The Gouty Kidney: A Reappraisal
Abstract
:1. Introduction
2. Early Works: Autopsy Findings
3. Imaging Studies
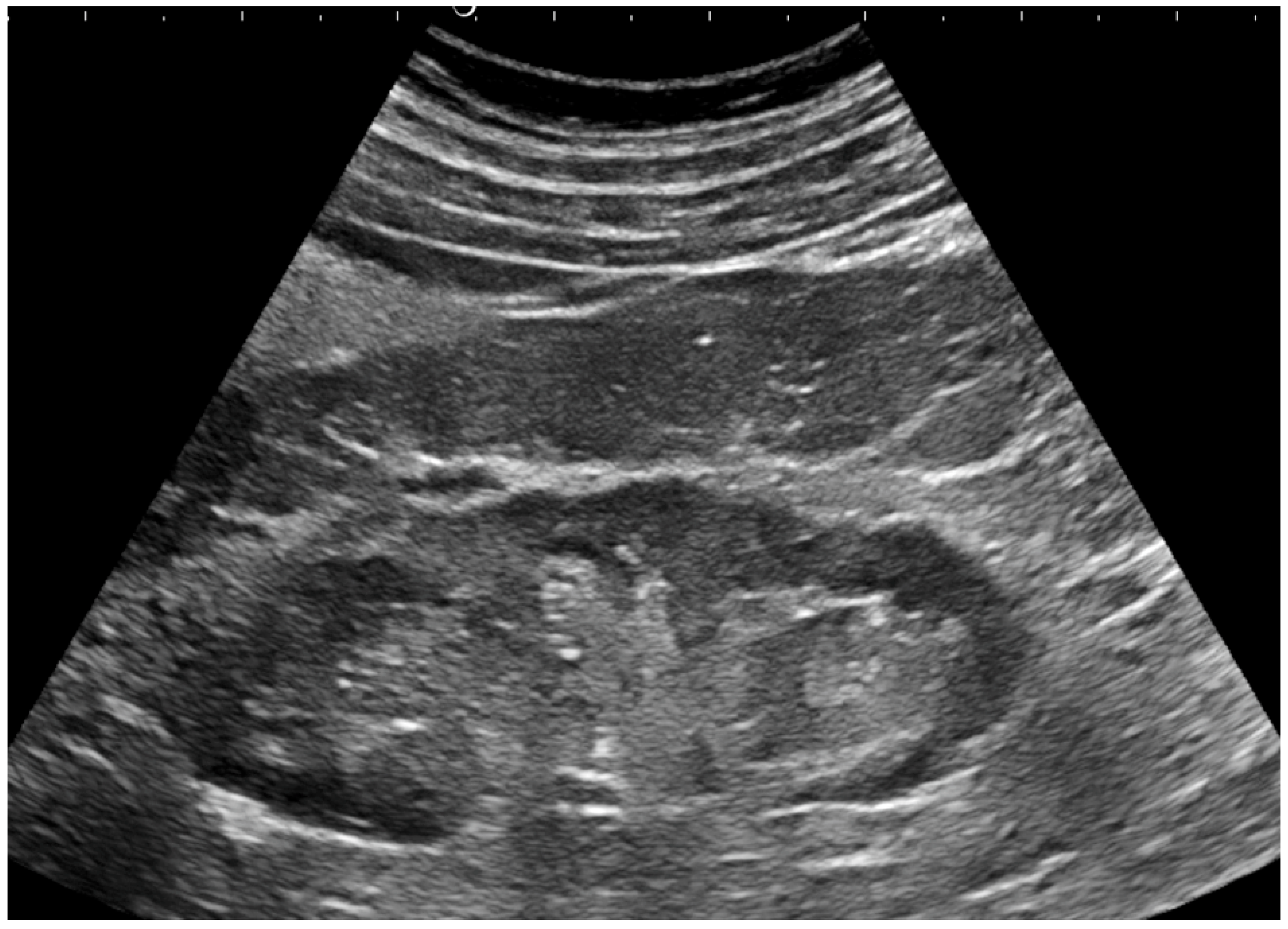
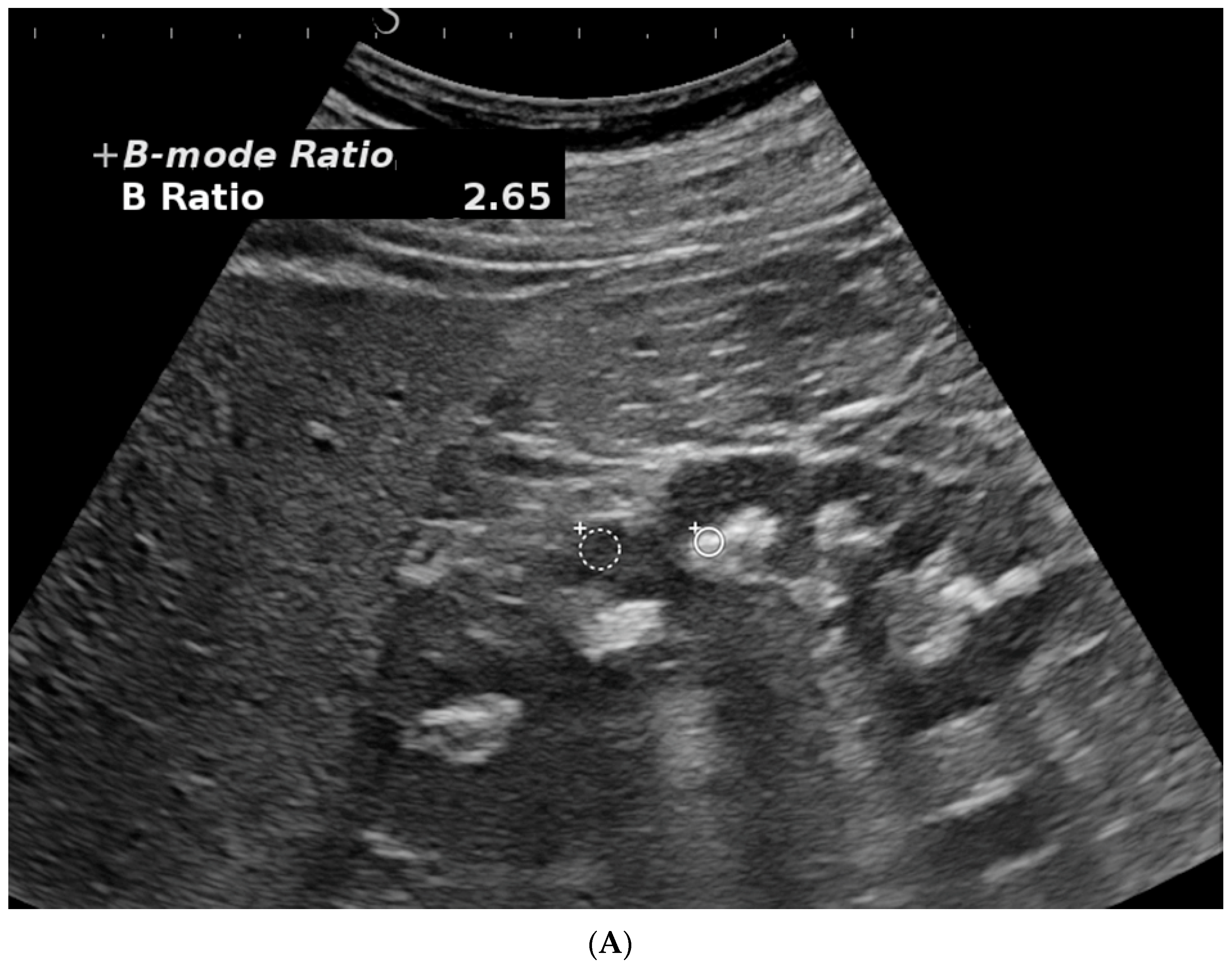
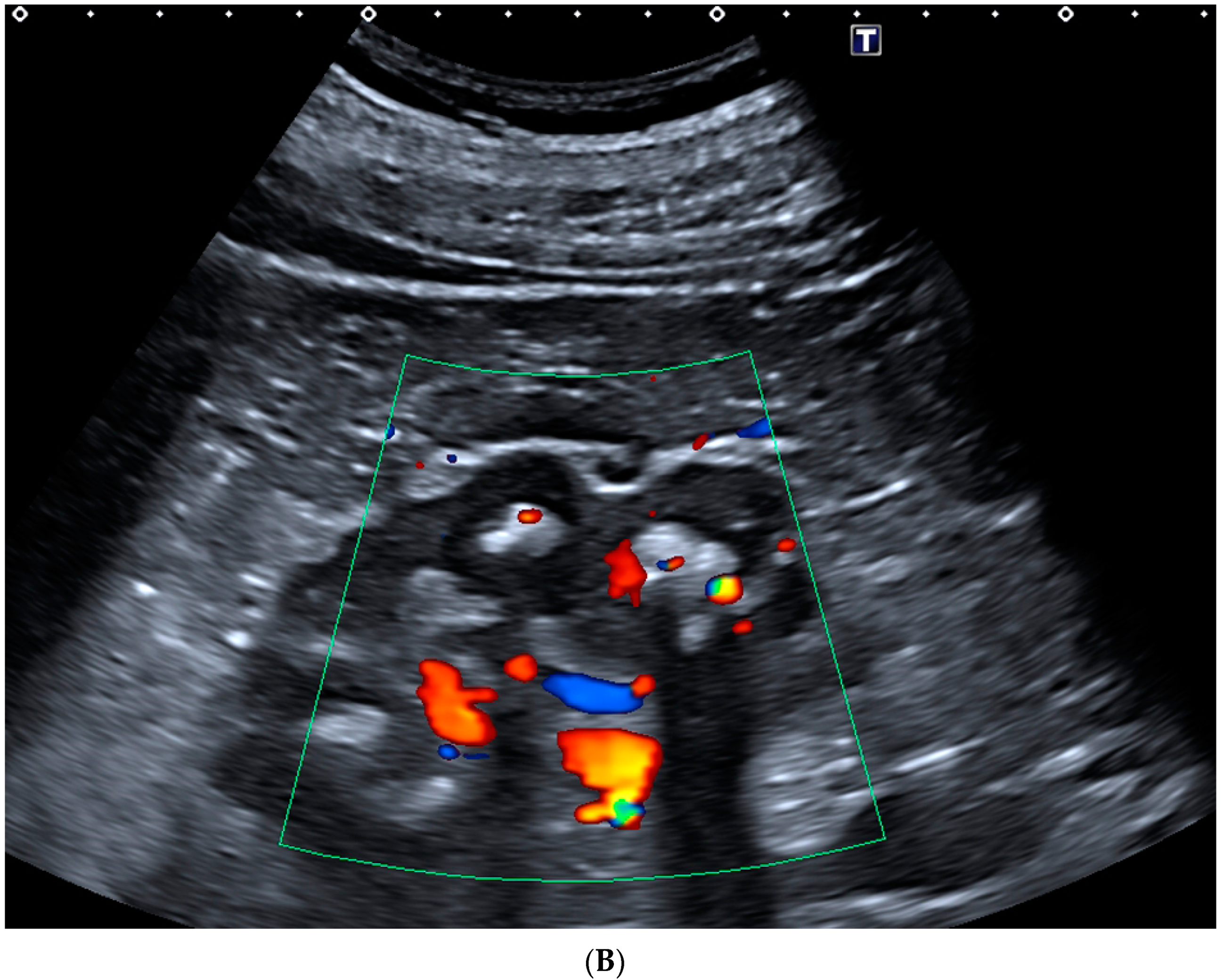
3.1. Kidney Ultrasonography (US)
3.2. Spectral CT
4. Gout and Microcrystalline Nephropathies
5. Experimental Models of Gouty Nephropathy
5.1. Inhibition of XO by Oxonate
5.2. Urate Oxidase-Knockout Mice
5.3. SLC2A9-Knockout Mice
5.4. Mechanism of Experimental Gouty Nephropathies
5.5. Comparison to Human Gouty Nephropathy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bardin, T.; Richette, P. Impact of comorbidities on gout and hyperuricaemia: An update on prevalence and treatment options. BMC Med. 2017, 15, 123. [Google Scholar] [CrossRef]
- Roughley, M.J.; Belcher, J.; Mallen, C.D.; Roddy, E. Gout and risk of chronic kidney disease and nephrolithiasis: Meta-analysis of observational studies. Arthritis Res. Ther. 2015, 17, 90. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, E. Reduced Glomerular Function and Prevalence of Gout: NHANES 2009–10. PLoS ONE 2012, 7, e50046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roughley, M.; Sultan, A.A.; Clarson, L.; Muller, S.; Whittle, R.; Belcher, J.; Mallen, C.D.; Roddy, E. Risk of chronic kidney disease in patients with gout and the impact of urate lowering therapy: A population-based cohort study. Arthritis Res. Ther. 2018, 20, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, K.H.; Kuo, C.F.; Luo, S.F.; See, L.C.; Chou, I.J.; Chang, H.C.; Chiou, M.J. Risk of end-stage renal disease associated with gout: A nationwide population study. Arthritis Res. Ther. 2012, 14, R83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stack, A.G.; Johnson, M.E.; Blak, B.; Klein, A.; Carpenter, L.; Morlock, R.; Maguire, A.R.; Parsons, V.L. Gout and the risk of advanced chronic kidney disease in the UK health system: A national cohort study. BMJ Open 2019, 9, e031550. [Google Scholar] [CrossRef]
- Simkin, P.; Bassett, J.; Lee, Q.P. Not water, but formalin, dissolves urate crystals in tophaceous tissue samples. J. Rheumatol. 1994, 21, 2320–2321. [Google Scholar]
- Nickeleit, V.; Mihatsch, M.J. Uric acid nephropathy and end-stage renal disease—Review of non-disease. Nephrol. Dial. Transplant. 1997, 12, 1832–1838. [Google Scholar] [CrossRef] [Green Version]
- Reif, M.C.; Constantiner, A.; Levitt, M.F. Chronic gouty nephropathy: A vanishing syndrome? N. Engl. J. Med. 1981, 304, 535–536. [Google Scholar] [CrossRef]
- Beck, L.H. Requiem for gouty nephropathy. Kidney Int. 1986, 30, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.J.; Nakagawa, T.; Jalal, D.; Sánchez-Lozada, L.G.; Kang, D.H.; Ritz, E. Uric acid and chronic kidney disease: Which is chasing which? Nephrol. Dial. Transpl. 2013, 28, 2221–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmerson, B.T.; Row, P.G. Editorial. An evaluation of the pathogenesis of the gouty kidney. Kidney Int. 1975, 8, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Bluestone, R.; Waisman, J.; Klinenberg, J.R. The gouty kidney. Semin. Arthritis Rheum. 1977, 7, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Charcot, J.; Cornil, V. Contribution à l’étude des altérations anatomiques de la goutte. CR Soc. Biol. 1864, 15, 139. [Google Scholar]
- Garrod, A.B. La Goutte, sa Nature, son Traitement et le Rhumatisme Goutteux; Charcot, J.M., Translator; Annotator; Adrien Delahaye: Paris, France, 1867. [Google Scholar]
- Khanna, P.; Johnson, R.J.; Marder, B.; LaMoreaux, B.; Ada Kumar, A. Systemic Urate Deposition: An Unrecognized Complication of Gout? J. Clin. Med. 2020, 9, 3204. [Google Scholar] [CrossRef]
- Brown, J.; Mallory, G.K. Renal Changes in Gout. N. Engl. J. Med. 1950, 243, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Sokoloff, L. The pathology of gout. Metabolism 1957, 6, 325–329. [Google Scholar] [CrossRef]
- Gonick, H.C.; Rubini, M.E.; Gleason, I.O.; Sommers, S.C. The renal lesion in gout. Ann. Intern. Med. 1965, 62, 667–674. [Google Scholar] [CrossRef]
- Verger, D.; Leroux-Robert, C.; Ganter, P.; Richet, G. Intra-renal deposits of urate in patients with chronic hyperuricemic renal insufficiency. J. Urologie Néphrol. 1967, 73, 314–318. (In French) [Google Scholar]
- Ostberg, Y. Renal urate deposits in chronic renal insufficiency. Acta Med. Scand. 1968, 183, 197–201. [Google Scholar] [CrossRef]
- Talbott, J.H.; Terplan, K.L. The kidney in gout. Medicine 1960, 39, 405–467. [Google Scholar] [CrossRef]
- Seegmiller, J.E.; Frazier, P.D. Biochemical considerations of the renal damage of gout. Ann. Rheum. Dis. 1966, 25, 668–672. [Google Scholar]
- Linnane, J.W.; Burry, A.F.; Emmerson, B.T. Urate Deposits in the Renal Medulla. Nephron 1981, 29, 216–222. [Google Scholar] [CrossRef]
- Ayoub, I.; Almaani, S.; Brodsky, S.; Nadasdy, T.; Prosek, J.; Hebert, L.; Rovin, B. Revisiting medullary tophi: A link between uric acid and progressive chronic kidney disease? Clin. Nephrol. 2016, 85, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Shultz, P.K.; Strife, J.L.; Strife, C.F.; McDaniel, J.D. Hyperechoic renal medullary pyramids in infants and children. Radiology 1991, 181, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Jequier, S.; Kaplan, B.S. Echogenic renal pyramids in children. J. Clin. Ultrasound 1991, 19, 85–92. [Google Scholar] [CrossRef]
- Nayir, A.; Kadioĝlu, A.; Şirin, A.; Emre, S.; Tonguç, E.; Bilge, I. Causes of increased renal medullary echogenicity in Turkish children. Pediatr. Nephrol. 1995, 9, 729–733. [Google Scholar] [CrossRef]
- Quaia, E.; Correas, J.M.; Mehta, M.; Murchison, J.T.; Gennari, A.G.; Van Beek, E.J. Gray Scale Ultrasound, Color Doppler Ultrasound, and Contrast-Enhanced Ultrasound in Renal Parenchymal Diseases. Ultrasound Q. 2018, 34, 250–267. [Google Scholar] [CrossRef]
- Toyoda, K.; Miyamoto, Y.; Ida, M.; Tada, S.; Utsunomiya, M. Hyperechoic medulla of the kidneys. Radiology 1989, 173, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Jeon, W.K.; Kim, H.K.; Kim, Y.T.; Han, S.T.; Kirn, Y.S.; Han, C.Y.; Lee, Y.W. Sonographic Findings in Gouty Nephropathy. J. Korean Radiol. Soc. 1994, 31, 523–527. [Google Scholar] [CrossRef] [Green Version]
- Bardin, T.; Nguyen, Q.D.; Tran, K.M.; Le, N.H.; Do, M.D.; Richette, P.; Letavernier, E.; Correas, J.-M.; Resche-Rigon, M. A cross- sectional study of 502 patients found a diffuse hyperechoic kidney medulla pattern in patients with sereve gout. Kidney Int. 2021, 99, 218–226. [Google Scholar] [CrossRef]
- Rosenfeld, D.L.; Preston, M.P.; Salvaggi-Fadden, K. Serial renal sonographic evaluation of patients with Lesch-Nyhan syndrome. Pediatr. Radiol. 1994, 24, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.K.; Parker, B.R. Renal oxypurine deposition in Lesch-Nyhan syndrome: Sonographic evaluation. Pediatr. Radiol. 1989, 19, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Rahmouni, A.; Bargoin, R.; Herment, A.; Bargoin, N.; Vasile, N. Color Doppler twinkling artifact in hyperechoic regions. Radiology 1996, 199, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, M.; Kadasne, R.D.; Elsobky, E.; Ali-El-Dein, B.; Monga, M. A prospective comparative study of color Doppler ultrasound with twinkling and non-contrast computerized tomography for the evaluation of acute renal colic. J. Urol. 2016, 196, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Hanafi, M.Q.; Fakhrizadeh, A.; Jaafaezadeh, E. An investigation into the clinical accuracy of twinkling artifacts in patients with urolithiasis smaller than 5 mm in comparison with computed tomography scanning. J. Fam. Med. Prim. Care 2019, 8, 401–406. [Google Scholar] [CrossRef]
- Shang, M.; Sun, X.; Liu, Q.; Li, J.; Shi, D.; Ning, S.; Cheng, L. Quantitative Evaluation of the Effects of Urinary Stone Composition and Size on Color Doppler Twinkling Artifact: A Phantom Study. J. Ultrasound Med. 2016, 36, 733–740. [Google Scholar] [CrossRef] [Green Version]
- Bardin, T.; Tran, K.M.; Nguyen, Q.D.; Sarfati, M.; Richette, P.; Vo, N.T.; Bousson, V.; Correas, J.-M. Renal medulla in severe gout: Typical findings on ultrasonography and dual-energy CT study in two patients. Ann. Rheum. Dis. 2018, 78, 433–434. [Google Scholar] [CrossRef]
- Gamala, M.; Jacobs, J.W.G.; Van Laar, J.M. The diagnostic performance of dual energy CT for diagnosing gout: A systematic literature review and meta-analysis. Rheumatology 2019, 58, 2117–2121. [Google Scholar] [CrossRef]
- Piani, F.; Johnson, R.J. Does gouty nephropathy exist, and is it more common than we think? Kidney Int. 2021, 99, 31–33. [Google Scholar] [CrossRef]
- Oh, Y.J.; Moon, K.W. Presence of tophi is associated with a rapid decline in the renal function in patients with gout. Sci. Rep. 2021, 1, 5684. [Google Scholar] [CrossRef]
- Cossey, L.N.; Dvanajscak, Z.; Larsen, C.P. A diagnostician’s field guide to crystalline nephropathies. Sem. Diagn. Pathol. 2020, 37, 135–142. [Google Scholar] [CrossRef]
- Mulay, S.R.; Anders, H.-J. Crystal nephropathies: Mechanisms of crystal-induced kidney injury. Nat. Rev. Nephrol. 2017, 13, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, C.A.; Batuman, V.; Behrens, J.; Bridoux, F.; Sirac, C.; Dispenzieri, A.; Herrera, G.A.; Lachmann, H. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat. Rev. Nephrol. 2012, 8, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Durfee, E.M. Tumor lysis syndrome. Crit Care Nurse 2022, 42, 19–25. [Google Scholar] [CrossRef]
- Landgren, A.J.; Jacobsson LT, H.; Lindström, U.; Sandström TZ, S.; Drivelegka, P.; Björkman, L.E.; Dehlin, M. Incidence of and risk factors for nephrolithiasis in patients with gout and the general population, a cohort study. Arthritis Res. Ther. 2017, 19, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daudon, M.; Traxer, O.; Conort, P.; Lacour, B.; Jungers, P. Type 2 diabetes increases the risk for uric acid stones. J. Am. Soc. Nephrol. 2006, 17, 2026–2033. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Dalbeth, N.; Yin, H.; Li, C.; Merriman, T.R.; Wei, W.-H. Mouse models for human hyperuricaemia: A critical review. Nat. Rev. Rheumatol. 2019, 15, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Preitner, F.; Bonny, O.; Laverriere, A.; Rotman, S.; Firsov, D.; Da Costa, A.; Metref, S.; Thorens, B. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc. Natl. Acad. Sci. USA 2009, 106, 15501–15506. [Google Scholar] [CrossRef] [Green Version]
- Stavric, B.; Johnson, W.J.; Grice, H.C. Uric nephropathy: An experimental model. Proc. Sot. Exp. Biol. Med. 1969, 130, 512–516. [Google Scholar] [CrossRef]
- Waisman, J.; Bluestone, R.; KLlinenberger, J.R. A preliminary report of nephropathy in hyperuricemic rats. Lab. Inv. 1974, 30, 716–722. [Google Scholar]
- Guan, J.; Huang, X.-Q.; Dong, J.-L.; Lu, H.-M.; Lin, Y.-W.; Liu, M.; Yi, Z.-B.; Wu, L.-M.; Huang, Y.-M.; Lan, T. A novel mouse model of hyperuricemia and gouty nephropathy. Chin. Med. J. 2020, 133, 2012–2014. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wakamiya, M.; Vaishnav, S.; Geske, R.; Montgomery, C.; Jones, P.; Bradley, A.; Caskey, C.T. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc. Natl. Acad. Sci. USA 1994, 91, 742–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Hou, X.; Yuan, X.; Cui, L.; Liu, Z.; Li, X.; Ma, L.; Cheng, X.; Xin, Y.; Wang, C.; et al. Knockout of the urate oxidase gene provides a stable mouse model of hyperuricemia associated with metabolic disorders. Kidney Int. 2018, 93, 69–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Zhang, N.; Dong, X.; Fan, N.; Wang, L.; Xu, Y.; Chen, H.; Duan, W. Uricase-deficient rat is generated with CRISPR/Cas9 technique. PeerJ 2020, 8, e8971. [Google Scholar] [CrossRef] [PubMed]
- Preitner, F.; Laverriere-Loss, A.; Metref, S.; Da Costa, A.; Moret, C.; Rotman, S.; Bazin, D.; Daudon, M.; Sandt, C.; Dessombz, A.; et al. Urate-induced acute renal failure and chronic inflammation in liver-specific Glut9 knockout mice. Am. J. Physiol. Physiol. 2013, 305, F786–F795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellmayr, M.; Petzsche, M.R.H.; Ma, Q.; Krüger, N.; Liapis, H.; Brink, A.; Lenz, B.; Angelotti, M.L.; Gnemmi, V.; Kuppe, C.; et al. Only Hyperuricemia with Crystalluria, but not Asymptomatic Hyperuricemia, Drives Progression of Chronic Kidney Disease. J. Am. Soc. Nephrol. 2020, 31, 2773–2792. [Google Scholar] [CrossRef]
- Preitner, F.; Pimentel, A.; Metref, S.; Berthonneche, C.; Sarre, A.; Moret, C.; Rotman, S.; Centeno, G.; Firsov, D.; Thorens, B. No development of hypertension in the hyperuricemic liver-Glut9 knockout mouse. Kidney Int. 2015, 87, 940–947. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Honarpisheh, M.; Li, C.; Sellmayr, M.; Lindenmeyer, M.; Böhland, C.; Romagnani, P.; Anders, H.-J.; Steiger, S. Soluble Uric Acid Is an Intrinsic Negative Regulator of Monocyte Activation in Monosodium Urate Crystal–Induced Tissue Inflammation. J. Immunol. 2020, 205, 789–800. [Google Scholar] [CrossRef]
- Jordan, D.M.; Choi, H.K.; Verbanck, M.; Topless, R.; Won, H.-H.; Nadkarni, G.; Merriman, T.R.; Do, R. No causal effects of serum urate levels on the risk of chronic kidney disease: A Mendelian randomization study. PLoS Med. 2019, 16, e1002725. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Meng, X.; Timofeeva, M.; Tzoulaki, I.; Tsilidis, K.K.; Ioannidis, J.P.; Campbell, H.; Theodoratou, E. Serum uric acid levels and multiple health outcomes: Umbrella review of evidence from observational studies, randomised controlled trials, and mendelian randomisation studies. BMJ 2017, 357, j2376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef] [Green Version]
- Klinkhammer, B.M.; Djudjaj, S.; Kunter, U.; Palsson, R.; Edvardsson, V.O.; Wiech, T.; Thorsteinsdottir, M.; Hardarson, S.; Foresto-Neto, O.; Mulay, S.R.; et al. Cellular and molecular mechanisms of kidney injury in 2,8-dihydroxyadenine nephropathy. J. Am. Soc. Nephrol. 2020, 31, 799–816. [Google Scholar] [CrossRef]
- Bouderlique, E.; Tang, E.; Perez, J.; Ea, H.K.; Renaudin, F.; Coudert, A.; Vandermeersch, S.; Bazin, D.; Haymann, J.-P.; Saint-Jacques, C.; et al. Inflammation plays a critical role in 2,8-dihydroxyadenine nephropathy. Comptes Rendus Chim. 2022, 25, 393–405. [Google Scholar] [CrossRef]
- Ankem, M.; Glazier, D.B.; Barone, J.G. Lesch-Nyhan syndrome presenting as acute renal failure secondary to obstructive uropathy. Urology 2000, 56, 1056. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardin, T.; Letavernier, E.; Correas, J.-M. The Gouty Kidney: A Reappraisal. Gout Urate Cryst. Depos. Dis. 2023, 1, 25-36. https://doi.org/10.3390/gucdd1010004
Bardin T, Letavernier E, Correas J-M. The Gouty Kidney: A Reappraisal. Gout, Urate, and Crystal Deposition Disease. 2023; 1(1):25-36. https://doi.org/10.3390/gucdd1010004
Chicago/Turabian StyleBardin, Thomas, Emmanuel Letavernier, and Jean-Michel Correas. 2023. "The Gouty Kidney: A Reappraisal" Gout, Urate, and Crystal Deposition Disease 1, no. 1: 25-36. https://doi.org/10.3390/gucdd1010004
APA StyleBardin, T., Letavernier, E., & Correas, J.-M. (2023). The Gouty Kidney: A Reappraisal. Gout, Urate, and Crystal Deposition Disease, 1(1), 25-36. https://doi.org/10.3390/gucdd1010004




