Abstract
Local heating was performed on a thermoplastic polymer film by contact with the tip of a soldering iron heated above the glass-transition temperature. The locally heated area was measured using microscopic Raman scattering spectroscopy, and the spatial distribution of the crystallinity was obtained from the low-frequency peak. The crystallinity distribution can be evaluated using the microscale spatial resolution. The temperature distribution around the locally heated area was calculated by applying the heat conduction equation, and good correspondence was obtained with the obtained crystallinity.
1. Introduction
Polyether ether ketone (PEEK, Figure 1) is a thermoplastic polymer with excellent mechanical, chemical, and thermal stabilities [1]. Compared with thermosetting polymers, it is expected to have shorter molding times, a lower cost, and higher productivity [2]. It is also attracting attention as a substrate for carbon fiber-reinforced plastics (CFRPs), which are expected to replace steel materials and are being considered as high-performance polymers for a wide range of applications, from automotive and aerospace to dental and orthopedic biomaterials. Its mechanical, optical, electrical, and other properties are highly dependent on its degree of crystallinity [3,4,5,6,7,8,9,10,11,12]. Therefore, evaluation of the degree of its crystallinity is of great importance [13,14,15].
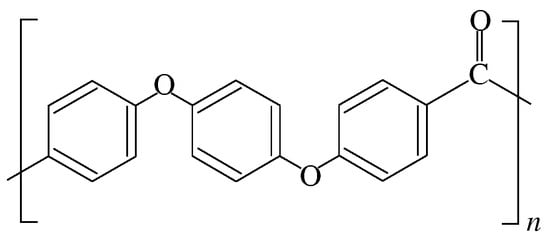
Figure 1.
Chemical structure of PEEK.
Various techniques have been used to evaluate the crystallinity of polymers. Representative techniques include wide-angle X-ray diffraction (WAXRD), differential scanning calorimetry (DSC) [16], Fourier-transform infrared spectroscopy [13], and Raman spectroscopy [14,15,17,18,19,20]. Raman spectroscopy is one of the most commonly used techniques for investigating polymer materials [21]. In addition, high spatial resolution can be achieved using microscopic optics. It has also been reported that carbon fibers promote crystallization on PEEK [22]. Therefore, high spatial resolution is a useful feature for comparing the degree of crystallinity near and far from carbon fibers in composite materials.
Using the high spatial resolution of micro-Raman scattering spectroscopy, Uematsu et al. reported that in carbon fiber-reinforced polyamide (PA6) composites, various polymorphs (α-phase, intermediate β-phase, and γ-phase crystals) exist near the carbon fiber [23]. The spatial distribution of crystallinity has also been reported using micro-Raman scattering spectroscopy. Yang et al. used Raman spectroscopy to investigate the spatial distribution of crystallinity in poly(ε-caprolactone) (PCL) films and reported that the crystallinity was not uniform throughout the film [24]. Doumeng et al. measured the spatial distribution of crystallinity in wear tracks in PEEK and glass-fiber-reinforced PEEK composites and reported that crystallinity was heterogeneous within the wear tracks [15]. Segawa et al. performed 3D Raman mapping measurements on poly(ε-caprolactone) (PCL) films during marine degradation and reported that crystallinity changed mainly near the film surface [25]. In particular, in recent years, Raman scattering in the low-frequency range has attracted attention in various fields, along with terahertz-range absorption spectroscopic techniques [26,27,28,29]. In some polymer materials, the peaks obtained by low-frequency Raman scattering spectroscopy [30,31,32,33] and THz vibrational spectroscopy [26,27,28,29] have been discussed as being related to intermolecular vibrational modes. It has also been reported that the intensity of low-frequency vibrational peaks in PEEK depends on the degree of crystallinity [34,35], and the origin of these peaks has been explained using molecular dynamics simulations [36].
Laser processing is expected to be applied to polymer materials, including CFRP, as the next-generation processing method [37,38]. It is expected that the crystallinity will change locally owing to the local heating caused by laser processing. Evaluating the spatial distribution of crystallinity owing to local heating in thermoplastic polymers is an important issue for high-quality processing.
In this study, low-frequency Raman scattering spectroscopy was used to measure the spatial distribution of crystallinity caused by crystallization in locally heated amorphous PEEK samples upon heating to the glass-transition temperature.
2. Materials and Methods
2.1. Sample Preparation
Sheets of PEEK polymer (Victrex, Cleveland, UK, Grade 2000-025G, 15 mm × 15 mm, t = 25 µm) were used as samples. Local heating was performed by bringing the tip (R = 0.6 mm) of a temperature-controlled soldering iron (Hozan Corporation, Osaka, Japan, HS-51, 25 mm tip curvature) in contact with the sample surface in atmosphere. As shown in Figure 2, the sample was placed on a microbalance (Conkoo, MH-500, 0.01–500 g), and the load on the microbalance was used to confirm that the tip of soldering iron and sample were in contact. Heating was performed under a load of 2.0 g. The temperature at the tip of the soldering iron was 300 °C, and the heating time was 60 s. After 60 s, the tip of the soldering iron was lifted, and the sample was left to cool in air.
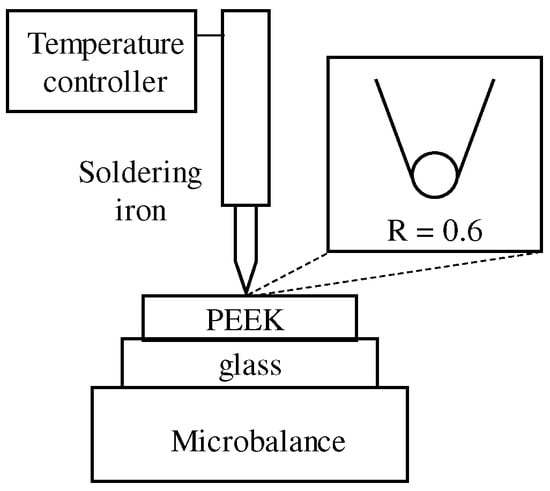
Figure 2.
Schematic diagram of point heating system.
2.2. Experiments
The local heated area was observed by optical microscopy, and the Raman spectra were obtained using micro-Raman spectroscopy (LabRAM HR Evolution, Horiba, Kyoto, Japan). The micro-Raman system consisted of laser (1064 nm, 200 mW), an edge filter (Horiba, Ultra low frequency Raman module, <20 cm−1) to remove the scattered excitation laser, a single spectrograph, and InGaAs detector cooled by liquid nitrogen. The focal length of the spectrograph was 800 mm, and a 300 L/mm grating was used. All measurements were performed at room temperature. The laser power was limited to approximately 200 mW on the sample to minimize the heating effects. A 50× objective lens was used to focus the laser spot to a diameter of approximately 1 µm. The samples were then moved under a microscope using a computer-controlled XY stage. The measurement time was 30 s, and the number of integration times was three. Line measurements were carried out with the center of heating as the origin, sufficiently far from the heated area.
3. Results
Optical microscopy images of the local heating area are shown in Figure 3(1). A circle with a radius of approximately 100 µm, where the tip is expected to be in contact, and a discolored region with a radius of approximately 500 µm centered on the heating point were observed. The Raman spectra of points A and B, indicated by arrows in Figure 3(1), are shown in Figure 3(2), where A and B are the typical Raman spectra of the heated and unheated regions, respectively. In spectrum A of the discolored region, three peaks at 50 cm−1, 97 cm−1, and 135 cm−1 were observed. The peak at 97 cm−1 is assigned to the Ph-O-Ph vibration, and the peaks at 50 cm−1 and 135 cm−1 are assigned to the Ph-CO-Ph vibration. In the spectrum B, the peaks at 50 cm−1 and 97 cm−1 have disappeared. In previous studies, spectra with this feature have been reported to be due to amorphous PEEK. The crystallinity of the area in which this spectrum was observed for amorphous PEEK was set as 0.
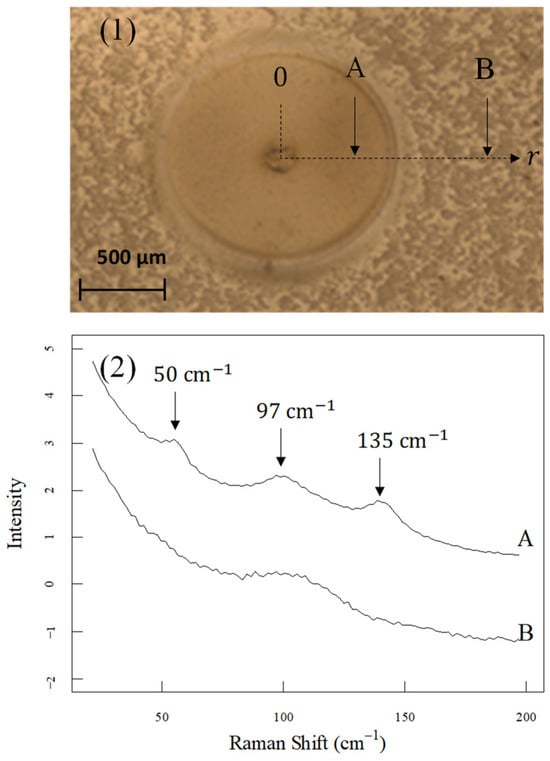
Figure 3.
(1) Optical microscope image of the heated spot. The positions where the Raman spectra were measured are indicated by arrows. (2) The Raman spectra were measured at positions A and B. The spectra were shifted to the ordinate for convenient display.
The crystallinity was calculated from the intensity ratio at 97 cm−1 and 135 cm−1 obtained by fitting analysis, as reported in a previous study [34]. The intensity of the Raman spectra in the low-frequency region is often corrected by the Bose–Einstein factor, as it is influenced by the measurement temperature [39]. To maintain consistency with a previous study [34] that calculated spectral intensity without the Bose–Einstein factor correction, our spectra have not been corrected. The two peaks are represented by a Gaussian function, and the baseline is a straight line. An example of the fitting analysis is presented in Figure 4. The open circle shows the experimental data, and the solid curve shows the fitting result. The model function accurately reproduced the experimental data. In addition, the fitting elements used are indicated by the dashed lines.
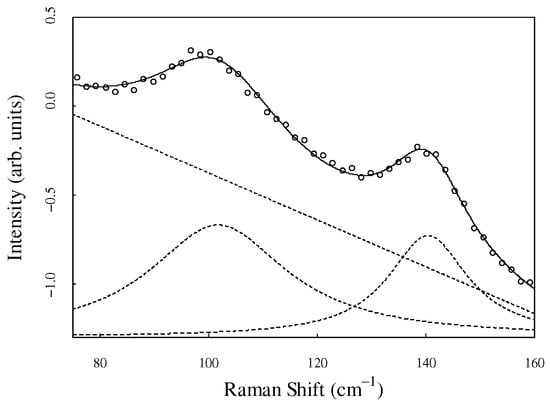
Figure 4.
Fitting results of Raman spectra. The open circles represent the experimental data. The solid lines represent the fitting results. Dashed lines are the decomposed elements (two Lorentzian functions and baseline).
Figure 5 shows the crystallinity distribution obtained using Raman spectroscopy. The horizontal axis represents the distance r from the center of the heating point. Crystallinity was almost constant up to approximately 100 µm. The point at which crystallinity began to decrease was designated as r1. r1 is thought to be the area where the tip is in contact with the polymer. The crystallinity gradually decreases from r1, dropping sharply at approximately 500 µm. The position at which the crystallinity became zero was designated as r2. The area outside r2 is thought to be the area where the temperature of the sample was below the glass-transition temperature. We demonstrated that the spatial distribution of crystallinity over a few microns caused by localized heating can be evaluated using Raman scattering spectroscopy.
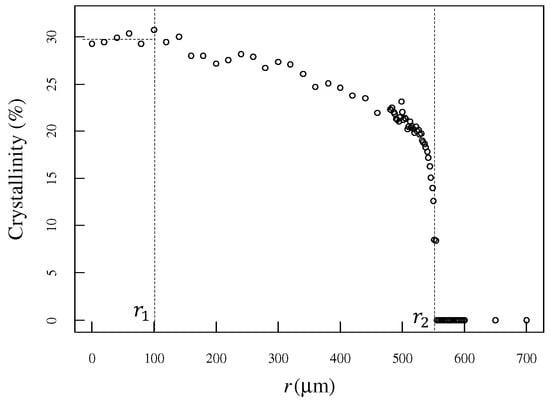
Figure 5.
The degree of crystallinity was evaluated in the radial direction, with the center of heating as the origin. The degree of crystallinity was constant at the center of the heating, and the position where the degree of crystallinity began to decrease was designated r1. The degree of crystallinity gradually decreased. The position where it decreased suddenly and where the degree of crystallinity became zero was designated as r2.
From the crystallinity obtained from Raman spectroscopy, we considered the temperature distribution within the film surface owing to localized heating. Assuming that the sample is sufficiently thin, the temperature is constant in the depth direction. The heat conduction equation at a distance r from the heating point is expressed as follows.
The radius of the heated area where the tip of the soldering iron was in contact was r1, the temperature of the soldering iron was T1, the position where the crystallinity was 0 was r2, and the glass-transition temperature was T2. The boundary condition is:
T1 and T2 were set to 300 °C, and the glass-transition temperature was set to 143 °C. As shown in Figure 4, r1 and r2 are set to 100 µm and 554 µm, respectively.
Solving Equation (1) under the conditions of Equation (2) yields:
According to Hertz’s contact theory, the contact radius between the copper and PEEK at room temperature is approximately 30 µm. The Young’s modulus of PEEK is known to increase sharply from the glass-transition temperature, and if it is estimated to be approximately 1% of the room temperature, the contact radius would be approximately 130 µm; therefore, r1 = 100 µm is considered reasonable.
Equation (3) was used to convert the distance r from the heating point to the temperature during heating. The relationship between the temperature distribution during heating and crystallinity is shown in Figure 6. The filled circles indicate the results of previously reported data on the heat treatment temperature and crystallinity of samples heat-treated in a conventional electric furnace [34]; those results are in good agreement with the results of this study.
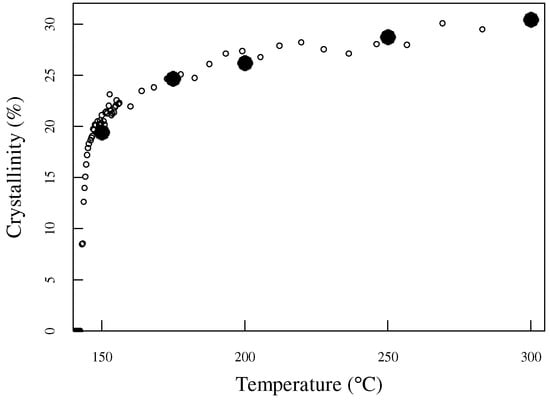
Figure 6.
The horizontal axis was converted to the temperature using the thermal conduction method. The results of a previous study, in which heat treatment was performed using an electric furnace, are shown with filled circles.
4. Conclusions
Local heating was performed on a thermoplastic polymer film by contact with the tip of a soldering iron heated above the glass-transition temperature. The locally heated area was measured using microscopic Raman scattering spectroscopy, and the spatial distribution of the crystallinity was obtained from the low-wavenumber peak. The crystallinity distribution can be evaluated using the microscale spatial resolution. The temperature distribution around the locally heated area was calculated by applying the heat conduction equation, and good correspondence was obtained with the obtained crystallinity. In applications that require a high degree of reliability in the mechanical strength of PEEK polymer, such as laser welding in the medical and aerospace fields, crystallinity with micro-level spatial resolution is essential, and it has been shown that evaluation of crystallinity using Raman scattering spectroscopy is useful.
Author Contributions
Conceptualization: T.S., K.C.G. and M.Y.; experiment: T.N. and M.Y.; validation: M.Y.; supervision, N.I. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI [grant number 23H01310].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Tomoko Numata was employed by the company Horiba Techno Service Co., Ltd. Author Naomoto Ishikawa was employed by the company Kiguchi Technics Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Blundell, D.J.; Osborn, B.N. The morphology of poly(aryl-ether-ether-ketone). Polymer 1983, 24, 953–958. [Google Scholar] [CrossRef]
- Biron, M. Handbook of Thermoplastic Elastomers; William Andrew Publishing: Norwich, NY, USA, 2012. [Google Scholar]
- Gao, S.-L.; Kim, J.-K. Cooling rate influences in carbon fibre/PEEK composites. Part 1. Crystallinity and interface adhesion. Compos. Part A Appl. Sci. Manuf. 2000, 31, 517–530. [Google Scholar] [CrossRef]
- Huo, P.; Cebe, P. Temperature-dependent relaxation of the crystal-amorphous interphase in poly(ether ether ketone). Macromolecules 1992, 25, 902–909. [Google Scholar] [CrossRef]
- Cogswell, F.N.; Hopprich, M. Environmental resistance of carbon fiber-reinforced polyether etherketone. Composites 1983, 14, 251–253. [Google Scholar] [CrossRef]
- Comer, A.J.; Ray, D.; Obande, W.O.; Jones, D.; Lyons, J.; Rosca, I.; O’Higgins, R.M.; McCarthy, M.A. Mechanical characterization of carbon fibre–PEEK manufactured by laser-assisted automated-tape-placement and autoclave. Compos. Part A Appl. Sci. Manuf. 2015, 69, 10–20. [Google Scholar] [CrossRef]
- Deignan, A.; Stanley, W.; McCarthy, M. Insights into wide variations in carbon fiber/polyetheretherketone rheology data under automated tape placement processing conditions. J. Compos. Mater. 2018, 52, 2213–2228. [Google Scholar] [CrossRef]
- Yang, D.; Cao, Y.; Zhang, Z.; Yin, Y.; Li, D. Effects of crystallinity control on mechanical properties of 3D-printed short-carbon-fiber-reinforced polyether ether ketone composites. Polym. Test. 2021, 97, 107149. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, C.; Chen, Z.; Huang, Z.; Zhou, H. Effect of crystallinity on optical properties of PEEK prepreg tapes for laser-assisted automated fiber placement. Compos. Commun. 2023, 38, 101490. [Google Scholar] [CrossRef]
- Rusakov, D.; Menner, A.; Spieckermann, F.; Wilhelm, H.; Bismarck, A. Morphology and properties of foamed high crystallinity PEEK prepared by high temperature thermally induced phase separation. J. Appl. Polym. Sci. 2021, 139, 5143. [Google Scholar] [CrossRef]
- Zhen, H.; Zhao, B.; Quan, L.; Fu, J. Effect of 3D Printing Process Parameters and Heat Treatment Conditions on the Mechanical Properties and Microstructure of PEEK Parts. Polymers 2023, 15, 2209. [Google Scholar] [CrossRef]
- Katsifis, G.A.; Suchowerska, N.; McKenzie, D.R. Optical properties of plasma-treated PEEK: Monitoring colour and crystallinity for applications in medicine and dentistry using ellipsometry. Plasma Process. Polym. 2022, 19, 2100241. [Google Scholar] [CrossRef]
- Regis, M.; Bellare, A.; Pascolini, T.; Bracco, P. Characterization of thermally annealed PEEK and CFR-PEEK composites: Structure-properties relationships. Polym. Degrad. Stab. 2017, 136, 121–130. [Google Scholar] [CrossRef]
- Doumeng, M.; Makhlouf, L.; Berthet, F.; Marsan, O.; Delbé, K.; Denape, J.; Chabert, F. A comparative study of the crystallinity of polyetheretherketone by using density, DSC, XRD, and Raman spectroscopy techniques. Polym. Test. 2021, 93, 106878. [Google Scholar] [CrossRef]
- Doumeng, M.; Ferry, F.; Delbé, K.; Mérian, T.; Chabert, F.; Berthet, F.; Marsan, O.; Nassiet, V.; Denape, J. Evolution of crystallinity of PEEK and glass-fibre reinforced PEEK under tribological conditions using Raman spectroscopy. Wear 2019, 426–427, 1040–1046. [Google Scholar] [CrossRef]
- Bas, C.; Battesti, P.; Albérola, N.D. Crystallization and melting behaviors of poly(aryletheretherketone) (PEEK) on origin of double melting peaks. J. Appl. Polym. Sci. 1994, 53, 1745–1757. [Google Scholar] [CrossRef]
- Louden, J.D. Crystallinity in poly(aryl ether ketone) films studied by raman spectroscopy. Polym. Commun. 1986, 27, 82–84. [Google Scholar]
- Everall, N.J.; Chalmers, J.M.; Ferwerda, R.; van der Maas, J.H.; Hendra, P.J. Measurement of poly(aryl ether ether ketone) crystallinity in isotropic and uniaxial samples using Fourier transform-Raman spectrocopy: A comparison of univariate and partial least-squares calibrations. J. Raman Spectrosc. 1994, 25, 43–51. [Google Scholar] [CrossRef]
- Briscoe, B.J.; Stuart, B.H.; Thomas, P.S.; Williams, D.R. A comparison of thermal- and solvent-induced relaxation of poly(ether ether ketone) using Fourier transform Raman spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1991, 47, 1299–1303. [Google Scholar] [CrossRef]
- Ellis, G.; Naffakh, M.; Marco, C.; Hendra, P.J. Fourier transform Raman spectroscopy in the study of technological polymers Part 1: Poly(aryl ether ketones), their composites and blends. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1997, 53, 2279–2294. [Google Scholar] [CrossRef]
- Hsu, S.L.; Patel, J.; Zhao, W. Molecular Characterization of Polymers: A Fundamental Guide; Chapter 10; Elsevier Science: Amsterdam, The Netherlands, 2021; p. 369. [Google Scholar]
- Lee, Y.; Porter, R.S. Crystallization of poly(etheretherketone) (PEEK) in carbon fiber composites. Polym. Eng. Sci. 1986, 26, 633–639. [Google Scholar] [CrossRef]
- Uematsu, H.; Kawasaki, T.; Koizumi, K.; Yamaguchi, A.; Sugihara, S.; Yamane, M.; Kawabe, K.; Ozaki, Y.; Tanoue, S. Relationship between crystalline structure of polyamide 6 within carbon fibers and their mechanical properties studied using Micro-Raman spectroscopy. Polymer 2021, 223, 123711. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, M.; Li, H.; Li, H. The degree of crystallinity exhibiting a spatial distribution in polymer films. Eur. Polym. J. 2018, 107, 303–307. [Google Scholar] [CrossRef]
- Segawa, T.; Ito, K.; Hatayama, M.; Maruyama, Y.; Gao, J.; Ueno, N.; Sato, H. Visualization of Changes in Crystallinity and Intermolecular Hydrogen Bonding of Poly(ε-caprolactone) during Marine Degradation Process by High- and Low-Frequency Three-Dimensional Raman Mapping. Appl. Polym. Mater. 2024, 6, 6408–6415. [Google Scholar] [CrossRef]
- Funaki, C.; Yamamoto, S.; Hoshina, H.; Ozaki, Y.; Sato, H. Three different kinds of weak C-H⋯O=C inter- and intramolecular interactions in poly(ε-caprolactone) studied by using terahertz spectroscopy, infrared spectroscopy and quantum chemical calculations. Polymer 2018, 137, 245–254. [Google Scholar] [CrossRef]
- Funaki, C.; Toyouchi, T.; Hoshina, H.; Ozaki, Y.; Sato, H. Terahertz imaging of the distribution of crystallinity and crystalline orientation in a poly(ε-caprolactone) film. Appl. Spectrosc. 2017, 71, 1537–1542. [Google Scholar] [CrossRef]
- Kaneko, T.; Hirai, N.; Ohki, Y. Terahertz absorption spectroscopy of poly(ether ether ketone). In Proceedings of the 2017 International Symposium on Electrical Insulating Materials, Toyohashi, Japan, 11–15 September 2017; pp. 539–542. [Google Scholar] [CrossRef]
- Hiroshiba, N.; Akiraka, M.; Kojima, H.; Ohnishi, S.; Ebata, A.; Tsuji, H.; Tanaka, S.; Koike, K.; Ariyoshi, S. Broadband infrared absorption spectroscopy of low-frequency inter-molecular vibrations in crystalline poly(L-lactide). Phys. B Condens. Matter 2023, 649, 414488. [Google Scholar] [CrossRef]
- Walker, G.; Römann, P.; Poller, B.; Löbmann, K.; Grohganz, H.; Rooney, J.S.; Huff, G.S.; Smith, G.P.S.; Rades, T.; Gordon, K.C.; et al. Probing pharmaceutical mixtures during milling: The potency of low-frequency raman spectroscopy in identifying disorder. Mol. Pharm. 2017, 14, 4675–4684. [Google Scholar] [CrossRef]
- Lipiäinen, T.; Fraser-Miller, S.J.; Gordon, K.C.; Strachan, C.J. Direct comparison of low- and mid-frequency Raman spectroscopy for quantitative solid-state pharmaceutical analysis. J. Pharm. Biomed. Anal. 2018, 149, 343–350. [Google Scholar] [CrossRef]
- Yamamoto, S.; Ohnishi, E.; Sato, H.; Hoshina, H.; Ishikawa, D.; Ozaki, Y. Low-frequency vibrational modes of Nylon 6 studied by using infrared and raman spectroscopies and density functional theory calculations. J. Phys. Chem. B 2019, 123, 5368–5376. [Google Scholar] [CrossRef]
- Yamamoto, S.; Miyada, M.; Sato, H.; Hoshina, H.; Ozaki, Y. Low-Frequency Vibrational Modes of poly(glycolic acid) and Thermal Expansion of Crystal Lattice Assigned on the Basis of DFT-Spectral Simulation Aided with a Fragment Method. J. Phys. Chem. B 2017, 121, 1128–1138. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Kobayasi, S.; Numata, T.; Kamihara, N.; Shimda, T.; Jikei, M.; Muraoka, M.; Barnsley, J.E.; Fraser-Miller, S.J.; Gordon, K.C. Evaluation of crystallinity in carbon fiber-reinforced poly(ether ether ketone) by using infrared low frequency Raman spectroscopy. J. Appl. Polym. Sci. 2022, 139, 51677. [Google Scholar] [CrossRef]
- Numata, T.; Ishikawa, N.; Shimada, T.; Gordon, K.C.; Yamaguchi, M. Low-Frequency Raman Spectroscopy on Amorphous Poly(Ether Ether Ketone) (PEEK). Materials 2024, 17, 3755. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yokokura, S.; Nagahama, T.; Yamaguchi, M.; Shimada, T. Molecular Dynamics simulation of poly(ether ether ketone) (PEEK) polymer to analyze intermolecular ordering by low wavenumber raman spectroscopy and X-ray diffraction. Polymers 2022, 14, 5406. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Brodesser, A.; Hustedt, M.; Bluemel, S.; Jaeschke, P.; Kaierle, S. Laser Processing of Carbon Fiber Reinforced Plastics—Release of Carbon Fiber Segments During Short-pulsed Laser Processing of CFRP. Phys. Procedia 2016, 83, 1021–1030. [Google Scholar] [CrossRef]
- Li, Q.; Perrie, W.; Tang, Y.; Allegre, O.; Ho, J.; Chalker, P.; Li, Z.; Edwardson, S.; Dearden, G. A study on ultrafast laser micromachining and optical properties of amorphous polyether(ether)ketone (PEEK) films. Procedia CIRP 2020, 94, 840–845. [Google Scholar] [CrossRef]
- Guinet, Y.; Paccou, L.; Hédoux, A. Low-Frequency Raman Spectroscopy: An Exceptional Tool for Exploring Metastability Driven States Induced by Dehydration. Pharmaceutics 2023, 15, 1955. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).