Effect of Acacia melanoxylon R. Br. Inclusion on the Chemical Composition, Fermentation Dynamics, and In Vitro Digestibility of Medicago sativa L. Silage
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Conditions
2.2. Sample Collection and Preparation
2.3. Silage Preparation
2.4. Chemical Analysis
2.5. In Vitro Digestibility, Gas Production, and Energy Estimation
2.6. Rumen Fluid Collection
2.7. Statistical Analysis
3. Results
3.1. Pre-Ensiling Chemical and Digestibility Profiles of M. sativa and A. melanoxylon
3.1.1. Pre-Ensiling Gas Production Profiles of M. sativa and A. melanoxylon
3.1.2. Effect of Ensiling on M. sativa Composition and Digestibility
3.2. Chemical Analysis of M. sativa Silages with Different Inclusion Levels of A. melanoxylon
3.2.1. Fermentation Profile of Silages
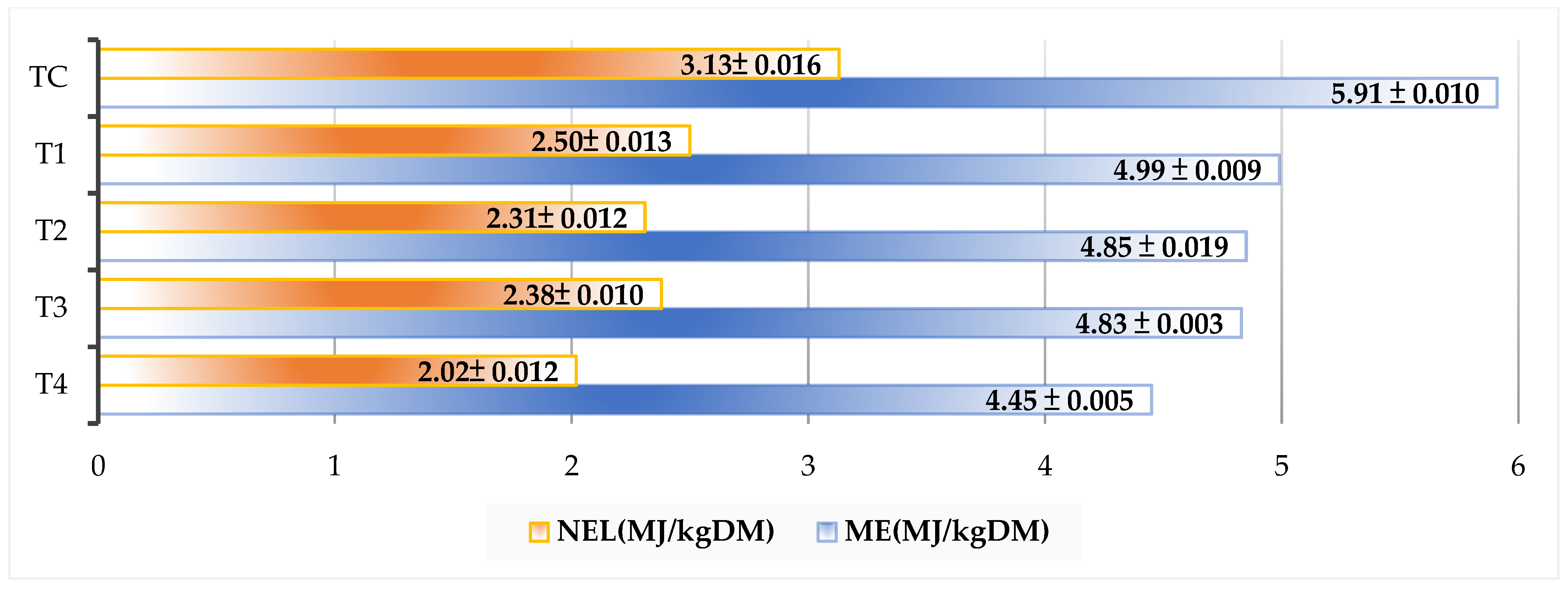
3.2.2. Estimated Energy Content of Silages
3.2.3. Gas Production Kinetics and Fermentability of Silages
4. Discussion
4.1. Impact of Ensiling on Fresh M. sativa Quality
4.2. Chemical Composition and Gas Production Kinetics Prior to Ensiling
4.3. Effect of A. melanoxylon Inclusion on Silage Composition and Digestibility
4.4. Energy Value and In Vitro Fermentation After Ensiling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Kung, L., Jr.; Shaver, R. Interpretation of silage fermentation analyses. Proc. Am. Forage Grassl. Counc. 2001, 29, 1–6. [Google Scholar]
- Gao, R.; Wang, B.; Jia, T.; Luo, Y.; Yu, Z. Effects of different carbohydrate sources on alfalfa silage quality at different ensiling days. Agriculture 2021, 11, 58. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Tetbury, UK, 1991. [Google Scholar]
- Rooke, J.A.; Hatfield, R.D. Biochemistry of Forage Cell Walls and Their Digestion. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 2003; pp. 95–139. [Google Scholar]
- Grum, D.E.; Shockey, W.L. Weiss Electrophoretic examination of alfalfa silage proteins. J. Dairy Sci. 1991, 74, 146–154. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Davies, D.R. The fermentation profile and effluent losses from silage. Grass Forage Sci. 2013, 68, 1–14. [Google Scholar] [CrossRef]
- Tao, L.; Zhou, H.; Zhang, N.; Si, B.; Tu, Y.; Ma, T.; Diao, Q. Effects of different source additives and wilt conditions on the pH value, aerobic stability, and carbohydrate and protein fractions of alfalfa silage. Anim. Sci. J. 2017, 88, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, Z.G.; Muck, R.E. New trends and opportunities in the development and use of inoculants for silage. FEMS Microbiol. Rev. 1996, 19, 53–68. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Dong, Z.; Chen, L.; Shao, T. Inclusion of alfalfa improves nutritive value and in vitro digestibility of various straw–grass mixed silages in Tibet. Grass Forage Sci. 2018, 73, 694–704. [Google Scholar] [CrossRef]
- Naumann, H.D.; Tedeschi, L.O.; Muir, J.P.; Lambert, B.D. Structural features of condensed tannins affect in vitro ruminal methane production and fermentation characteristics. J. Agric. Sci. 2016, 154, 1474–1487. [Google Scholar]
- Lee, M.R.F.; Fishwick, J.; Toovey, A.F.; Woodward, S.L.; Scollan, N.D. Red clover silage improves nitrogen utilisation and reduces ammonia emissions. Anim. Feed Sci. Technol. 2020, 265, 114507. [Google Scholar]
- Zhang, B.; Xu, Q.; Qin, G.; Yan, T. Effects of mixed ration silages combining legume forage with woody biomass. Bioresour. Technol. 2021, 326, 124733. [Google Scholar]
- Vieites-Blanco, C.; González-Prieto, S.J. Invasiveness, ecological impacts and control of acacias in southwestern Europe—A review. Web Ecol. 2020, 20, 33–51. [Google Scholar] [CrossRef]
- Pereira, A.; Ferreira, V.; Figueiredo, A. Invasive Acacia tree species affect instream litter decomposition through changes in water nitrogen concentration and litter characteristics. Microb. Ecol. 2021, 82, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, R.C.; Shelton, H.M. Forage Tree Legumes in Tropical Agriculture, 2nd ed.; FAO: Rome, Italy, 2005. [Google Scholar]
- Maduro Dias, C.S.A.M.; Nunes, H.; Vouzela, C.; Madruga, J.; Borba, A. In Vitro Rumen Fermentation Kinetics Determination and Nutritional Evaluation of Several Non-Conventional Plants with Potential for Ruminant Feeding. Fermentation 2023, 9, 416. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Wilkinson, J.M. Silage. In Quantitative Aspects of Ruminant Digestion and Metabolism, 2nd ed.; Dijkstra, J., Forbes, J.M., France, J., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 259–272. [Google Scholar]
- Maduro Dias, C.; Machado, M.; Nunes, H.; Borba, A.; Madruga, J.; Monjardino, P. Nitrogen Fertilization Using Conventional and Slow-Release Fertilizers at Multiple Levels in Lolium multiflorum Lam. Pastures. Agronomy 2024, 14, 2191. [Google Scholar] [CrossRef]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis, 12th ed.; AOAC: Washington, DC, USA, 1999. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analysis (Apparatus, Reagents, Procedures, and Some Applications); Agriculture Handbook No. 379; USDA-ARS: Washington, DC, USA, 1970.
- Kung, L.; Grieve, D.B.; Thomas, J.W.; Huber, J.T. Added ammonia or microbial inocula for fermentation and nitrogenous compounds of alfalfa ensiled at various percents of dry matter. J. Dairy Sci. 1984, 67, 299–306. [Google Scholar] [CrossRef]
- Moselhy, M.A.; Borba, J.P.; Borba, A.E.S. Improving the nutritive value, in vitro digestibility and aerobic stability of Hedychium gardnerianum silage through application of additives at ensiling time. Anim. Feed Sci. Technol. 2015, 206, 8–18. [Google Scholar] [CrossRef]
- Tilley, J.M.A.; Terry, R.A. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Alexander, R.H.; McGowan, M. The routine determination of in vitro digestibility of organic matter in forages. An investigation of the problems associated with continuous large-scale operation. Grass Forage Sci. 1966, 21, 140–147. [Google Scholar] [CrossRef]
- McDonald, P. The Biochemistry of Silage; John Wiley and Sons Ltd.: Chichester, UK, 1981. [Google Scholar]
- Ørskov, E.R.; McDonald, P. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Dias, C.S.A.M.M.; Nunes, H.P.B.; Vouzela, C.F.M.; Madruga, J.S.; Borba, A.E.S. Influence of the Season on the Nutritive Value and Gas Production of Opuntia ficus-indica and Agave americana L. in Ruminant Feed. Animals 2023, 13, 1008. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, F.; Wang, Y.; Fan, X.; Feng, C.; Wang, Y. Dynamics of fermentation parameters and bacterial community in alfalfa silage ensiled at different DM contents. Microorganisms 2021, 9, 1225. [Google Scholar] [CrossRef]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Mesquita, C.P.; Duarte, A.M.; Garcia, P.; Belo, A.F.; Portugal, A.V. Effects of storage conditions and conservation period on the quality of dehydrated lucerne (Medicago sativa L.). Agriculture 2024, 15, 1438. [Google Scholar]
- Dewhurst, R.J.; Scollan, N.D.; Lee, M.R.F.; Ougham, H.J.; Humphreys, M.O. Forage breeding goals to increase the beneficial fatty acid content of ruminant products. Proc. Nutr. Soc. 2003, 62, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Getachew, G.; Robinson, P.H.; DePeters, E.J.; Taylor, S.J. Relationships between chemical composition, dry matter degradation and in vitro gas production of several ruminant feeds. Anim. Feed Sci. Technol. 2004, 111, 57–71. [Google Scholar] [CrossRef]
- Makkar, H.P.S. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Rumin. Res. 2003, 49, 241–256. [Google Scholar] [CrossRef]
- Ben Salem, H.; Smith, T. Feeding strategies to increase small ruminant production in dry environments. Small Rumin. Res. 2008, 77, 174–194. [Google Scholar] [CrossRef]
- Franco, M.; Rinne, M. Dry matter content and additives with different modes of action modify the preservation characteristics of grass silage. Fermentation 2023, 9, 640. [Google Scholar] [CrossRef]
- Marcinčák, S.; Sabolová, D.; Váradyová, Z.; Mellen, M.; Gálik, B.; Ondrašovičová, S.; Tóthová, C.; Kiššová, Z.; Vršková, M.; Marcinčáková, D. The use of plant extracts and phytogenic feed additives in ruminant nutrition. Animals 2022, 12, 831. [Google Scholar]
- Lima, R.; Ribeiro Alves Lourenço, M.; Díaz, R.F.; Castro, A.; Fievez, V. Effect of combined ensiling of sorghum and soybean with or without molasses and lactobacilli on silage quality and in vitro rumen fermentation. Anim. Feed Sci. Technol. 2010, 155, 122–131. [Google Scholar] [CrossRef]
- Moore, K.J.; Jung, H.G. Lignin and fiber digestion. J. Range Manag. 2001, 54, 420–430. [Google Scholar] [CrossRef]
- Vargas-Ortiz, L.; Andrade-Yucailla, V.; Barros-Rodríguez, M.; Lima-Orozco, R.; Macías-Rodríguez, E.; Contreras-Barros, K.; Guishca-Cunuhay, C. Influence of Acacia mearnsii fodder on rumen digestion and mitigation of greenhouse gas production. Animals 2022, 12, 2250. [Google Scholar] [CrossRef] [PubMed]
- Lagrange, S.; Lobón, S.; Villalba, J. Gas production kinetics and in vitro degradability of tannin-containing legumes, alfalfa and their mixtures. Anim. Feed Sci. Technol. 2019, 253, 52–60. [Google Scholar] [CrossRef]
- Buxton, D.R.; Redfearn, D.D. Plant limitations to fiber digestion and utilization. J. Nutr. 1997, 127, 814S–818S. [Google Scholar] [CrossRef]
- Aderao, G.N.; Sahoo, A.; Bhatt, R.S.; Kumawat, P.K.; Soni, L. In vitro rumen fermentation kinetics, metabolite production, methane and substrate degradability of polyphenol-rich plant leaves and their component complete feed blocks. J. Anim. Sci. Technol. 2018, 60, 26. [Google Scholar] [CrossRef]
- Castro-Montoya, J.; Dickhoefer, U. Effects of tropical legume silages on intake, digestibility and performance in large and small ruminants: A review. Grass Forage Sci. 2018, 73, 26–39. [Google Scholar] [CrossRef]

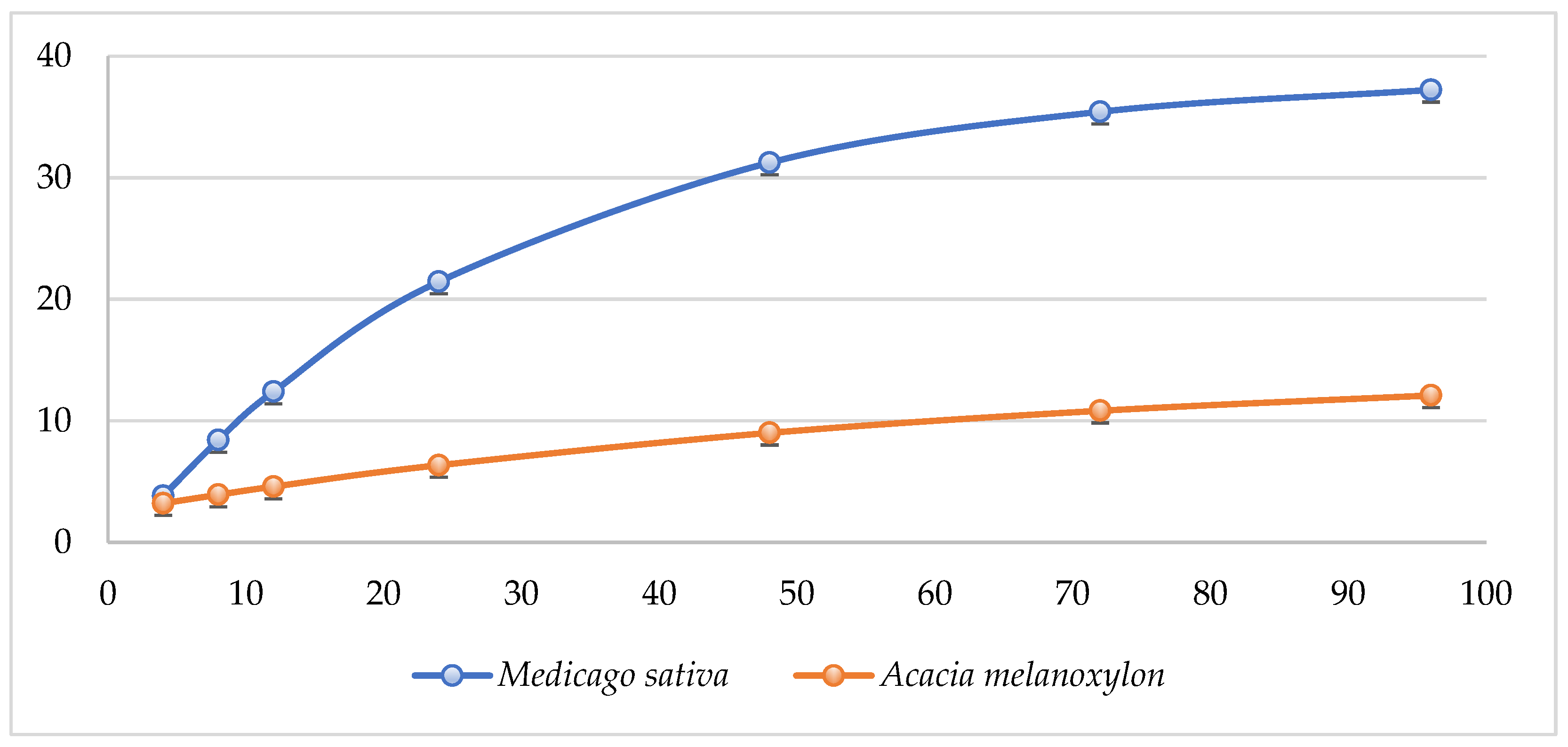

| A. melanoxylon | M. sativa | p Value | |
|---|---|---|---|
| Kinetics of Reaction | |||
| a (mL/0.2 g DM) | 2.46 ± 0.1242 | −1.6244 ± 0.2035 | 0.091 |
| b (mL/0.2 g DM) | 12.36 ± 0.1595 | 39.9572 ± 0.2374 | <0.001 |
| c (mL/h) | 0.0250 ± 0.0100 | 0.0364 ± 0.0006 | <0.001 |
| Lag t (h) | 0.00 ± 0.00 | 1.00 ± 0.0156 | <0.001 |
| Gas production (mL/0.2DM) | |||
| 4 h | 3.33 ± 0.24 | 3.98 ± 0.15 | 0.063 |
| 8 h | 3.91 ± 0.03 | 8.38 ± 0.14 | <0.001 |
| 12 h | 4.64 ± 0.11 | 12.54 ± 0.21 | <0.001 |
| 24 h | 6.35 ± 0.26 | 21.47 ± 0.25 | <0.001 |
| 48 h | 8.92 ± 0.12 | 31.26 ± 0.25 | <0.001 |
| 72 h | 10.95 ± 0.3 | 35.59 ± 0.17 | <0.001 |
| 96 h | 12.15 ± 0.19 | 37.29 ± 0.15 | <0.001 |
| Parameters | Treatment | |||||
|---|---|---|---|---|---|---|
| TC | T1 | T2 | T3 | T4 | ||
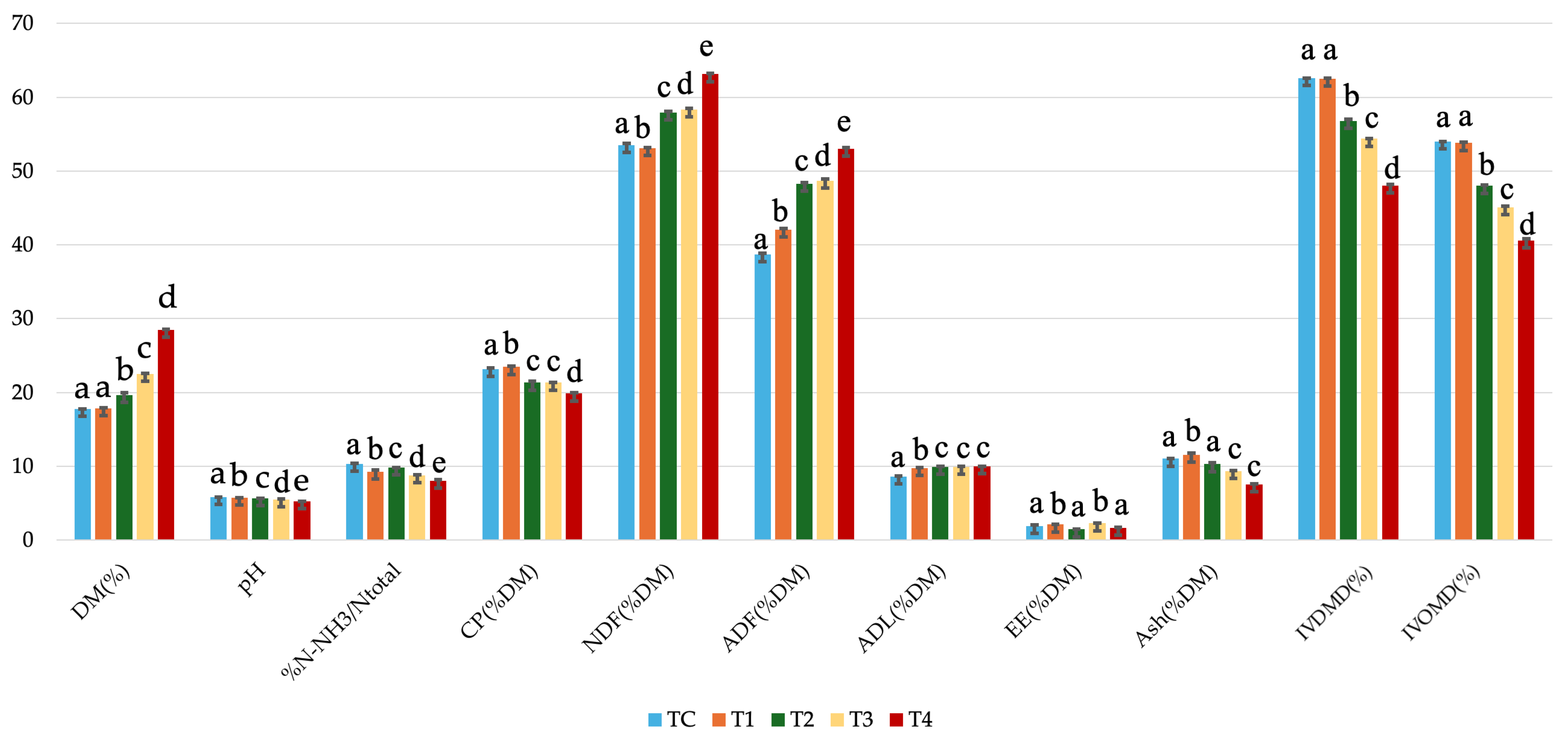
| DM (%) | Mean ± SEM | 17.75 ± 0.04 | 17.84 ± 0.09 | 19.65 ± 0.31 | 22.53 ± 0.06 | 28.45 ± 0.11 |
| CV (%) | 0.2 | 0.48 | 1.6 | 0.28 | 0.38 | |
| CP (%DM) | Mean ± SEM | 22.85 ± 0.19 | 23.45 ± 0.15 | 21.32 ± 0.15 | 21.31 ± 0.05 | 19.85 ± 0.09 |
| CV (%) | 0.82 | 0.62 | 0.7 | 0.22 | 0.45 | |
| NDF (%DM) | Mean ± SEM | 53.94 ± 0.20 | 53.09 ± 0.05 | 57.95 ± 0.13 | 58.36 ± 0.13 | 63.12 ± 0.08 |
| CV (%) | 0.37 | 0.1 | 0.22 | 0.22 | 0.13 | |
| ADF (%DM) | Mean ± SEM | 35.29 ± 0.20 | 42.08 ± 0.11 | 48.25 ± 0.15 | 48.70 ± 0.17 | 53.01 ± 0.17 |
| CV (%) | 0.57 | 0.25 | 0.31 | 0.36 | 0.31 | |
| ADL (%DM) | Mean ± SEM | 7.48 ± 0.08 | 9.74 ± 0.05 | 9.91 ± 0.05 | 9.93 ± 0.03 | 9.98 ± 0.03 |
| CV (%) | 1.01 | 0.54 | 0.53 | 0.27 | 0.27 | |
| EE (%DM) | Mean ± SEM | 1.61 ± 0.15 | 2.10 ± 0.05 | 1.43 ± 0.06 | 2.25 ± 0.07 | 1.62 ± 0.12 |
| CV (%) | 9.41 | 2.52 | 4.25 | 3.03 | 7.71 | |
| ASH (%DM) | Mean ± SEM | 10.45 ± 0.05 | 11.56 ± 0.23 | 10.28 ± 0.19 | 9.35 ± 0.09 | 7.53 ± 0.07 |
| CV (%) | 0.44 | 2 | 1.83 | 0.98 | 0.87 | |
| IVDMD (%) | Mean ± SEM | 62.61 ± 0.05 | 62.50 ± 0.06 | 56.79 ± 0.20 | 54.36 ± 0.07 | 48.02 ± 0.16 |
| CV (%) | 0.08 | 0.1 | 0.36 | 0.13 | 0.33 | |
| IVOMD (%) | Mean ± SEM | 54.14 ± 0.04 | 53.79 ± 0.14 | 47.99 ± 0.12 | 45.07 ± 0.12 | 40.61 ± 0.22 |
| CV (%) | 0.06 | 0.26 | 0.25 | 0.28 | 0.54 | |
| Parameters | Treatment | |||||
|---|---|---|---|---|---|---|
| TC | T1 | T2 | T3 | T4 | ||
| pH | Mean ± SEM | 5.86 ± 0.01 | 5.72 ± 0.01 | 4.85 ± 0.01 | 4.76 ± 0.01 | 4.53 ± 0.01 |
| CV (%) | 0.11 | 0.17 | 0.18 | 0.18 | 0.19 | |
| N-NH3/N (%) | Mean ± SEM | 11.38 ± 0.10 | 9.25 ± 0.20 | 9.82 ± 0.01 | 8.76 ± 0.08 | 8.05 ± 0.10 |
| CV (%) | 0.84 | 2.19 | 0.10 | 0.89 | 1.22 | |
| Treatment | p Value | |||||
|---|---|---|---|---|---|---|
| TC | T1 | T2 | T3 | T4 | ||
| Kinetics of Reaction | ||||||
| a (mL/0.2 g DM) | −1.95 ± 0.15 | −0.87 ± 0.08 | −0.15 ± 0.05 | −1.25 ± 0.1 | −0.03 ± 0.12 | 0.06 |
| b (mL/0.2 g DM) | 32.72 ± 0.48 | 28.43 ± 0.52 | 22.97 ± 0.23 | 25.08 ± 0.59 | 24.72 ± 0.22 | <0.001 |
| c (mL/h) | 0.04 ± 0.002 | 0.02 ± 0.002 | 0.01 ± 0.001 | 0.03 ± 0.002 | 0.02 ± 0.001 | <0.001 |
| Lag t (h) | 1.67 ± 0.06 | 1.31 ± 0.05 | 0.4 ± 0.08 | 1.86 ± 0.11 | 0.12 ± 0.11 | <0.001 |
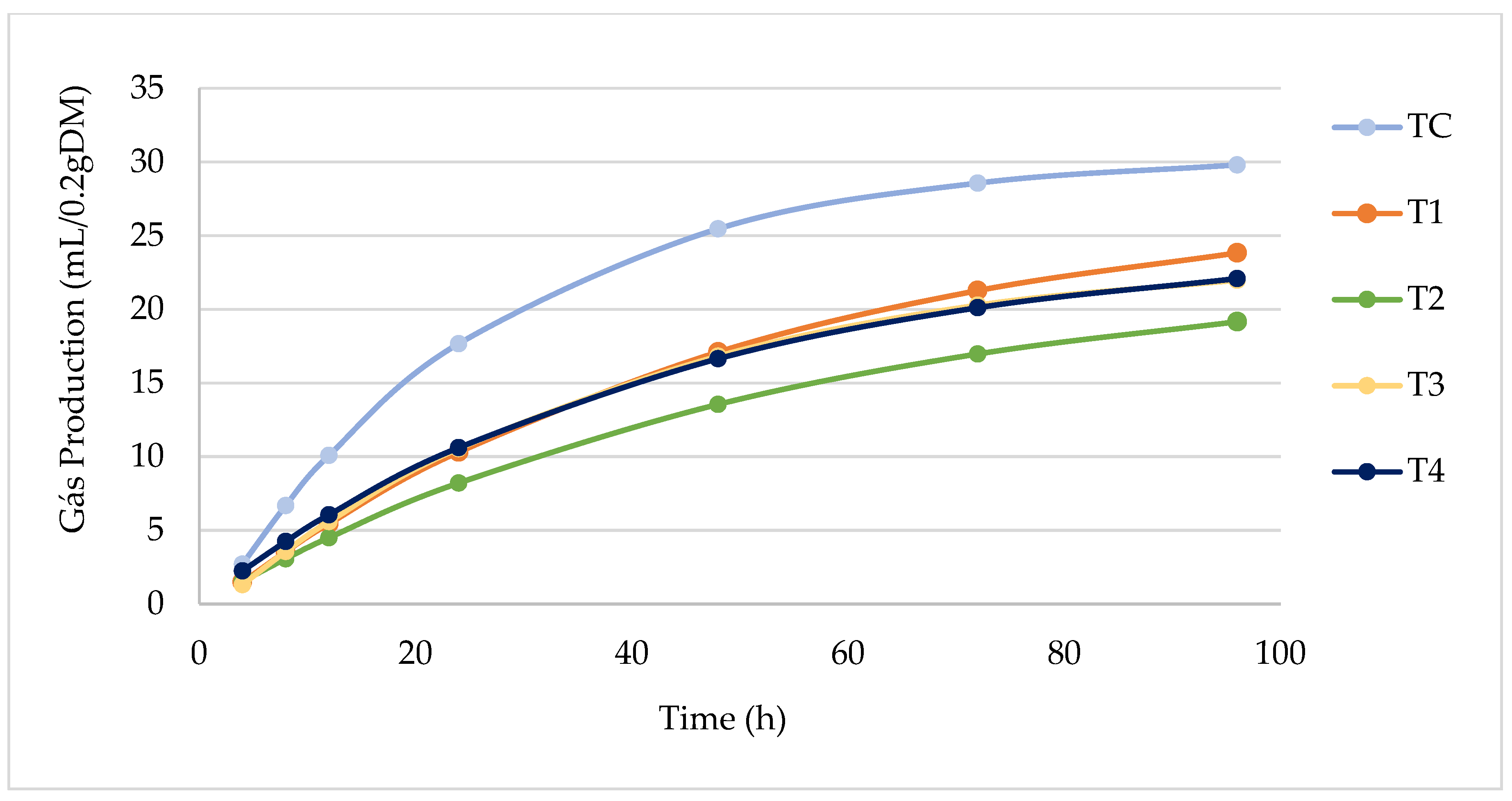
| Gas Production (mL/0.2 g DM) | ||||||
| 4 h | 2.75 ± 0.13 | 1.48 ± 0.12 | 1.44 ± 0.28 | 1.28 ± 0.4 | 2.18 ± 0.4 | 0.10 |
| 8 h | 6.65 ± 0.17 | 3.47 ± 0.23 | 3.12 ± 0.24 | 3.47 ± 0.32 | 4.17 ± 0.14 | <0.001 |
| 12 h | 10.14 ± 0.31 | 5.36 ± 0.2 | 4.52 ± 0.1 | 5.59 ± 0.13 | 5.98 ± 0.16 | <0.001 |
| 24 h | 17.76 ± 0.26 | 10.35 ± 0.19 | 8.24 ± 0.14 | 10.64 ± 0.08 | 10.6 ± 0.26 | <0.001 |
| 48 h | 25.51 ± 0.05 | 17.2 ± 0.07 | 13.51 ± 0.1 | 16.91 ± 0.38 | 16.58 ± 0.18 | <0.001 |
| 72 h | 28.59 ± 0.27 | 21.26 ± 0.04 | 17.05 ± 0.25 | 20.32 ± 0.09 | 20.2 ± 0.17 | <0.001 |
| 96 h | 29.74 ± 0.26 | 23.9 ± 0.26 | 19.07 ± 0.21 | 21.95 ± 0.19 | 22.04 ± 0.09 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, C.M.; Melo, V.; Nunes, H.; Borba, A. Effect of Acacia melanoxylon R. Br. Inclusion on the Chemical Composition, Fermentation Dynamics, and In Vitro Digestibility of Medicago sativa L. Silage. Grasses 2025, 4, 37. https://doi.org/10.3390/grasses4030037
Dias CM, Melo V, Nunes H, Borba A. Effect of Acacia melanoxylon R. Br. Inclusion on the Chemical Composition, Fermentation Dynamics, and In Vitro Digestibility of Medicago sativa L. Silage. Grasses. 2025; 4(3):37. https://doi.org/10.3390/grasses4030037
Chicago/Turabian StyleDias, Cristiana Maduro, Vanessa Melo, Helder Nunes, and Alfredo Borba. 2025. "Effect of Acacia melanoxylon R. Br. Inclusion on the Chemical Composition, Fermentation Dynamics, and In Vitro Digestibility of Medicago sativa L. Silage" Grasses 4, no. 3: 37. https://doi.org/10.3390/grasses4030037
APA StyleDias, C. M., Melo, V., Nunes, H., & Borba, A. (2025). Effect of Acacia melanoxylon R. Br. Inclusion on the Chemical Composition, Fermentation Dynamics, and In Vitro Digestibility of Medicago sativa L. Silage. Grasses, 4(3), 37. https://doi.org/10.3390/grasses4030037






