Association Between Frailty Scoring and Cardiopulmonary Exercise Testing: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
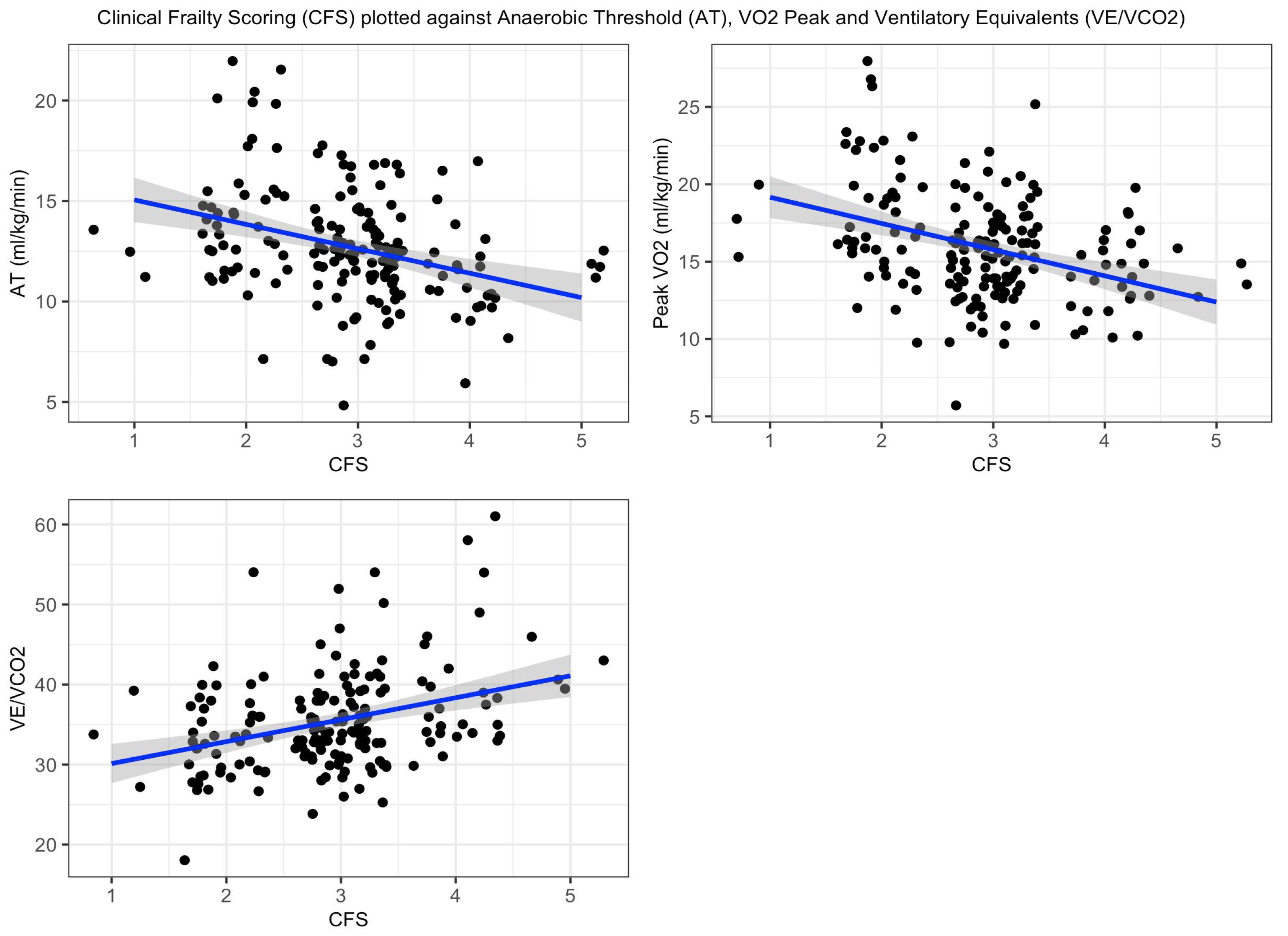
3.1. CFS Score and CPET Values
3.2. One-Year Mortality Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moran, J.; Wilson, F.; Guinan, E.; McCormick, P.; Hussey, J.; Moriarty, J. Role of cardiopulmonary exercise testing as a risk-assessment method in patients undergoing intra-abdominal surgery: A systematic review. Br. J. Anaesth. 2016, 116, 177–191. Available online: https://pubmed.ncbi.nlm.nih.gov/26787788/ (accessed on 20 August 2024). [CrossRef] [PubMed]
- Hennis, P.J.; Meale, P.M.; Grocott, M.P.W. Cardiopulmonary exercise testing for the evaluation of perioperative risk in non-cardiopulmonary surgery. Postgrad. Med. J. 2011, 87, 550–557. Available online: https://pubmed.ncbi.nlm.nih.gov/21693573/ (accessed on 20 August 2024). [CrossRef] [PubMed]
- Reeves, T.; Bates, S.; Sharp, T.; Richardson, K.; Bali, S.; Plumb, J.; Anderson, H.; Prentis, J.; Swart, M.; Levett, D.Z.H.; et al. Cardiopulmonary exercise testing (CPET) in the United Kingdom-a national survey of the structure, conduct, interpretation and funding. Perioper. Med. 2018, 7, 2. Available online: https://pubmed.ncbi.nlm.nih.gov/29423173/ (accessed on 15 August 2023). [CrossRef]
- Ferguson, M.; Shulman, M. Cardiopulmonary Exercise Testing and Other Tests of Functional Capacity. Curr. Anesthesiol. Rep. 2022, 12, 26. Available online: https://link.springer.com/article/10.1007/s40140-021-00499-6 (accessed on 20 August 2024). [CrossRef] [PubMed]
- Preoperative Assessment and Optimisation for Adult Surgery including consideration of COVID-19 and its implications. Available online: https://cpoc.org.uk/sites/cpoc/files/documents/2021-06/Preoperative%20assessment%20and%20optimisation%20guidance.pdf (accessed on 19 December 2024).
- Riedel, B.; Li, M.H.G.; Lee, C.H.A.; Ismail, H.; Cuthbertson, B.H.; Wijeysundera, D.N.; Ho, K.M.; Wallace, S.; Thompson, B.; Ellis, M.; et al. A simplified (modified) Duke Activity Status Index (M-DASI) to characterise functional capacity: A secondary analysis of the Measurement of Exercise Tolerance before Surgery (METS) study. Br. J. Anaesth. 2021, 126, 181–190. Available online: http://www.bjanaesthesia.org.uk/article/S0007091220304621/fulltext (accessed on 16 December 2024). [CrossRef] [PubMed]
- Swarbrick, C.J.; Williams, K.; Evans, B.; Blake, H.A.; Poulton, T.; Nava, S.; Shah, A.; Martin, P.; Partridge, J.S.L.; Moppett, I.K. Characteristics of older patients undergoing surgery in the UK: SNAP-3, a snapshot observational study. Br. J. Anaesth. 2025. Available online: http://www.bjanaesthesia.org/article/S0007091224007013/fulltext (accessed on 15 January 2025).
- Lamperti, M.; Romero, C.S.; Guarracino, F.; Cammarota, G.; Vetrugno, L.; Tufegdzic, B.; Lozsan, F.; Macias, F.; Juan, J.; Duma, A.; et al. Preoperative assessment of adults undergoing elective noncardiac surgery: Updated guidelines from the European Society of Anaesthesiology and Intensive Care. Eur. J. Anaesthesiol. 2024, 42, 1–35. Available online: https://journals.lww.com/ejanaesthesiology/fulltext/2025/01000/preoperative_assessment_of_adults_undergoing.1.aspx (accessed on 15 January 2025). [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. Available online: https://pubmed.ncbi.nlm.nih.gov/16129869/ (accessed on 20 August 2024). [CrossRef] [PubMed]
- Parini, S.; Azzolina, D.; Massera, F.; Garlisi, C.; Papalia, E.; Baietto, G.; Bora, G.; Mastromarino, M.G.; Barini, M.; Ruffini, E.; et al. Comparison of frailty indexes as predictors of clinical outcomes after major thoracic surgery. J. Thorac. Dis. 2024, 16, 3192–3203. Available online: https://pubmed.ncbi.nlm.nih.gov/38883684/ (accessed on 20 August 2024). [CrossRef] [PubMed]
- Fagard, K.; Leonard, S.; Deschodt, M.; Devriendt, E.; Wolthuis, A.; Prenen, H.; Flamaing, J.; Milisen, K.; Wildiers, H.; Kenis, C. The impact of frailty on postoperative outcomes in individuals aged 65 and over undergoing elective surgery for colorectal cancer: A systematic review. J Geriatr. Oncol. 2016, 7, 479–491. Available online: https://pubmed.ncbi.nlm.nih.gov/27338516/ (accessed on 20 August 2024). [CrossRef] [PubMed]
- O’Mahony, M.; Mohammed, K.; Kasivisvanathan, R. Cardiopulmonary Exercise Testing Versus Frailty, Measured by the Clinical Frailty Score, in Predicting Morbidity in Patients Undergoing Major Abdominal Cancer Surgery. World J. Surg. 2021, 45, 116–125. Available online: https://pubmed.ncbi.nlm.nih.gov/32935139/ (accessed on 20 August 2024). [CrossRef] [PubMed]
- Levett, D.Z.H.; Jack, S.; Swart, M.; Carlisle, J.; Wilson, J.; Snowden, C.; Riley, M.; Danjoux, G.; Ward, S.; Older, P.; et al. Perioperative cardiopulmonary exercise testing (CPET): Consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br. J. Anaesth. 2018, 120, 484–500. Available online: https://pubmed.ncbi.nlm.nih.gov/29452805/ (accessed on 15 August 2023). [CrossRef] [PubMed]
- Snitkjær, C.; Jensen, L.R.; Soylu, L.; Hauge, C.; Kvist, M.; Jensen, T.K.; Kokotovic, D.; Burcharth, J. Impact of clinical frailty on surgical and non-surgical complications after major emergency abdominal surgery. BJS Open 2024, 8, zrae039. Available online: https://pubmed.ncbi.nlm.nih.gov/38788680/ (accessed on 8 December 2024). [CrossRef] [PubMed]
| Domain | Median (IQR) |
|---|---|
| Age | 73.8 (69.0–79.0) |
| Male | 70% (n = 121) |
| Ischaemic heart disease | 18% (n = 32) |
| Atrial fibrillation | 14% (n = 25) |
| Stroke/TIA | 8% (n = 14) |
| Diabetes | 23% (n = 40) |
| Heart failure | 7% (n = 12) |
| COPD | 16% (n = 27) |
| Hypertension | 45% (n = 79) |
| Surgery Type | (n) |
|---|---|
| Cystectomy | 76 |
| Nephrectomy | |
| 35 | |
| Bowel resection | |
| 36 | |
| Lung resection | |
| 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunter, A.; Roche, M.; Robeel, M.; Hodgson, L. Association Between Frailty Scoring and Cardiopulmonary Exercise Testing: A Retrospective Cohort Study. Anesth. Res. 2025, 2, 6. https://doi.org/10.3390/anesthres2010006
Hunter A, Roche M, Robeel M, Hodgson L. Association Between Frailty Scoring and Cardiopulmonary Exercise Testing: A Retrospective Cohort Study. Anesthesia Research. 2025; 2(1):6. https://doi.org/10.3390/anesthres2010006
Chicago/Turabian StyleHunter, Alex, Matthew Roche, Moheb Robeel, and Luke Hodgson. 2025. "Association Between Frailty Scoring and Cardiopulmonary Exercise Testing: A Retrospective Cohort Study" Anesthesia Research 2, no. 1: 6. https://doi.org/10.3390/anesthres2010006
APA StyleHunter, A., Roche, M., Robeel, M., & Hodgson, L. (2025). Association Between Frailty Scoring and Cardiopulmonary Exercise Testing: A Retrospective Cohort Study. Anesthesia Research, 2(1), 6. https://doi.org/10.3390/anesthres2010006






