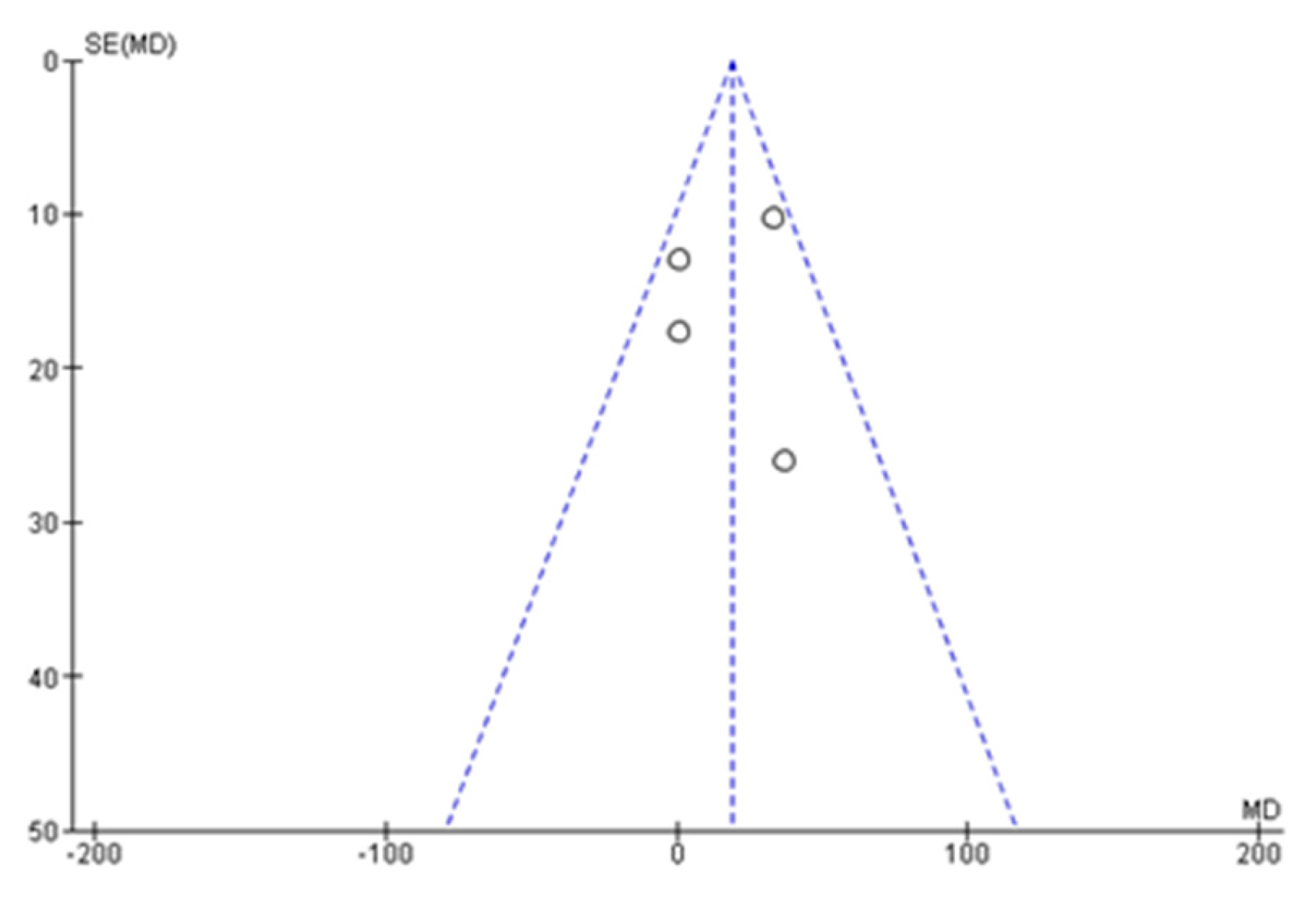
3.2. Risk of Bias Analysis
3.3. Evidence Quality Assessment
4. Discussion
5. Conclusions
Key Messages
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Section and Topic | Item # | Checklist Item | Location Where Item is Reported |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review. | 01 |
| Abstract | |||
| Abstract | 2 | See the PRISMA 2020 for Abstract’s checklist. | 01 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | 01, 02 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | 03 |
| Methods | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | 03 |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists, and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | 03 |
| Search strategy | 7 | Present the full search strategies for all databases, registers, and websites, including any filters and limits used. | 02, 03 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | 03 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | 03, 04 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | 04 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | 04 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess the risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study, whether they worked independently, and if applicable, details of automation tools used in the process. | 04 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | 04 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | 04 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | 04 | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | 04 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | 04 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | 04 | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | 04 | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | 04 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | 04 |
| Results | |||
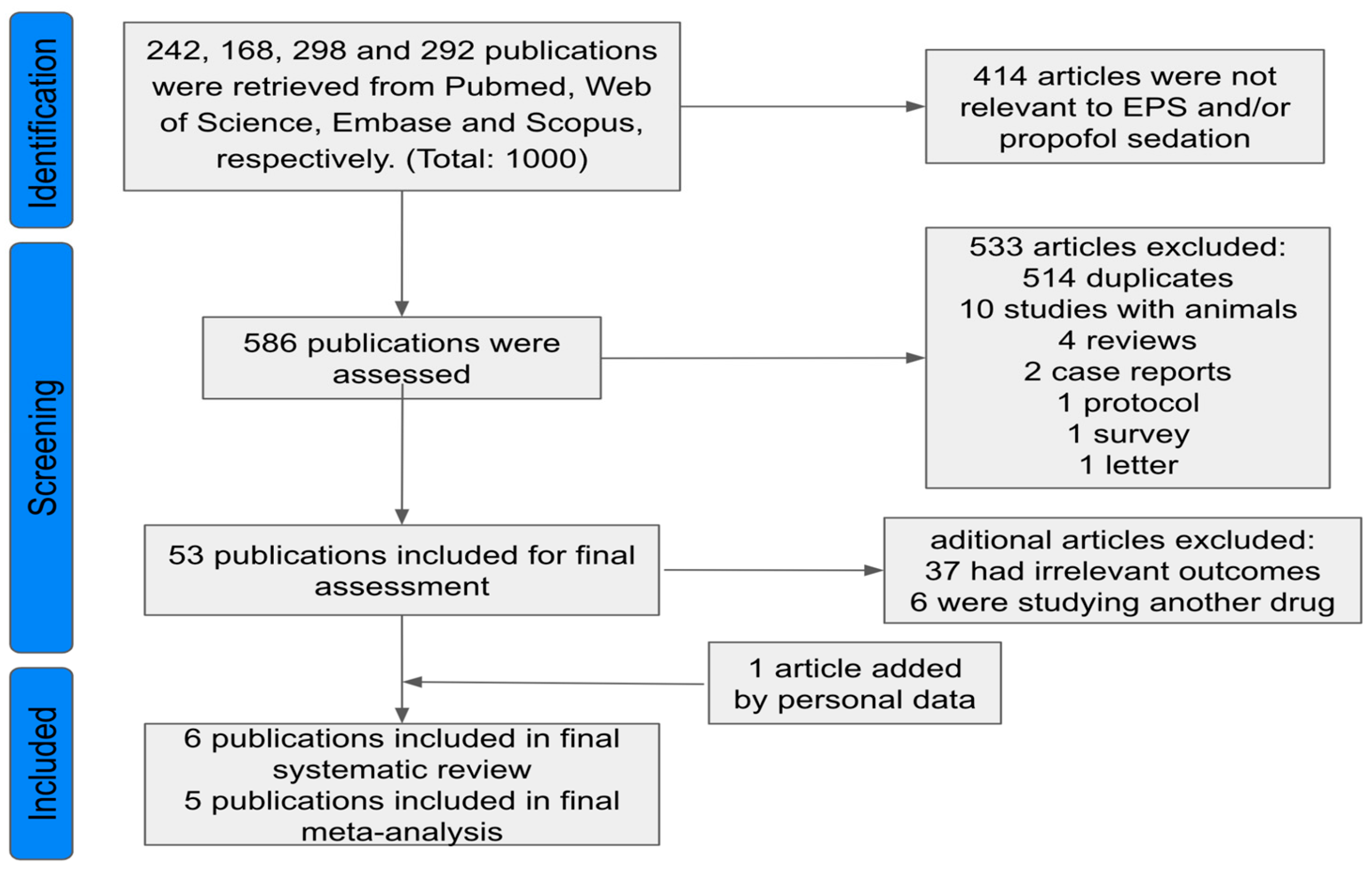
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | 05 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | 05 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | 05, 06 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | 11 |
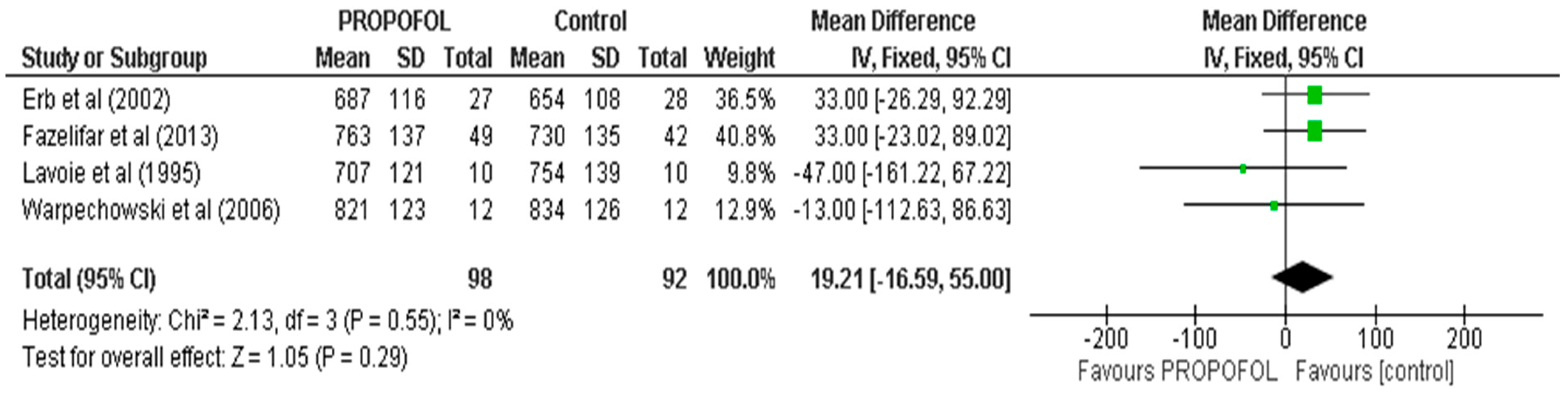
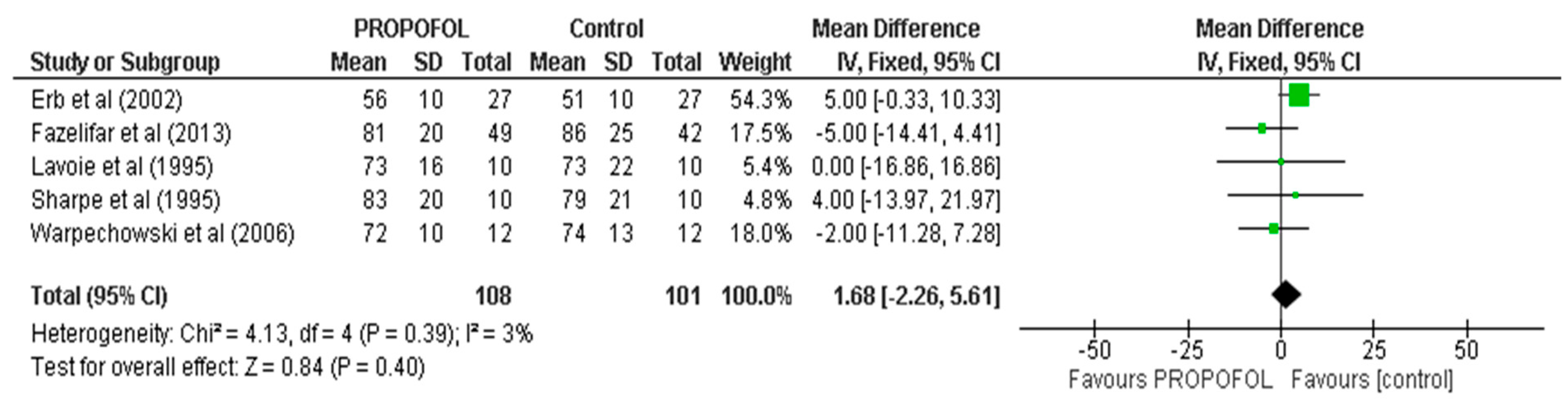
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | 06, 07, 08, 09 |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies. | 07, 08 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was conducted, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | 09, 10 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | 09, 10, 11 | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | 09, 10 | |
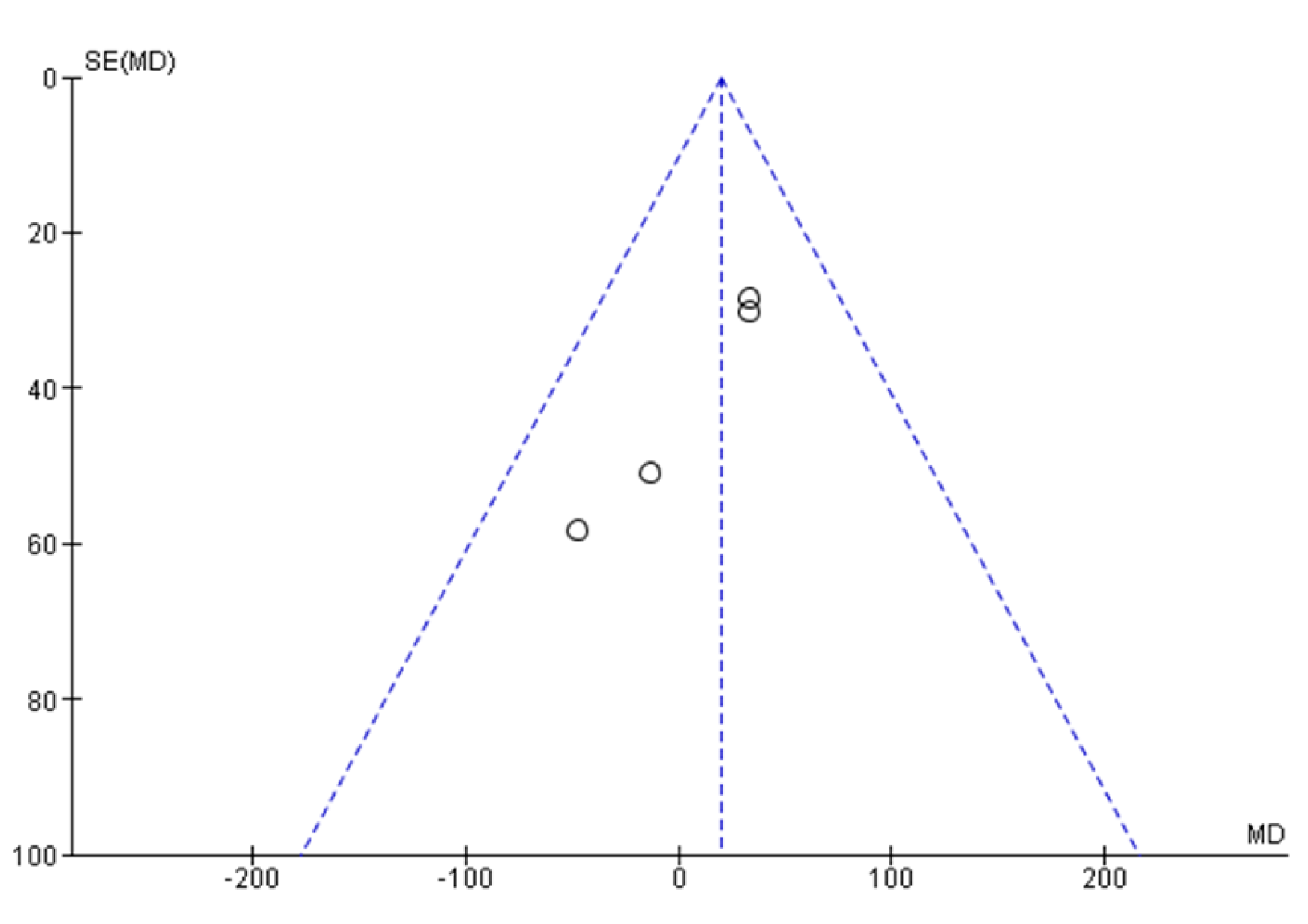
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | 11 |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | 11, 12 |
| Discussion | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | 12 |
| 23b | Discuss any limitations of the evidence included in the review. | 12, 13 | |
| 23c | Discuss any limitations of the review processes used. | 13, 14 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | 14, 15 | |
| Other Information | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | 02 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | 02, 15 | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | 02 | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | 15 |
| Competing interests | 26 | Declare any competing interests of review authors. | 15 |
| Availability of data, code, and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; and any other materials used in the review. | 4, 5, 15 |
References
- Lau, W.; Kovoor, P.; Ross, D.L. Cardiac electrophysiologic effects of midazolam combined with fentanyl. Am. J. Cardiol. 1993, 72, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Vladinov, G.; Fermin, L.; Longini, R.; Ramos, Y.; Maratea, E. Choosing the anesthetic and sedative drugs for supraventricular tachycardia ablations: A focused review. Pacing Clin. Electrophysiol. 2018, 41, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Terashima, S.; Muroya, T.; Ikegawa, H.; Kajino, K.; Sakuramoto, K.; Yui, R.; Kishimoto, M.; Takahashi, H.; Nakajima, M.; Nakamura, F.; et al. Propofol suppresses ventricular arrhythmias: A case report of acute caffeine intoxication. Acute Med. Surg. 2020, 7, 514. [Google Scholar] [CrossRef] [PubMed]
- Miró, O.; de la Red, G.; Fontanals, J. Cessation of paroxysmal atrial fibrillation during acute intravenous propofol administration. Anesthesiol. 2000, 92, 910. [Google Scholar] [CrossRef]
- Thomson, S.J.; Yate, P.M. Bradycardia after propofol infusion. Anaesthesia 1987, 42, 430. [Google Scholar] [CrossRef]
- Shannon, K.; Saltzman, D.; Li, I.; Mokszycki, R.; Galletta, G. Ventricular tachycardia converts to sinus rhythm after propofol administration. Am. J. Emerg. Med. 2021, 48, 377.e1–377.e3. [Google Scholar] [CrossRef]
- Hug, C.C.; McLeskey, C.H.; Nahrwold, M.L.; Roizen, M.F.; Stanley, T.H.; Thisted, R.A.; Walawander, C.A.; White, P.F.; Apfelbaum, J.L.; Grasela, T.H. Hemodynamic effects of propofol: Data from over 25,000 patients. Anesth. Analg. 1993, 77, 21–29. [Google Scholar]
- Vuyk, J.; Engbers, F.H.M.; Lemmens, H.J.M.; Burm, A.G.L.; Vletter, A.A.; Gladines, M.P.R.R.; Bovill, J.G. Pharmacodynamics of propofol in female patients. Anesthesiology 1992, 77, 3–9. [Google Scholar] [CrossRef]
- Kannan, S.; Sherwood, N. Termination of supraventricular tachycardia by propofol. Br. J. Anaesth. 2002, 88, 874–875. [Google Scholar] [CrossRef]
- Tramèr, M.R.; Moore, R.A.; McQuay, H.J. Propofol and bradycardia: Causation, frequency and severity. Br. J. Anaesth. 1997, 78, 642–651. [Google Scholar] [CrossRef]
- Wu, M.-H.; Lin, J.-L.; Lai, L.-P.; Young, M.-L.; Lu, C.-W.; Chang, Y.-C.; Wang, J.-K.; Lue, H.-C. Radiofrequency catheter ablation of tachycardia in children with and without congenital heart disease: Indications and limitations. Int. J. Cardiol. 2000, 72, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Alphin, R.S.; Martens, J.R.; Dennis, D.M. Frequency-dependent effects of propofol on atrioventricular nodal conduction in guinea pig isolated heart. Mechanism and potential antidysrhythmic properties. Anesthesiology 1995, 83, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.A.; Huang, S.K.; Wagshal, A.B.; Kulkarni, R.S. Electrophysiological effects of propofol on the normal cardiac conduction system. Cardiology 1996, 87, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Su, M.J.; Sun, S.S. Age-related propofol effects on electrophysiological properties of isolated hearts. Anesth. Analg. 1997, 84, 964–971. [Google Scholar] [CrossRef]
- Warpechowski, P.; Lima, G.G.; Medeiros, C.M.; Santos, A.T.L.; Kruse, M.; Migloransa, M.H.; Kalil, R.A. Randomized study of propofol effect on electrophysiological properties of the atrioventricular node in patients with nodal reentrant tachycardia. Pacing Clin. Electrophysiol. 2006, 29, 1375–1382. [Google Scholar] [CrossRef]
- Warpechowski, P.; dos Santos, A.T.L.; Pereira, P.J.I.; de Lima, G.G. Effects of propofol on the cardiac conduction system. Rev. Bras. Anestesiol. 2010, 60, 438–444. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Romano, R.; Ciccaglioni, A.; Fattorini, F.; Quaglione, R.; Favaro, R.; Arcioni, R.; Conti, G.; Gasparetto, A. Effects of propofol on the human heart electrical system: A transesophageal pacing electrophysiologic study. Acta Anaesthesiol. Scand. 1994, 38, 30–32. [Google Scholar] [CrossRef]
- Lai, L.; Lin, J.; Wu, M.; Wang, M.; Huang, C.; Yeh, H.; Tseng, Y.; Lien, W.; Huang, S.K.S. Usefulness of Intravenous Propofol Anesthesia for Radiofrequency Catheter Ablation in Patients with Tachyarrhythmias: Infeasibility for Pediatric Patients with Ectopic Atrial Tachycardia. Pacing Clin. Electrophysiol. 1999, 22, 1358–1364. [Google Scholar] [CrossRef]
- Pérez, E.R.; Bartolomé, F.B.; Carretero, P.S.; Fernández, C.S.; Mateos, E.J.; Tarlovsky, L.G. Efectos electrofisiológicos del sevoflurano versus propofol en niños con síndrome de Wolff-Parkinson-White. Rev. Española Anestesiol. Reanim. 2008, 55, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Wutzler, A.; Huemer, M.; Boldt, L.-H.; Parwani, A.S.; Attanasio, P.; Tscholl, V.; Haverkamp, W. Effects of deep sedation on cardiac electrophysiology in patients undergoing radiofrequency ablation of supraventricular tachycardia: Impact of propofol and ketamine. EP Eur. 2013, 15, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Paech, C.; Wagner, F.; Strehlow, V.; Gebauer, R.A. Drug-Induced Loss of Preexcitation in Pediatric Patients with WPW Pattern During Electrophysiologic Study. Pediatr. Cardiol. 2019, 40, 194–197. [Google Scholar] [CrossRef]
- Matsushima, M.; Kimura, S.; Kitaura, A.; Hamasaki, S.; Iwamoto, T.; Mino, T.; Masui, K.; Nakao, S. Propofol suppresses the His-ventricular conduction in paediatric patients. J. Clin. Pharm. Ther. 2021, 46, 433–439. [Google Scholar] [CrossRef]
- Lavoie, J.; Walsh, E.P.; Burrows, F.A.; Laussen, P.; Lulu, J.A.; Hansen, D.D. Effects of propofol or isoflurane anesthesia on cardiac conduction in children undergoing radiofrequency catheter ablation for tachydysrhythmias. Anesthesiology 1995, 82, 884–887. [Google Scholar] [CrossRef]
- Sharpe, M.D.; Dobkowski, W.B.; Murkin, J.M.; Klein, G.; Yee, R. Propofol has no direct effect on sinoatrial node function or on normal atrioventricular and accessory pathway conduction in Wolff-Parkinson-White syndrome during alfentanil/midazolam anesthesia. Anesthesiology 1995, 82, 888–895. [Google Scholar] [CrossRef]
- Erb, T.O.; Kanter, R.J.; Hall, J.M.; Gan, T.J.; Kern, F.H.; Schulman, S.R. Comparison of Electrophysiologic Effects of Propofol and Isoflurane-based Anesthetics in Children Undergoing Radiofrequency Catheter Ablation for Supraventricular Tachycardia. Anesthesiology 2002, 96, 1386–1394. [Google Scholar] [CrossRef]
- Fazelifar, A.; Eskandari, A.; Hashemi, M.; Alavi, M.; Totounchi, M.; Forghanian, A.; Zeighami, M.; Emkanjoo, Z.; Haghjoo, M. Deep sedation in patients undergoing atrioventricular nodal reentry tachycardia ablation. Res. Cardiovasc. Med. 2013, 2, 176. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Oxman, A.D.; Brozek, J.; Glasziou, P.; Jaeschke, R.; Vist, G.E.; Williams, J.W., Jr.; Kunz, R.; Craig, J.; Montori, V.M.; et al. GRADE: Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008, 336, 1106–1110. [Google Scholar] [CrossRef]
- Granholm, A.; Alhazzani, W.; Møller, M.H. Use of the GRADE approach in systematic reviews and guidelines. Br. J. Anaesth. 2019, 123, 554–559. [Google Scholar] [CrossRef]
- Zipes, D.P. Guidelines for clinical intracardiac electrophysiological and catheter ablation procedures. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Intracardiac Electrophysiologic and Catheter Ablation Procedures), developed in collaboration with the North American Society of Pacing and Electrophysiology. J. Am. Coll. Cardiol. 1995, 26, 555–573. [Google Scholar] [PubMed]
- Aliot, E.M.; Stevenson, W.G.; Almendral-Garrote, J.M.; Bogun, F.; Calkins, C.H.; Delacretaz, E.; Della Bella, P.; Hindricks, G.; Jaïs, P.; Josephson, M.E.; et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: Developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Europace: European pacing, arrhythmias, and cardiac electrophysiology: Journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. EP Eur. 2009, 11, 771–817. [Google Scholar]
- Magaldi, M.; Fontanals, J.; Pérez, J. Sinusal reversion of atrial flutter after intravenous propofol administration. Med. Intensiv. 2014, 38, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Xu, M.; Kong, A.; Liu, Q.; Chen, R.; Dai, Q.; Wang, L.; Sun, B. Propofol terminates ventricular fibrillation storm caused by pulmonary embolism. Chin. Med. J. 2014, 127, 3840. [Google Scholar] [CrossRef]
- Harris, A.P.; Fleisher, L.A. Changes in Heart Rate Variability Under Propofol Anesthesia: A Possible Explanation for Propofol-Induced Bradycardia. Anesth. Analg. 1994, 79, 373–377. [Google Scholar]
- Napolitano, C.A.; Raatikainen, M.J.; Martens, J.R.; Dennis, D.M. Effects of intravenous anesthetics on atrial wavelength and atrioventricular nodal conduction in guinea pig heart. Potential antidysrhythmic properties and clinical implications. Anesthesiology 1996, 85, 393–402. [Google Scholar] [CrossRef]
- Kurokawa, H.; Murray, P.A.; Damron, D.S. Propofol attenuates beta-adrenoreceptor-mediated signal transduction via a protein kinase C-dependent pathway in cardiomyocytes. Anesthesiology 2002, 96, 688–698. [Google Scholar] [CrossRef]
- Burjorjee, J.E.; Milne, B. Propofol for electrical storm; a case report of cardioversion and suppression of ventricular tachycardia by propofol. Can. J. Anaesth. 2002, 49, 973–977. [Google Scholar] [CrossRef]
- Hermann, R.; Vettermann, J. Change of Ectopic Supraventricular Tachycardia to Sinus Rhythm During Administration of Propofol. Anesth. Analg. 1992, 75, 1030–1032. [Google Scholar] [CrossRef]
- Mulpuru, S.K.; Patel, D.V.; Wilbur, S.L.; Vasavada, B.C.; Furqan, T. Electrical storm and termination with propofol therapy: A case report. Int. J. Cardiol. 2008, 128, 6–8. [Google Scholar] [CrossRef]
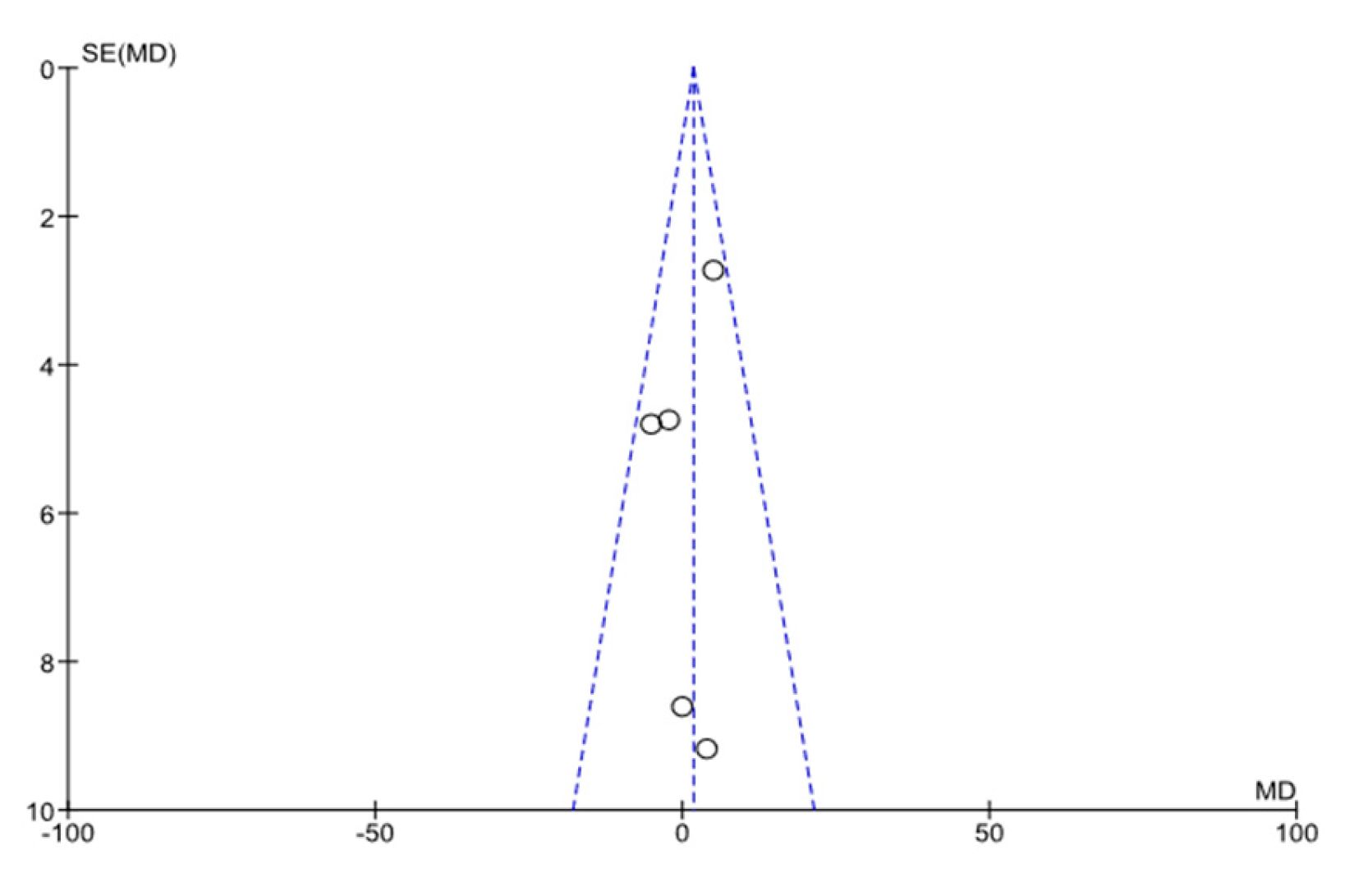

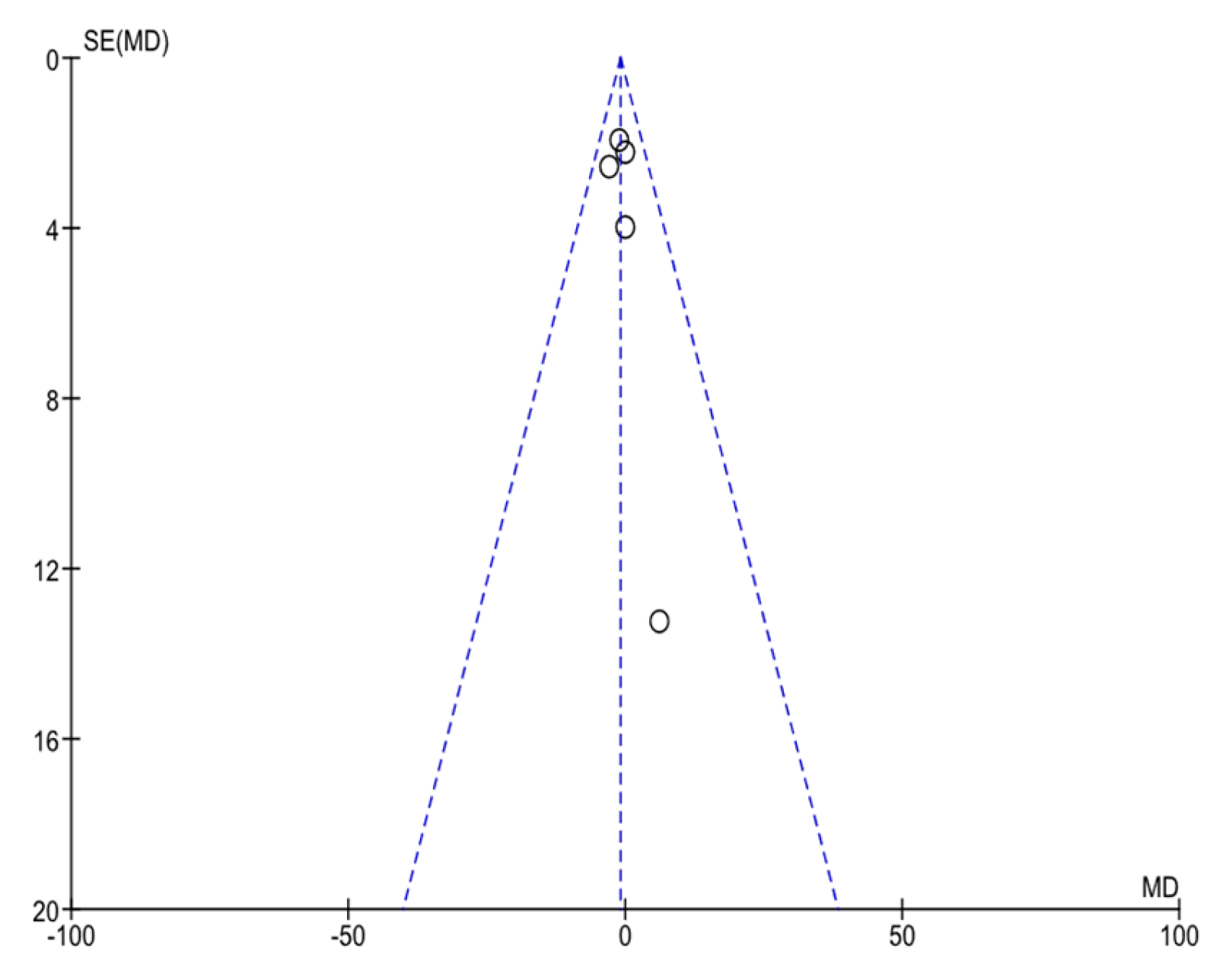
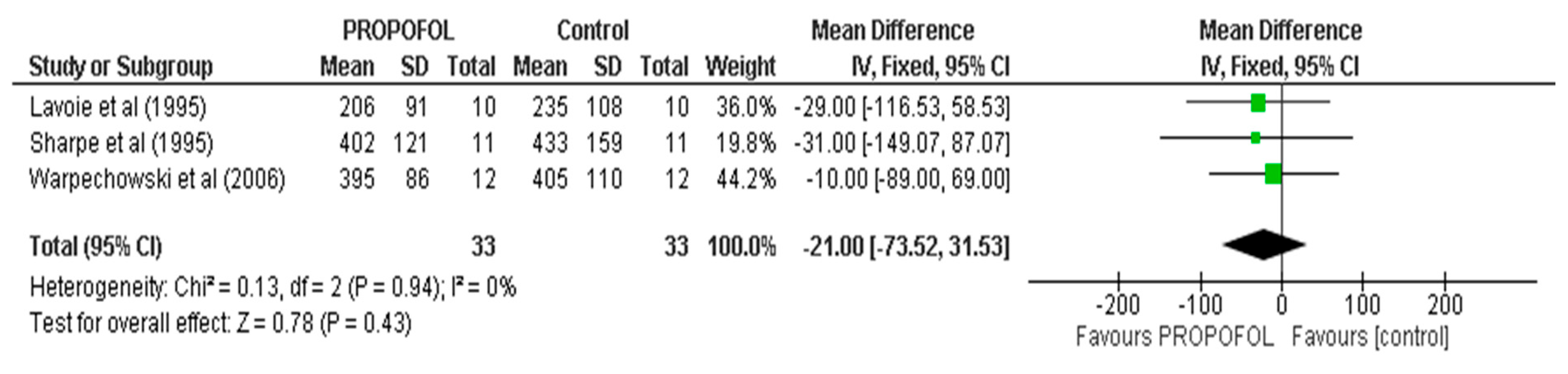
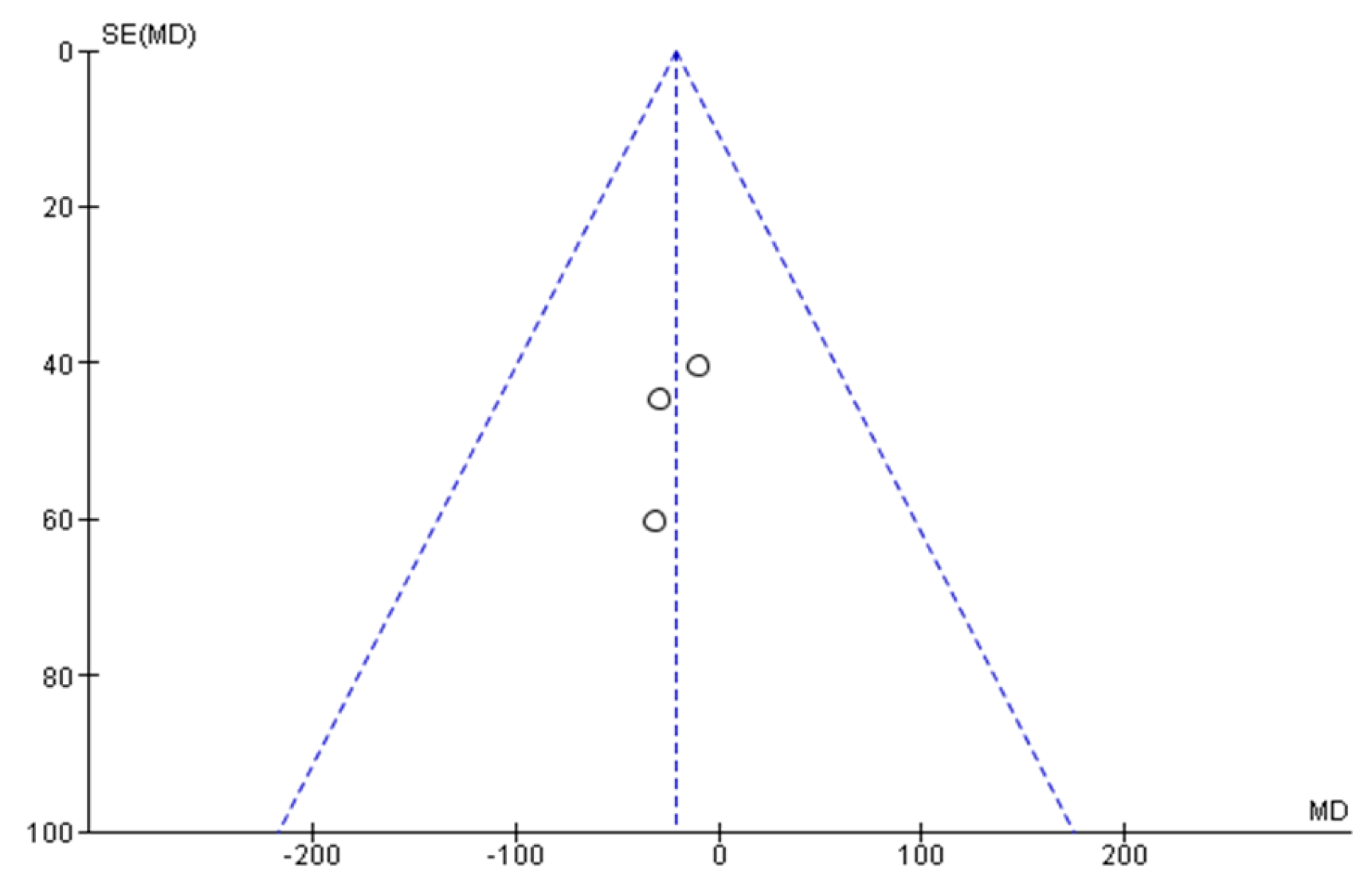

| Conector | Search Terms |
|---|---|
| “Propofol” [Mesh] OR “2,6-Diisopropylphenol” [TITLE/ABSTRACT] OR “2,6 Diisopropylphenol” [TITLE/ABSTRACT] OR “2,6-Bis(1-methylethyl)phenol” [TITLE/ABSTRACT] OR “Disoprofol” [TITLE/ABSTRACT] OR “Diprivan” [TITLE/ABSTRACT] OR “Disoprivan” [TITLE/ABSTRACT] OR “Fresofol” [TITLE/ABSTRACT] OR “Ivofol” [TITLE/ABSTRACT] OR “Propofol Fresenius” [TITLE/ABSTRACT] OR “Propofol MCT” [TITLE/ABSTRACT] OR “Propofol-Lipuro” [TITLE/ABSTRACT] OR “Recofol” [TITLE/ABSTRACT] OR “Aquafol” [TITLE/ABSTRACT] OR “Propofol Abbott” [TITLE/ABSTRACT] | |
| AND | “Arrhythmias, Cardiac” [Mesh] OR “Arrhythmia, Cardiac” [TITLE/ABSTRACT] OR “Cardiac Dysrhythmia” [TITLE/ABSTRACT] OR “Dysrhythmia, Cardiac” [TITLE/ABSTRACT] OR “Cardiac Arrhythmia” [TITLE/ABSTRACT] OR “Cardiac Arrhythmias” [TITLE/ABSTRACT] OR “Arrhythmia” [TITLE/ABSTRACT] OR “Arrythmia” [TITLE/ABSTRACT] |
| AND | “Cardiac Electrophysiology” [Mesh] OR “Electrophysiology, Cardiac” [TITLE/ABSTRACT] OR “Electrophysiologic Techniques, Cardiac” [Mesh] OR “Cardiac Electrophysiologic Technique” [TITLE/ABSTRACT] OR “Electrophysiological Techniques, Intracardiac” [TITLE/ABSTRACT] OR “Transesophageal Electrophysiologic Study” [TITLE/ABSTRACT] OR “Atrial Electrograms” [TITLE/ABSTRACT] OR “Electrophysiologic Study, Cardiac” [TITLE/ABSTRACT] OR “His Bundle Electrogram” [TITLE/ABSTRACT] OR “Catheter Ablation” [Mesh] OR “Ablation, Catheter” [TITLE/ABSTRACT] OR “Catheter Ablation, Radiofrequency” [TITLE/ABSTRACT] OR “Radiofrequency Catheter Ablation” [TITLE/ABSTRACT] OR “Ablation, Radiofrequency Catheter” [TITLE/ABSTRACT] |
| Author and Year | Study Design | Sample Size | Gender Male/Fem | Mean Age | Type of Arrhythmia | Loss of Pre-Excitation | Arrhythmia Induction | No Induction of Arrhythmia | Prevented Ablation | Supressed AV Conduction |
|---|---|---|---|---|---|---|---|---|---|---|
| Romano R, 1994 [19] | Prospective cohort | 10 | 8/2 | 46 ± 19 | No base pathology | No | 10 | No | No | No |
| Lai LP, 1999 [20] | Prospective cohort | 150 | 78/72 | Adults (n = 86) 44 ± 16 Ped (n = 64) 11 ± 3.3 | 24 flutter 31 NRT 68 AVRT 12 EVT 17 EAT | No | 148 | 4 | 4 out of 7 Ped with EAT | No |
| Pérez, 2008 [21] | Prospective cohort | 15 | 11/4 | 9.3 ± 3.6 | WPW | No | 15 | No | No | No |
| Wutzler, 2013 [22] | Prospective cohort | 31 | 15/16 | 52 ± 16 | WPW | No | 31 | No | No | No |
| Paech, 2019 [23] | Prospective cohort | 37 | 24/13 | 13 ± 4 | WPW | 6 | 37 | No | No | No |
| Matsushima, 2021 [24] | Prospective cohort | 23 | 16/7 | 6 ± 1.5 | 12 WPW 8 SVT 3 TVE | No | 23 | No | No | No |
| Studies | Control Group for Placebo Effect | Randomized Group Allocation | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Selective Reporting (Reporting Bias) |
|---|---|---|---|---|---|
| Lavoie, 1995 [25] |  |  |  |  |  |
| Sharpe, 1995 [26] |  |  |  |  |  |
| Erb, 2002 [27] |  |  |  |  |  |
| Warpechowski, 2006 [15] |  |  |  |  |  |
| Fazelifar, 2013 [28] |  |  |  |  |  |
 Low risk of bias,
Low risk of bias,  Uncertain risk of bias,
Uncertain risk of bias,  High risk of bias.
High risk of bias.| Outcome | Number of Studies | Number of Participants | Statistical Method | Effect Size | p-Value | Heterogeneity (I2) | Certainty of Evidence (Grade) |
|---|---|---|---|---|---|---|---|
| CL | 4 | 200 | MD, Random | 19.21 (16.59, 55) | 0.55 | 0 | ⨁⨁⨁⨁ |
| AH | 5 | 209 | MD, Random | 1.68 (2.26, 6.51) | 0.39 | 0 | ⨁⨁⨁⨁ |
| HV | 5 | 195 | MD, Random | −0.39, 1.38 | 0.89 | 0 | ⨁⨁⨁⨁ |
| CSNRT | 3 | 66 | MD, Random | 21 (7.53, 52.31) | 0.46 | 0 | ⨁⨁⨁⨁ |
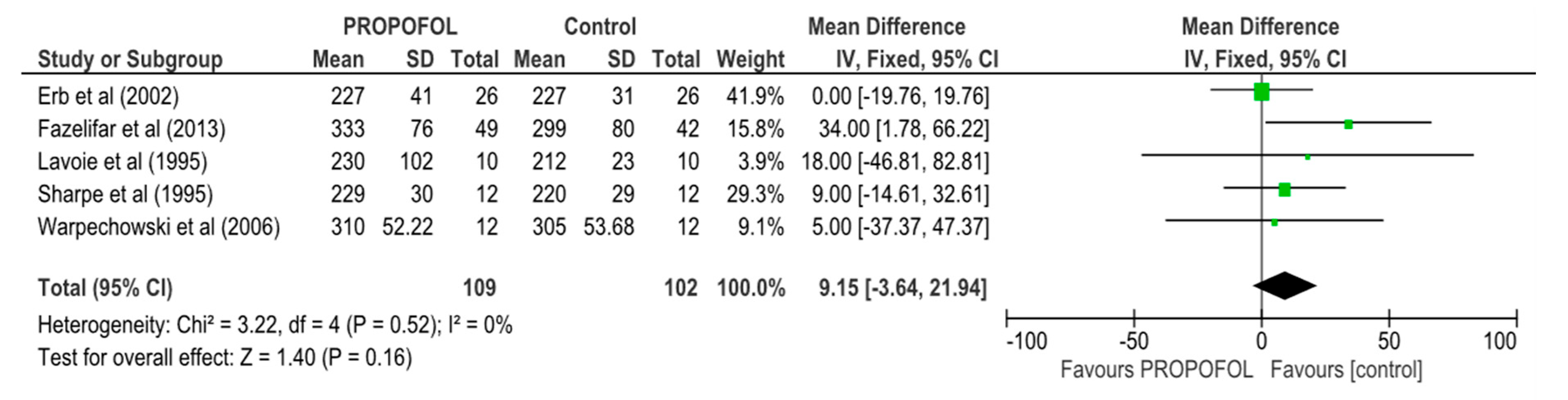
| AERP/FP | 5 | 211 | MD, Random | 9.15 (3.64, 21.94) | 0.52 | 0 | ⨁⨁⨁⨁ |
| AVNERP | 4 | 180 | MD, Random | 18.67 (−4.86, 32.47) | 0.15 | 0 | ⨁⨁⨁◯ |
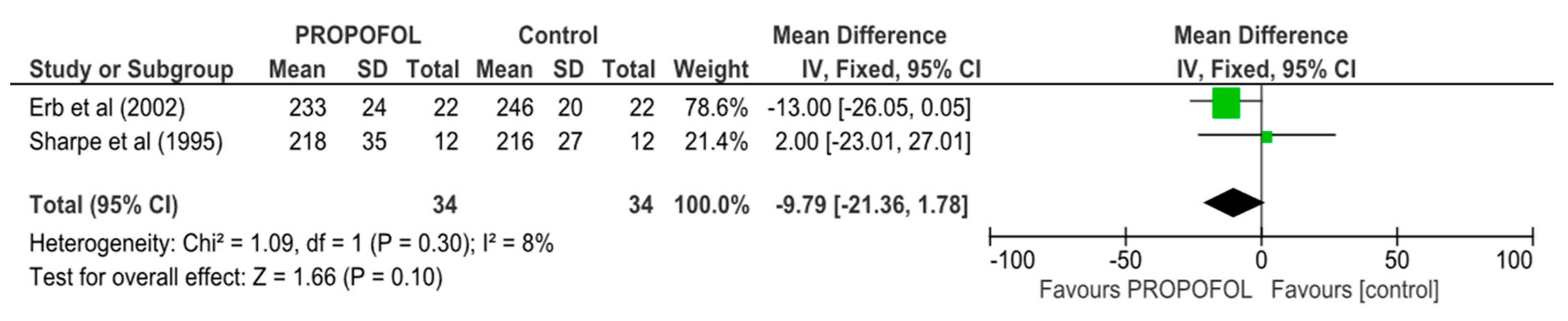
| VERP | 2 | 68 | MD, Random | 9.79 (2.16, 17.86) | 0.13 | 0 | ⨁⨁⨁◯ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warpechowski, P.; Warpechowski, R.B.; De Lima, B.A.; Pinto, E.F.A.; Bastos, M.L.S.; Eibel, B.; Trindade, R.D.; Leiria, T.L. Effects of Propofol in the Cardiac Conduction System in Electrophysiologic Study: Systematic Review and Meta-Analysis. Anesth. Res. 2025, 2, 16. https://doi.org/10.3390/anesthres2030016
Warpechowski P, Warpechowski RB, De Lima BA, Pinto EFA, Bastos MLS, Eibel B, Trindade RD, Leiria TL. Effects of Propofol in the Cardiac Conduction System in Electrophysiologic Study: Systematic Review and Meta-Analysis. Anesthesia Research. 2025; 2(3):16. https://doi.org/10.3390/anesthres2030016
Chicago/Turabian StyleWarpechowski, Paulo, Rodrigo B. Warpechowski, Barbara A. De Lima, Emanuella F. A. Pinto, Mariana L. S. Bastos, Bruna Eibel, Rubens D. Trindade, and Tiago L. Leiria. 2025. "Effects of Propofol in the Cardiac Conduction System in Electrophysiologic Study: Systematic Review and Meta-Analysis" Anesthesia Research 2, no. 3: 16. https://doi.org/10.3390/anesthres2030016
APA StyleWarpechowski, P., Warpechowski, R. B., De Lima, B. A., Pinto, E. F. A., Bastos, M. L. S., Eibel, B., Trindade, R. D., & Leiria, T. L. (2025). Effects of Propofol in the Cardiac Conduction System in Electrophysiologic Study: Systematic Review and Meta-Analysis. Anesthesia Research, 2(3), 16. https://doi.org/10.3390/anesthres2030016






