Ocular Fat Embolism Syndrome Following Surgical Repair of a Long Bone Fracture
Abstract
1. Introduction
2. Materials and Methods
3. Case Presentation
3.1. History of Injury and Clinical Course
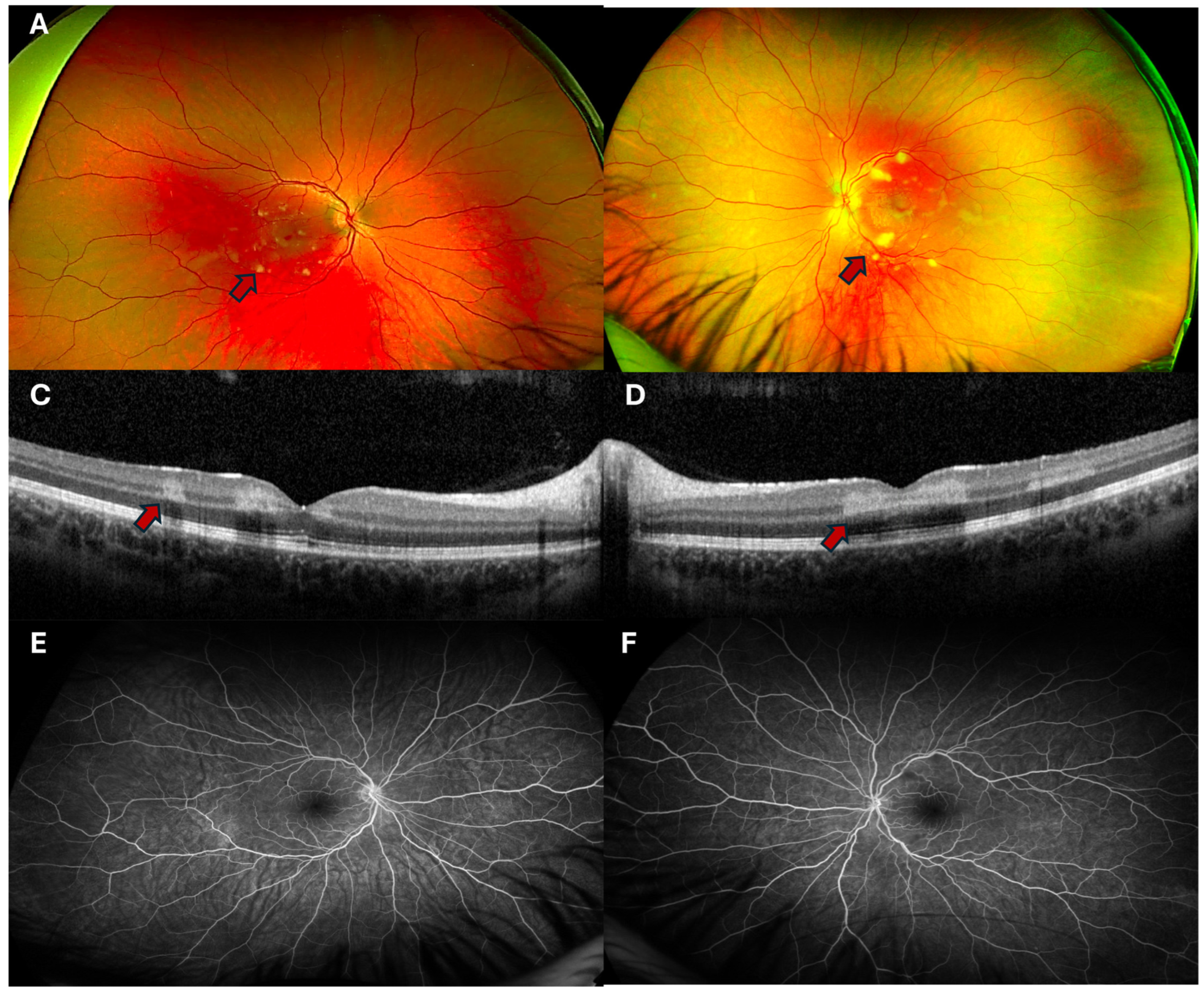
3.2. Ophthalmic Findings
3.3. Clinical Workup
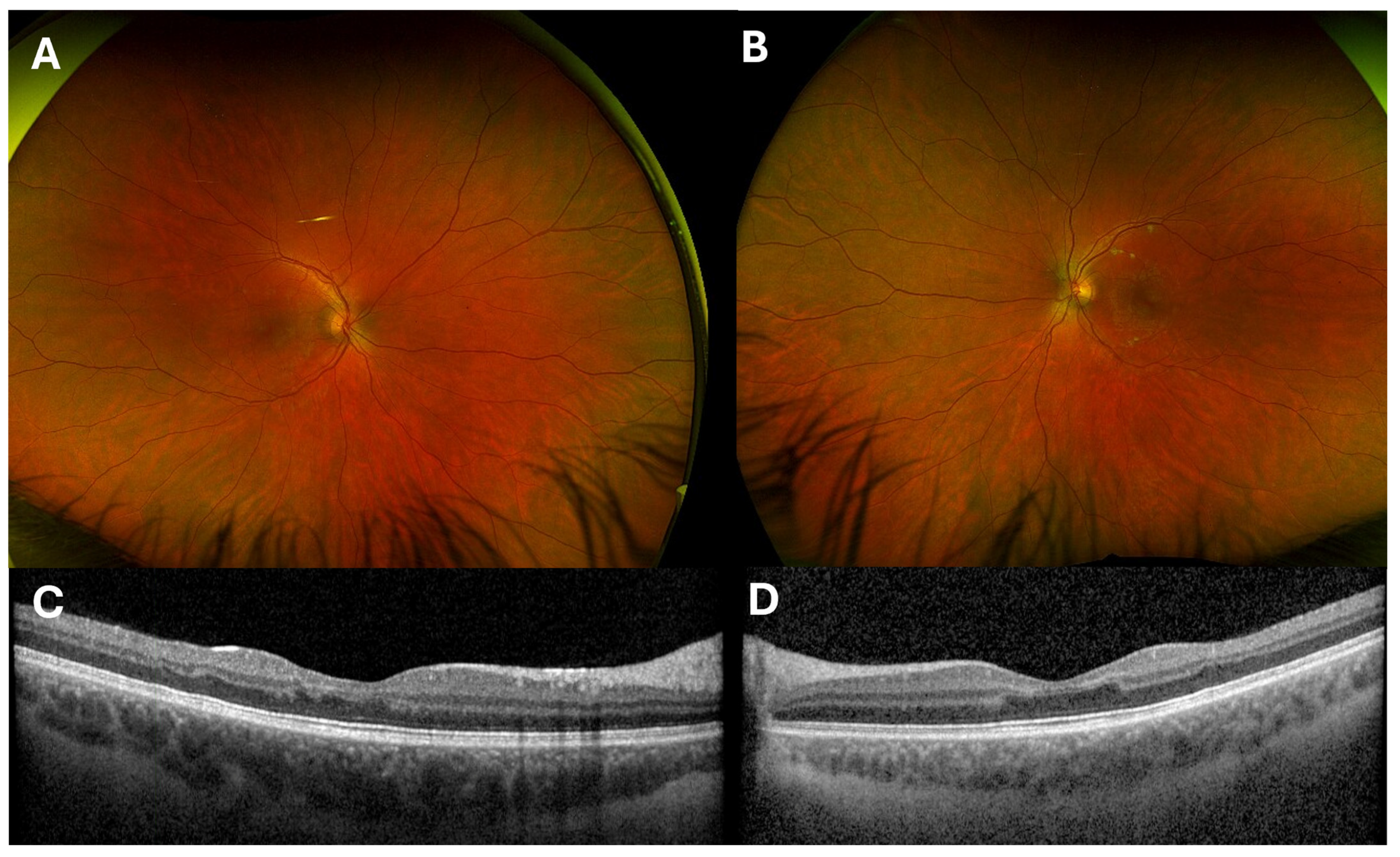
3.4. Diagnosis, Differential and Course of Recovery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AVM | Arteriovenous malformation |
| AVSD | Atrioventricular septal defect |
| CTPA | Computed tomography pulmonary angiogram |
| CWS | Cotton wool spots |
| FA | Fluorescein angiography |
| FES | Fat embolism syndrome |
| FLAIR | Fluid-attenuated inversion recovery |
| MRI | Magnetic resonance imaging |
| OCT | optical coherence tomography |
| PAMM | Paracentral acute middle maculopathy |
| PR | Purtscher retinopathy |
| PLR | Purtscher-like retinopathy |
References
- Rothberg, D.L.; Makarewich, C.A. Fat Embolism and Fat Embolism Syndrome. J. Am. Acad. Orthop. Surg. 2019, 27, e346–e355. [Google Scholar] [CrossRef] [PubMed]
- Adeyinka, A.; Pierre, L. Fat Embolism. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Mellor, A.; Soni, N. Fat embolism. Anaesthesia 2001, 56, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Bracco, D.; Favre, J.B.; Joris, R.; Ravussin, A. Fatal fat embolism syndrome: A case report. J. Neurosurg. Anesthesiol. 2000, 12, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Kobayashi, T.; Yoshihara, T.; Tsukamoto, M.; Kai, K.; Mawatari, M. Fatal fat embolism syndrome during posterior spinal fusion surgery: A case report and literature review. Medicine 2021, 100, e28381. [Google Scholar] [CrossRef] [PubMed]
- Mohar, J. Fatal Fulminant Fat Embolism Syndrome in Adult Spine Deformity Surgery: A Case Report. JBJS Case Connect. 2022, 12, e22. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Inokuchi, G.; Tsuneya, S.; Hoshioka, Y.; Chiba, F.; Yoshida, M.; Makino, Y.; Iwase, H. A case of fatal fulminant fat embolism syndrome following multiple fractures resulting from a fall. J. Forensic Sci. 2022, 67, 2115–2121. [Google Scholar] [CrossRef] [PubMed]
- Nentwich, M.M.; Remy, M.; Schaller, U.C. Ocular fat embolism syndrome. Int. Ophthalmol. 2011, 31, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Lyons, L.J.; Yarrabolu, T.; Kuffel, R.R., Jr.; Bishop, J.E. Juvenile Central Retinal Artery Occlusion Associated with Atrial Septal Defect. J. Pediatr. Ophthalmol. Strabismus 2019, 56, e73–e75. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Liu, Y.H.; Chou, H.D.; Tseng, H.J.; Fu, Y.C.; Liu, W.C. Concomitant post-traumatic ocular and cerebral fat embolism syndrome and thrombotic pulmonary embolism: A case report. Medicine 2022, 101, e29331. [Google Scholar] [CrossRef] [PubMed]
- Roden, D.; Fitzpatrick, G.; O’Donoghue, H.; Phelan, D. Purtscher’s retinopathy and fat embolism. Br. J. Ophthalmol. 1989, 73, 677–679. [Google Scholar] [CrossRef] [PubMed]
- Meling, T.; Harboe, K.; Søreide, K. Incidence of traumatic long-bone fractures requiring in-hospital management: A prospective age- and gender-specific analysis of 4890 fractures. Injury 2009, 40, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Almigdad, A.; Mustafa, A.; Alazaydeh, S.; Alshawish, M.; Bani Mustafa, M.; Alfukaha, H. Bone Fracture Patterns and Distributions according to Trauma Energy. Adv. Orthop. 2022, 2022, 8695916. [Google Scholar] [CrossRef] [PubMed]
- Serhan, H.A.; Abuawwad, M.T.; Taha, M.J.J.; Hassan, A.K.; Abu-Ismail, L.; Delsoz, M.; Alrawashdeh, H.M.; Alkorbi, H.A.; Moushmoush, O.; Elnahry, A.G. Purtscher’s and Purtscher-like retinopathy etiology, features, management, and outcomes: A summative systematic review of 168 cases. PLoS ONE 2024, 19, e0306473. [Google Scholar] [CrossRef] [PubMed]
- Burrowes, K.S.; Clark, A.R.; Tawhai, M.H. Blood flow redistribution and ventilation-perfusion mismatch during embolic pulmonary arterial occlusion. Pulm. Circ. 2011, 1, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Lovering, A.T.; Elliott, J.E.; Beasley, K.M.; Laurie, S.S. Pulmonary pathways and mechanisms regulating transpulmonary shunting into the general circulation: An update. Injury 2010, 41 (Suppl. S2), S16–S23. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, K.; Patel, B.C. Purtscher Retinopathy. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Meng, Y.; Sawut, A.; Tian, M.; Li, Y.; Cai, L.; Xiao, D.; Yi, Z.; Chen, C. Purtscher-like retinopathy and paracentral acute middle maculopathy associated with improper antihypertensive drug use: A case report. Front. Med. 2024, 11, 1394614. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; McKibbin, M. Purtscher’s retinopathy: Epidemiology, clinical features and outcome. Br. J. Ophthalmol. 2007, 91, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Beckingsale, A.B.; Rosenthal, A.R. Early fundus fluorescein angiographic findings and sequelae in traumatic retinopathy: Case report. Br. J. Ophthalmol. 1983, 67, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Mirza, K.; Acharya, P.U.; Austine, J. Transient cortical blindness in fat embolism syndrome—A diagnostic enigma. Chin. J. Traumatol. 2021, 24, 79–82. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hughes, L.P.A.; Dragoman, R.M.; Stephenson, K.A.J.; Kirker, A.W. Ocular Fat Embolism Syndrome Following Surgical Repair of a Long Bone Fracture. Complications 2025, 2, 6. https://doi.org/10.3390/complications2010006
Hughes LPA, Dragoman RM, Stephenson KAJ, Kirker AW. Ocular Fat Embolism Syndrome Following Surgical Repair of a Long Bone Fracture. Complications. 2025; 2(1):6. https://doi.org/10.3390/complications2010006
Chicago/Turabian StyleHughes, Lauren P. A., Ryan M. Dragoman, Kirk A. J. Stephenson, and Andrew W. Kirker. 2025. "Ocular Fat Embolism Syndrome Following Surgical Repair of a Long Bone Fracture" Complications 2, no. 1: 6. https://doi.org/10.3390/complications2010006
APA StyleHughes, L. P. A., Dragoman, R. M., Stephenson, K. A. J., & Kirker, A. W. (2025). Ocular Fat Embolism Syndrome Following Surgical Repair of a Long Bone Fracture. Complications, 2(1), 6. https://doi.org/10.3390/complications2010006







