Severe Postoperative Complications Following Bilateral DIEP Flap Breast Reconstruction in a High-Risk Patient: A Case Report
Abstract
:1. Introduction
1.1. Evolution of Breast Reconstruction and the Rise of the DIEP Flap
1.2. Patients’ Selection for DIEP Flap
1.3. Complications of DIEP Flap Surgery
2. Case Presentation
2.1. Surgical Procedure
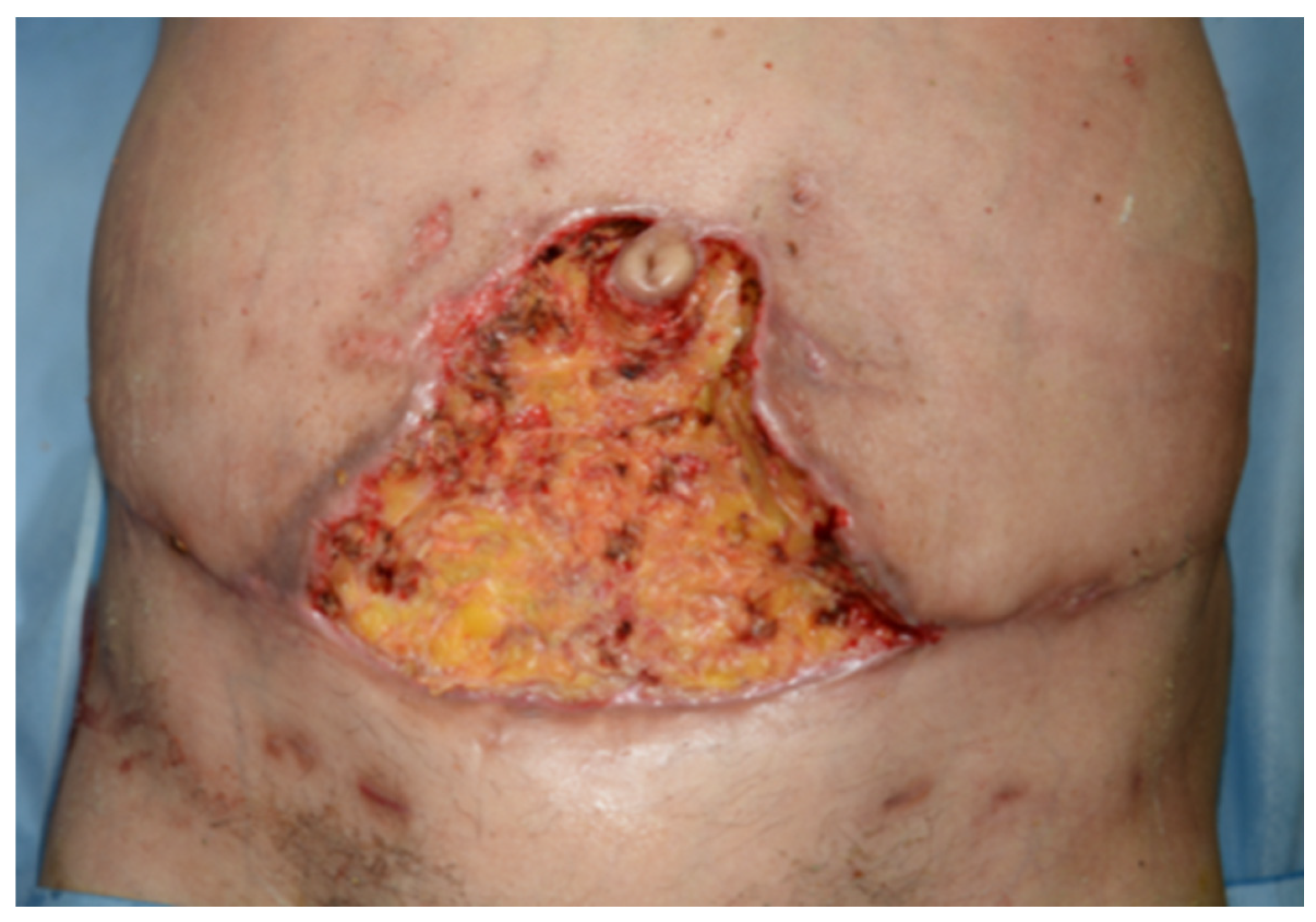
2.2. Postoperative Course
3. Discussion
- Pelvic or abdominal thrombosis due to vessel injury or stasis during dissection.
- Post-COVID-19 hypercoagulable state as the patient had an asymptomatic infection eight months earlier.
- Intraoperative thrombosis due to venous clamping or impaired revascularization.
- Paradoxical embolism via a potential patent foramen ovale.
- In situ pulmonary thrombosis as a result of systemic coagulopathy.
| Topic | Reference |
|---|---|
| Venous congestion management and surgical refinements | Pignatti et al. [31] proposed multiple venous drainage configurations and a decision-making algorithm to reduce venous congestion in DIEP flaps. |
| Preoperative vascular imaging and detection of venous anomalies | Hennessy et al. [32] reviewed the utility of infrared thermography for perforator mapping; Davis et al. [33] demonstrated that CTA can identify atypical venous connections predictive of congestion. |
| Fat necrosis and perfusion analysis | Yoo et al. [34] showed that intraoperative ICG angiography significantly reduces fat necrosis by guiding resection of poorly perfused areas. |
| General surgical complications (infection, seroma, dehiscence) and individual risk factors | Modarressi et al. [35] highlighted systemic and procedural risk factors influencing postoperative morbidity in a large DIEP flap cohort. |
| VTE risk in immediate reconstruction after mastectomy | Pannucci et al. [41] identified increased thrombotic risk in patients undergoing immediate autologous breast reconstruction; Mandalà et al. [42] and Chew et al. [43] discussed the role of cancer therapy in enhancing VTE incidence. |
| Higher VTE risk in autologous vs. implant-based reconstruction | Lemaine et al. [44] quantified VTE rates in DIEP patients on LMWH prophylaxis; Masoomi et al. [36] identified predictive risk factors for VTE in autologous reconstructions. |
| Elevated risk in bilateral DIEP flap procedures | Wormald et al. [45] conducted a meta-analysis showing increased flap failure and complications in bilateral vs. unilateral DIEP reconstructions; Schaverien et al. [46] provided prospective data confirming this risk. |
| Importance of Caprini score and healthcare team training | Pannucci et al. [37] validated the Caprini risk assessment model in reconstructive surgery; Gould et al. [38] provided guideline-based VTE prevention strategies in surgical patients. |
| Comparison of short vs. extended chemoprophylaxis in DIEP flap patients | Awaida et al. [54], in a cohort of 424 patients, found no significant difference in VTE incidence between short-term and extended prophylaxis but confirmed higher risk with Caprini ≥ 8. |
| Temporal pattern of VTE and implications for prophylaxis duration | Rochlin et al. [57], in a cohort of 12,778 patients, reported that over 65% of VTE events occurred post-discharge, mostly within the first 24 days. |
| Post-COVID prothrombotic state as emerging risk factor | Gao W et al. [59] demonstrated that DIEP flap reconstruction post-COVID remains safe overall, although 0.8% of post-COVID patients developed VTE despite standard prophylaxis. |
| Persistent endothelial dysfunction post-COVID and implications for microvascular risk | Gao Y-P et al. [60] showed significantly reduced flow-mediated dilation (FMD) in COVID-19 survivors nearly one year after infection, linked to TNF-α elevation, suggesting long-term endothelial damage and higher thrombotic potential. |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Champaneria, M.C.; Wong, W.W.; Hill, M.E.; Gupta, S.C. The Evolution of Breast Reconstruction: A Historical Perspective. World J. Surg. 2012, 36, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Hartrampf, C.R.; Scheflan, M.; Black, P.W. Breast reconstruction with a transverse abdominal island flap. Plast. Reconstr. Surg. 1982, 69, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Holstrom, H. The free abdominoplasty flap and its use in breast reconstruction. An experimental study and clinical case report. Scand. J. Plast. Reconstr. Surg. 1979, 13, 423–427. [Google Scholar]
- Blondeel, N.; Vanderstraeten, G.G.; Monstrey, S.J.; Van Landuyt, K.; Tonnard, P.; Lysens, R.; Boeckx, W.D.; Matton, G. The donor site morbidity of free DIEP flaps and free TRAM flaps for breast reconstruction. Br. J. Plast. Surg. 1997, 50, 322–330. [Google Scholar] [CrossRef]
- Allen, R.J.; Treece, P. Deep inferior epigastric perforator flap for breast reconstruction. Ann. Plast. Surg. 1994, 32, 32. [Google Scholar] [CrossRef]
- Futter, C.M.; Webster, M.H.C.; Hagen, S.; Mitchell, S.L. A retrospective comparison of abdominal muscle strength following breast reconstruction with a free TRAM or DIEP flap. Br. J. Plast. Surg. 2000, 53, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Nahabedian, M.Y. The deep inferior epigastric perforator flap: Where we started and where we are now. Gland. Surg. 2023, 12, 696–703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’connor, E.F.; Rozen, W.M.; Chowdhry, M.; Patel, N.G.; Chow, W.T.; Griffiths, M.; Ramakrishnan, V.V. The microvascular anastomotic coupler for venous anastomoses in free flap breast reconstruction improves outcomes. Gland. Surg. 2016, 5, 88–92. [Google Scholar]
- Newman, M.I.; Samson, M.C. The application of laser-assisted indocyanine green fluorescent dye angiography in microsurgical breast reconstruction. J. Reconstr. Microsurg. 2009, 25, 21–226. [Google Scholar] [CrossRef]
- Winters, H.; Tielemans, H.; Hummelink, S.; Slater, N.; Ulrich, D. DIEP flap breast reconstruction combined with vascularized lymph node transfer for patients with breast cancer-related lymphedema. Eur. J. Surg. Oncol. 2022, 48, 1718–1722. [Google Scholar] [CrossRef]
- Aiello, C.; Choe, J.; Suri, K.; Smith, M.L.; Selber, J.C.; Sugiyama, G.; Tanna, N. Advancing breast reconstruction: Next-generation robotic-assisted deep inferior epigastric perforator flap techniques. Int. Microsurg. J. 2024, 8, 3. [Google Scholar] [CrossRef]
- Garza, R.I.; Ochoa, O.M.; Chrysopoulo, M.M. Post-mastectomy breast reconstruction with autologous tissue: Current methods and techniques. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3433. [Google Scholar] [CrossRef]
- Stefura, T.; Rusinek, J.; Wątor, J.; Zagórski, A.; Zając, M.; Libondi, G.; Wysocki, W.M.; Koziej, M. Implant vs. autologous tissue-based breast reconstruction: A systematic review and meta-analysis of the studies comparing surgical approaches in 55,455 patients. J. Plast. Reconstr. Aesthetic Surg. 2023, 77, 346–358. [Google Scholar] [CrossRef]
- Song, Y.; Zeng, J.; Tian, X.; Zheng, H.; Wu, X. A review of different breast reconstruction methods. Am. J. Transl. Res. 2023, 15, 3846–3855. [Google Scholar] [PubMed] [PubMed Central]
- Tallroth, L.; Velander, P.; Klasson, S. A short-term comparison of expander prosthesis and DIEP flap in breast reconstructions: A prospective randomized study. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Borrero, M.; Hilaire, H.S.; Allen, R. Modern approaches to abdominal-based breast reconstruction. Clin. Plast. Surg. 2023, 50, 267–279. [Google Scholar] [CrossRef]
- Murphy, B.D.; Kerrebijn, I.; Farhadi, J.; Masia, J.; Hofer, S.O.P. Indications and Controversies for Abdominally-Based Complete Autologous Tissue Breast Reconstruction. Clin. Plast. Surg. 2018, 45, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ménez, T.; Michot, A.; Tamburino, S.; Weigert, R.; Pinsolle, V. Multicenter evaluation of quality of life and patient satisfaction after breast reconstruction, a long-term retrospective study. Ann. Chir. Plast. Esthet. 2018, 63, 126–133. [Google Scholar] [CrossRef]
- Opsomer, D.; Vyncke, T.; Depypere, B.; Stillaert, F.; Blondeel, P.M.; Van Landuyt, K.M. Lumbar Flap versus the Gold Standard: Comparison to the DIEP Flap. Plast. Reconstr. Surg. 2020, 145, 706e–714e. [Google Scholar] [CrossRef]
- Bar-Meir, E.D.; Reish, R.G.; Yueh, J.H.; McArdle, C.; Tobias, A.M.; Lee, B.T. The Maylard incision: A low transverse incision variant seen in DIEP flap breast reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, e447–e452. [Google Scholar] [CrossRef]
- Hsieh, F.; Kumiponjera, D.; Malata, C.M. An algorithmic approach to abdominal flap breast reconstruction in patients with pre-existing scars—Results from a single surgeon’s experience. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 1650–1660. [Google Scholar] [CrossRef] [PubMed]
- Ribuffo, D.; Marcellino, M.; Barnett, G.R.; Houseman, N.D.; Scuderi, N. Do prior abdominal surgeries increase complications in abdominally based breast reconstructions? Ann. Plast. Surg. 2015, 75, 526–533. [Google Scholar]
- Ribuffo, D.; Marcellino, M.; Barnett, G.R.; Houseman, N.D.; Scuderi, N. Breast reconstruction with abdominal flaps after abdominoplasties. Plast. Reconstr. Surg. 2000, 108, 1604–1608. [Google Scholar] [CrossRef]
- De Frene, B.; Van Landuyt, K.; Hamdi, M.; Blondeel, P.; Roche, N.; Voet, D.; Monstrey, S. Free DIEAP and SGAP flap breast reconstruction after abdominal/gluteal liposuction. J. Plast. Reconstr. Aesthetic Surg. 2006, 59, 1031–1036. [Google Scholar] [CrossRef]
- Klasson, S.; Nyman, J.; Svensson, H.; Velander, P. Smoking increases donor site complications in breast reconstruction with DIEP flap. J. Plast. Surg. Hand Surg. 2016, 50, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Schaverien, M.V.; McCulley, S.J. Effect of obesity on outcomes of free autologous breast reconstruction: A meta-analysis. Microsurgery 2014, 34, 484–497. [Google Scholar] [CrossRef]
- Wang, T.Y.; Serletti, J.M.; Cuker, A.; McGrath, J.; Low, D.W.; Kovach, S.J.; Wu, L.C. Free tissue transfer in the hypercoagulable patient: A review of 58 flaps. Plast. Reconstr. Surg. 2012, 129, 443–453. [Google Scholar] [CrossRef]
- Ellis, A.J.; Hendrick, V.M.; Williams, R.; Komm, B.S. Selective estrogen receptor modulators in clinical practice: A safety overview. Expert. Opin. Drug Saf. 2015, 14, 921–934. [Google Scholar] [CrossRef]
- Mirzabeigi, M.N.; Nelson, J.A.; Fischer, J.P.; Kovach, S.J.; Serletti, J.M.; Wu, L.C.; Kanchwala, S. Tamoxifen (selective estrogen-receptor modulators) and aromatase inhibitors as potential perioperative thrombotic risk factors in free flap breast reconstruction. Plast. Reconstr. Surg. 2015, 135, 670e–679e. [Google Scholar] [CrossRef]
- Pignatti, M.; Pinto, V.; Giorgini, F.A.; Lozano Miralles, M.E.; Cannamela, G.; D’Arpa, S.; Cipriani, R.; De Santis, G. Meta-analysis of the effects of venous super-drainage in deep inferior epigastric artery perforator flaps for breast reconstruction. Microsurgery 2021, 41, 186–195. [Google Scholar] [CrossRef]
- Pignatti, M.; Pinto, V.; Giorgini, F.A.; Lozano Miralles, M.E.; D’Arpa, S.; Cipriani, R.; De Santis, G. Different hydraulic constructs to optimize the venous drainage of DIEP flaps in breast reconstruction: Decisional algorithm and review of the literature. J. Reconstr. Microsurg. 2021, 37, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, O.; Potter, S.M. Use of infrared thermography for the assessment of free flap perforators in autologous breast reconstruction: A systematic review. JPRAS Open 2020, 23, 60–70. [Google Scholar] [CrossRef]
- Davis, C.R.; Jones, L.; Tillett, R.L.; Richards, H.; Wilson, S.M. Predicting venous congestion before DIEP breast reconstruction by identifying atypical venous connections on preoperative CTA imaging. Microsurgery 2019, 39, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Yoo, A.; Palines, P.A.; Mayo, J.L.; Bartow, M.J.; Danos, D.M.; St Hilaire, H.; Wise, M.W.; Stalder, M.W. The impact of indocyanine green angiography on fat necrosis in deep inferior epigastric perforator flap breast reconstruction. Ann. Plast. Surg. 2022, 88, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Modarressi, A.; Schettini, A.V.; Rüegg, E.M.; Pittet-Cuénod, B. Venous thromboembolism events after breast reconstructions with DIEP free flaps in 192 consecutive case. Ann. Chir. Plast. Esthet. 2018, 63, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Masoomi, H.; Paydar, K.Z.; Wirth, G.A.; Aly, A.; Kobayashi, M.R.; Evans, G.R. Predictive risk factors of venous thromboembolism in autologous breast reconstruction surgery. Ann. Plast. Surg. 2014, 72, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Pannucci, C.J.; Bailey, S.H.; Dreszer, G.; Fisher Wachtman, C.; Zumsteg, J.W.; Jaber, R.M.; Hamill, J.B.; Hume, K.M.; Rubin, J.P.; Neligan, P.C.; et al. Validation of the Caprini risk assessment model in plastic and reconstructive surgery patients. J. Am. Coll. Surg. 2011, 212, 105–112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gould, M.K.; Garcia, D.A.; Wren, S.M.; Karanicolas, P.J.; Arcelus, J.I.; Heit, J.A.; Samama, C.M. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e227S–e277S, Erratum in Chest 2012, 141, 1369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kesson, E.M.; Allardice, G.M.; George, W.D.; Burns, H.J.; Morrison, D.S. Effectsofmulti- disciplinary team working on breast cancer survival: Retrospective, compara- tive, interventional cohort study of 13 722 women. BMJ 2012, 344, e2718. [Google Scholar] [CrossRef]
- Mutebi, M.; Anderson, B.O.; Duggan, C.; Adebamowo, C.; Agarwal, G.; Ali, Z.; Bird, P.; Bourque, J.; DeBoer, R.; Gebrim, L.H.; et al. Breast cancer treatment: A phased approach to implementation. Cancer 2020, 126 (Suppl. S10), 2365–2378. [Google Scholar] [CrossRef] [PubMed]
- Pannucci, C.J.; Chang, E.Y.; Wilkins, E.G. Venous thromboembolic disease in autogenous breast reconstruction. Ann. Plast. Surg. 2009, 63, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Mandalà, M.; Tondini, C. Adjuvant therapy in breast cancer and venous thromboembolism. Thromb. Res. 2012, 130 (Suppl. S1), S66–S70. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.K.; Wun, T.; Harvey, D.J.; Zhou, H.; White, R.H. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J. Clin. Oncol. 2007, 25, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Lemaine, V.; McCarthy, C.; Kaplan, K.; Mehrara, B.; Pusic, A.L.; Cordeiro, P.G.; Disa, J.J. Venous thromboembolism following microsurgical breast reconstruction: An objective analysis in 225 consecutive patients using low-molecular-weight heparin prophylaxis. Plast. Reconstr. Surg. 2011, 127, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Wormald, J.C.; Wade, R.G.; Figus, A. The increased risk of adverse outcomes in bilateral deep inferior epigastric artery perforator flap breast reconstruction compared to unilateral reconstruction: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthetic Surg. 2014, 67, 143–156. [Google Scholar] [CrossRef]
- Schaverien, M.V.; Butler, C.E. Complications in DIEP Flap Breast Reconstruction After Mastectomy for Breast Cancer: A Prospective Cohort Study Comparing Unilateral and Bilateral Reconstructions. Ann. Surg. Oncol. 2017, 24, 1451–1453. [Google Scholar] [CrossRef] [PubMed]
- Veeramani, A.; McCarty, J.C.; Vieira, B.L.; Karinja, S.; Pusic, A.L.; Carty, M.J.; Erdmann-Sager, J. Safety of DIEP Flap Reconstruction in Patients with Factor V Leiden: A Retrospective Cohort Study. Plast. Reconstr. Surg. Glob. Open. 2022, 10, e4244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Awaida, C.; Trabelsi, N.; Bou-Merhi, J.; Bernier, C.; Gagnon, A.; Harris, P.; Tchakmakian, A.; Dragomir, A.; Odobescu, A. Short-term versus extended chemoprophylaxis against venous thromboembolism in DIEP flap breast reconstruction: A retrospective study of 424 patients. Ann. Chir. Plast. Esthet. 2024, 69, 384–390. [Google Scholar] [CrossRef]
- Caprini, J.A.; Arcelus, J.I.; Reyna, J.J. Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease. Semin. Hematol. 2001, 38 (Suppl. S5), 12–19. [Google Scholar] [CrossRef] [PubMed]
- Ricci, J.A.; Crawford, K.; Ho, O.A.; Lee, B.T.; Patel, K.M.; Iorio, M.L. Practical Guidelines for Venous Thromboembolism Prophylaxis in Free Tissue Transfer. Plast. Reconstr. Surg. 2016, 138, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Goldhaber, S.Z. Pulmonary embolism. Lancet 2004, 363, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Karius, A.K.; Chen, J.; Tiongco, R.F.P.; Lagziel, T.; Cooney, C.M.; Broderick, K.P. Prior COVID-19 Infection Predisposes to Worse Outcomes After Autologous Breast Reconstruction: A Propensity Score-Matched Analysis. Ann. Plast. Surg. 2023, 90 (Suppl. S5), S639–S644. [Google Scholar] [CrossRef] [PubMed]
- Angelini, D.E.; Kaatz, S.; Rosovsky, R.P.; Zon, R.L.; Pillai, S.; Robertson, W.E.; Elavalakanar, P.; Patell, R.; Khorana, A. COVID-19 and venous thromboembolism: A narrative review. Res. Pract. Thromb. Haemost. 2022, 6, e12666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamdi, M.; Kapila, A.K.; Waked, K. Current status of autologous breast reconstruction in Europe: How to reduce donor site morbidity. Gland. Surg. 2023, 12, 1760–1773. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sweetland, S.; Green, J.; Liu, B.; de González, A.B.; Canonico, M.; Reeves, G.; Beral, V.; Mws on behalf of the Million Women Study collaborators. Duration and magnitude of the postoperative risk of venous thromboembolism in middle-aged women: Prospective cohort study. BMJ 2009, 339, b4583. [Google Scholar] [CrossRef]
- Fischer, J.P.; Wes, A.M.; Tuggle, C.T.; Wu, L.C. Venous thromboembolism risk in mastectomy and immediate breast reconstruction: Analysis of NSQIP data sets. Plast. Reconstr. Surg. 2014, 133, 263e–273e. [Google Scholar]
- Rochlin, D.H.; Sheckter, C.C.; Pannucci, C.; Momeni, A. Venous thromboembolism following microsurgical breast reconstruction: A longitudinal analysis of 12,778 patients. Plast. Reconstr. Surg. 2020, 146, 465–473. [Google Scholar] [CrossRef]
- Pittelkow, E.M.; DeBrock, W.C.; Mailey, B.; Ballinger, T.J.; Socas, J.; Lester, M.E.; Hassanein, A.H.M. Evaluation of an extended-duration chemoprophylaxis regimen for VTE after microsurgical breast reconstruction. Plast. Reconstr. Surg. Glob. Open. 2021, 9, e3741. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Lu, Y.; Huang, O.; Chen, X.; He, J.; Chen, W.; Li, Y.; Xu, H.; Wu, J.; Shen, K. Safety analysis of immediate breast reconstruction with a deep inferior epigastric perforator (DIEP) flap in the post-COVID-19 era: A comparison between pre- and post-pandemic cohorts. Gland. Surg. 2023, 12, 1475–1484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, Y.-P.; Zhou, W.; Huang, P.-N.; Liu, H.-Y.; Bi, X.-J.; Zhu, Y.; Sun, J.; Tang, Q.-Y.; Li, L.; Zhang, J.; et al. Persistent Endothelial Dysfunction in Coronavirus Disease-2019 Survivors Late After Recovery. Front. Med. 2022, 9, 809033. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]





| Indications | Contraindications |
|---|---|
| Patient preference | Underweight patients (without abdominal fat and skin suitable for surgery) or obese and severe obese patients |
| Severe soft tissue damage (ex: after radiation therapy) | Anatomical unsuitability (vasculopathic patients or patients with previous abdomen surgery) |
| Overweight patients with excess of abdominal skin and fat) | Strong active smokers |
| Unilateral reconstruction | Medical unsuitability for surgery |
| Failed or unpleasant implant reconstruction | Delays in oncologic treatment |
| Parameter | Value |
|---|---|
| Caprini risk score | 8 (high risk for thromboembolic events) |
| Thromboprophylaxis | Enoxaparin (Inhixa) 4000 IU SC pre-op, elastic compression stockings, pneumatic compression devices, postoperative enoxaparin 4000 IU daily |
| ASA classification | III |
| Mallampati score | I |
| Home medications | Letrozole, ramipril, omeprazole |
| Laboratory findings (06/14/2024) | Hb: 14.1 g/dL, Hct: 43.2%, PLT: 299 × 109/L, PT: 1.18, aPTT: 1.13 |
| ECG (06/14/2024) | Sinus bradycardia (heart rate: 55 bpm) and first-degree atrioventricular block (1° AV block). |
| Chest X-ray (06/14/2024) | No significant findings |
| CT neck, chest, abdomen (06/12/2024) | No metastatic lesions; mild pericardial effusion and small hepatic nodule (9 mm, S2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marena, F.; Grosso, M.; De Col, A.; Bassetto, F.; Brambullo, T. Severe Postoperative Complications Following Bilateral DIEP Flap Breast Reconstruction in a High-Risk Patient: A Case Report. Complications 2025, 2, 12. https://doi.org/10.3390/complications2020012
Marena F, Grosso M, De Col A, Bassetto F, Brambullo T. Severe Postoperative Complications Following Bilateral DIEP Flap Breast Reconstruction in a High-Risk Patient: A Case Report. Complications. 2025; 2(2):12. https://doi.org/10.3390/complications2020012
Chicago/Turabian StyleMarena, Francesco, Marco Grosso, Alessia De Col, Franco Bassetto, and Tito Brambullo. 2025. "Severe Postoperative Complications Following Bilateral DIEP Flap Breast Reconstruction in a High-Risk Patient: A Case Report" Complications 2, no. 2: 12. https://doi.org/10.3390/complications2020012
APA StyleMarena, F., Grosso, M., De Col, A., Bassetto, F., & Brambullo, T. (2025). Severe Postoperative Complications Following Bilateral DIEP Flap Breast Reconstruction in a High-Risk Patient: A Case Report. Complications, 2(2), 12. https://doi.org/10.3390/complications2020012







