Nutritional and Structural Components of Forage Sorghum Subjected to Nitrogen Fertilization and Molybdenum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Plant Material
2.2. Climatic Conditions and Soil Classification
2.3. Experimental Conduction
2.4. Irrigation
2.5. Biometric Analyses
2.6. Yield
2.7. Bromatological Analyses
2.8. Statistical Analysis
3. Results
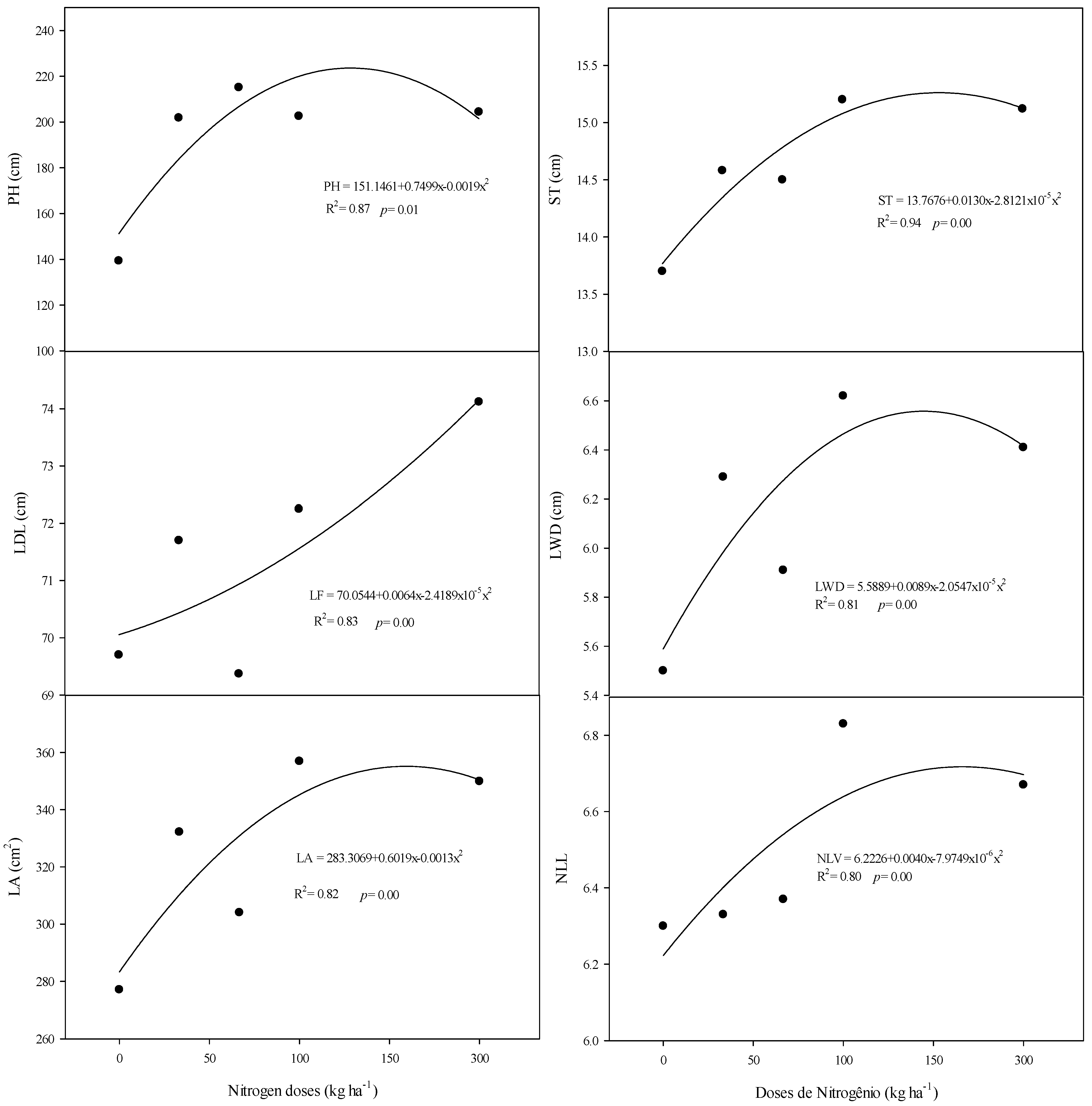
3.1. Morphometric Characteristics
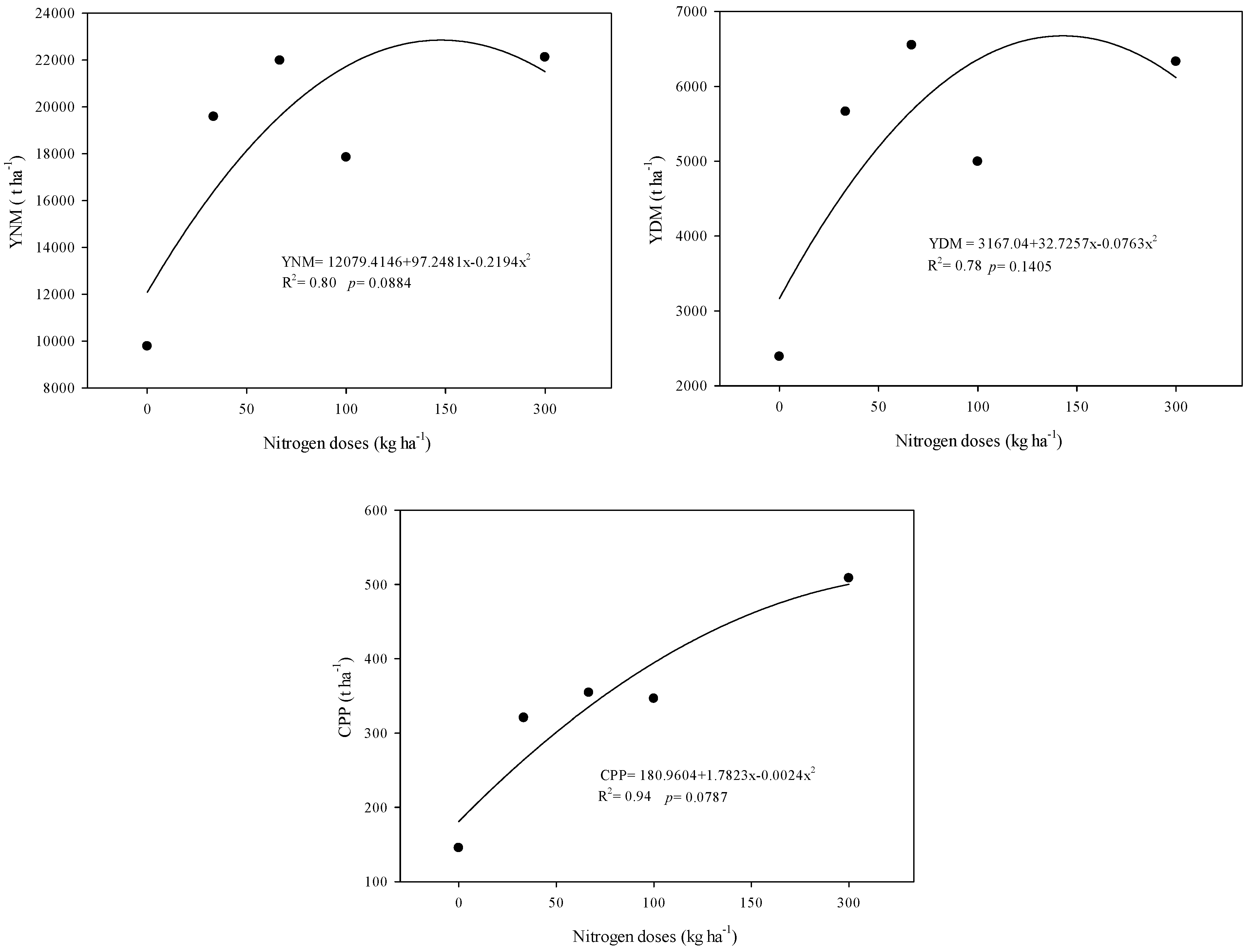
3.2. Productive Characteristics
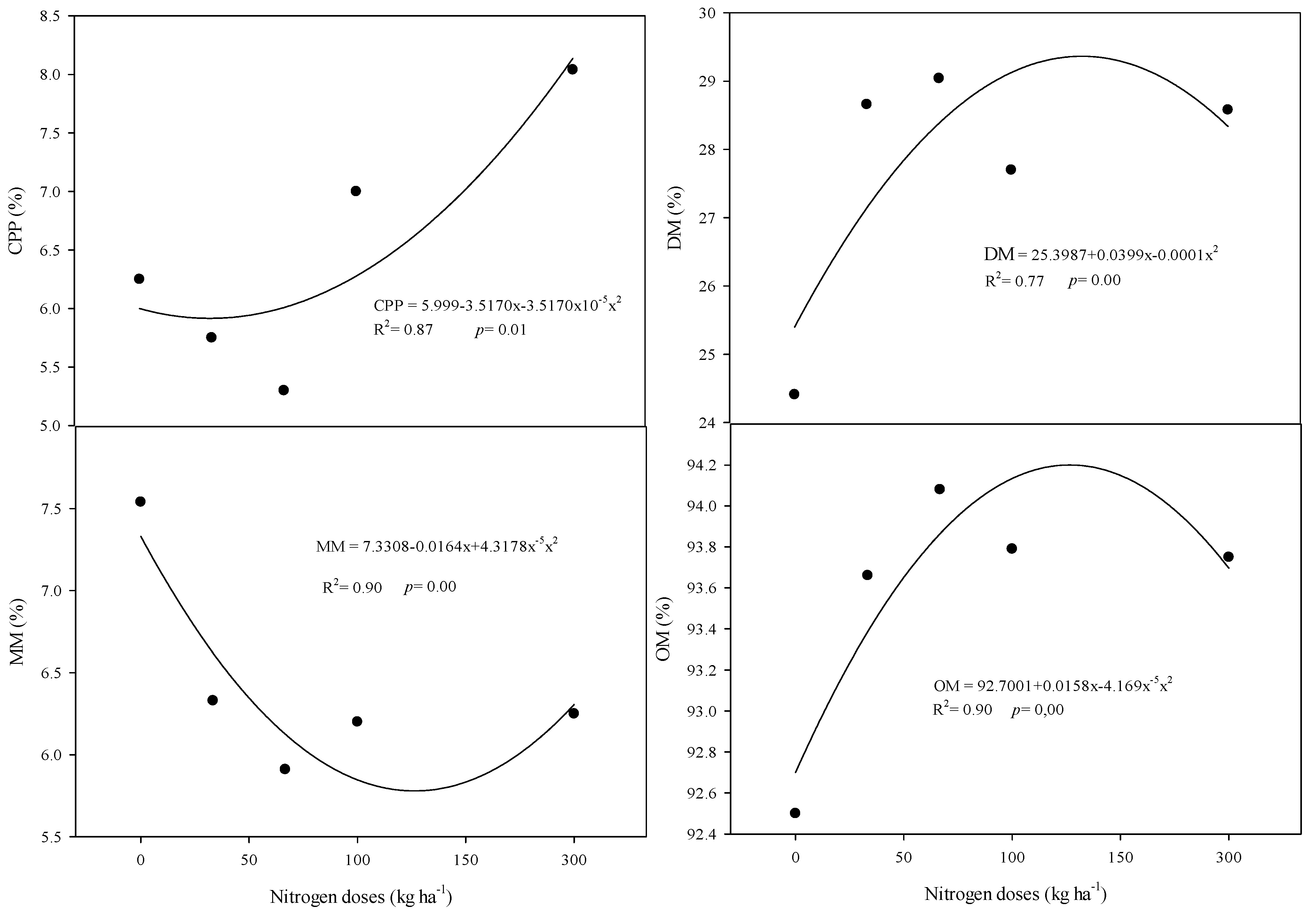
3.3. Bromatological Components
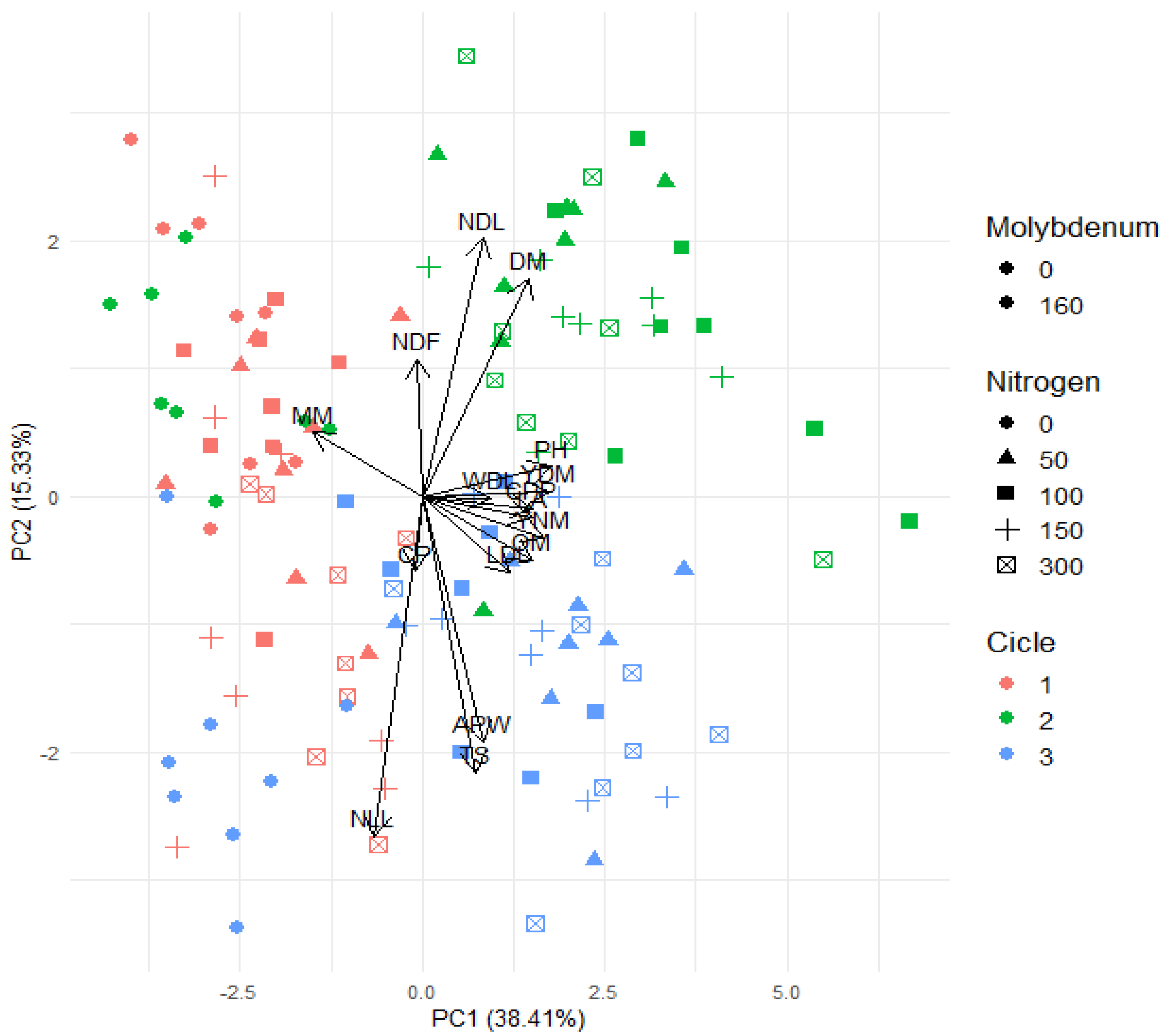
3.4. Relationship Between Analyzed Variables and Nitrogen and Molybdenum Fertilization Management in Consecutive Cycles of Forage Sorghum Under Water Deficit
4. Discussion
4.1. The Morphometric Characteristics of Sorghum Are Influenced by Consecutive Cycles, Nitrogen Doses, and Molybdenum
4.2. The Productive Characteristics of Sorghum SF-15 Are Influenced by the Productive Cycle and Nitrogen
4.3. Bromatological Components Are Positively Influenced by Cycle, Nitrogen, and Molybdenum
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alves, C.P.; Jardim, A.M.; Júnior, G.D.; de Souza, L.S.; de Araújo, G.G.; de Souza, C.A.; da Silva Salvador, K.R.; Leite, R.M.; Pinheiro, A.G.; da Silva, T.G. How to enhance the agronomic performance of cactus-sorghum intercropped system: Planting configurations, density and orientation. Ind. Crop. Prod. 2022, 184, 115059. [Google Scholar] [CrossRef]
- Oliveira, L.B.; Macêdo, A.J.d.S.; Santos, E.M. Interaction Between Native and Cultivated Forage Species in Semi-Arid Conditions. Arq. Ciênc. Vet. Zool. UNIPAR. 2019, 22, 127–138. [Google Scholar] [CrossRef]
- Uzcátegui, J.P.V.; Chompre, K.; Castillo, D.; Rangel, S.; Rangel, A.B.; Piña, A. Nutritional assessment of tropical pastures as a sustainability strategy in dual-purpose cattle ranching in the South of Lake Maracaibo, Venezuela. J. Saudi Soc. Agric. Sci. 2021, 21, 432–439. [Google Scholar] [CrossRef]
- Akin, D.E. Histological and Physical Factors Affecting Digestibility of Forages. Agron. J. 1989, 81, 17–25. [Google Scholar] [CrossRef]
- Nunes, J.d.S.L.; Silva, T.G.F.; de Souza, L.S.B.; Jardim, A.M.d.R.F.; Alves, H.K.M.N.; da Cruz Neto, J.F.; Leite, R.M.C.; Pinheiro, A.G. Morphogenesis of forage cactus under modification of the growth environment. Agrometeoros 2020, 27, 367–375. [Google Scholar] [CrossRef]
- Perazzo, A.F.; Santos, E.M.; Pinho, R.M.A.; Campos, F.S.; Ramos, J.P.d.F.; de Aquino, M.M.; da Silva, T.C.; Bezerra, H.F.C. Agronomic characteristics and rain use efficiency in sorghum cultivars in the semi-arid region. Cienc. Rural. 2000, 30, 365–372. [Google Scholar] [CrossRef]
- Silva, H.W.; Guimaraes, C.R.B.; Andriguetti, S.C.; Godinho, A.M.M.; dos Santos, A.T.; Araújo, E.B.; Fachiano Junior, L.C.; Pereira, C.W. de J. Forage sorghum productivity as a function of cutting interval and regrowth. Braz. J. Dev. 2020, 6, 14441–14450. [Google Scholar] [CrossRef]
- Jardim, A.M.d.R.F.; de Souza, L.S.B.; Alves, C.P.; de Araújo, J.F.N.; de Souza, C.A.A.; Pinheiro, A.G.; de Araújo, G.G.L.; Campos, F.S.; Tabosa, J.N.; da Silva, T.G.F. Intercropping forage cactus and sorghum in a semi-arid environment improves biological efficiency and competitive ability through interspecific complementarity. J. Arid Environ. 2021, 188, 104464. [Google Scholar] [CrossRef]
- Moura, M.M.A.; Pires, D.A.d.A.; Rodrigues, J.A.S.; Sales, E.C.J.; Costa, R.F.; Tolentino, D.C. Chemical composition of sorghum genotypes silages. Acta Sci. 2016, 38, 369–373. [Google Scholar] [CrossRef]
- Pino, F.; Heinrichs, A.J. Sorghum forage in precision-fed dairy heifer diets. J. Dairy Sci. 2017, 100, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Oresca, D.; de Oliveira, A.C.; da Silva, T.G.F.; Pessoa, L.G.M.; de Souza, J.C.G. Agronomic performance of forage sorghum and millet grown under irrigation with saline water and nitrogen doses in the Brazilian semi-arid. Res. Soc. Dev. 2021, 10, 1–18. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Growth and Development. In Plant Physiology and Development, 6th ed.; Lima, L.B., Preparação, A.S., Neumann, L.P., Arte, S.G., Eds.; Artmed: Porto Alegre, Rio Grande do Sul, Brazil, 2017; pp. 377–731. [Google Scholar]
- Bredemeier, C.; Mundstock, C.M. Regulation of nitrogen absorption and assimilation in plants. Cienc. Rural. 2013, 43, 1771–1776. [Google Scholar] [CrossRef]
- Ahmed, S.O.; Abdalla, A.W.H.; Inoue, T.; Ping, A.; Babiker, E.E. Nutritional quality of grains of sorghum cultivar grown under different levels of micronutrients fertilization. Food. Chem. 2014, 159, 374–380. [Google Scholar] [CrossRef]
- Albino, U.B.; CAMPO, R. JEffect of molybdenum sources and doses on Bradyrhizobium survival and biological nitrogen fixation in soybean. Pesqui. Agropecu. Bras. 2001, 36, 527–534. [Google Scholar] [CrossRef]
- Oliveira, S.L.; Crusciol, C.A.C.; Rodrigues, V.A.; Galeriani, T.M.; Portugal, J.R.; Bossolani, J.W.; Moretti, L.G.; Calonego, J.C.; Cantarella, H. Molybdenum Foliar Fertilization Improves Photosynthetic Metabolism and Grain Yields of Field-Grown Soybean and MaizeFront. Plant Sci. 2022, 13, 887682. [Google Scholar] [CrossRef]
- Ferreira, A.C.d.B.; Araújo, A.D.A.; Pereira, P.R.G.; Cardoso, A.A. Agronomic and nutritional characteristics of corn fertilized with nitrogen, molybdenum and zinc. Sci. Agric. 2001, 58, 131–138. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; De Moraes, J.L.G.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Silva, A.d.S.; Mendes, A.M.S.; Silva, A.F.; de Souza, A.V.; Neves, A.L.A. Characterization of the Brazilian Semiarid: Natural and human factors. In Brazilian Semiarid: Research, Development and Innovation; Sá, I.B., da Silva, P.C.G., Eds.; Embrapa Semiarid: Petrolina, Brazil, 2010; pp. 17–48. [Google Scholar]
- Cirino Júnior, B.; Henrique, J.C.G.S.; Maciel, L.H.; Souza, C.M.P.G.; De Alcântara, E.B.; Da Silva, N.S.G.; Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A. Diagnostic attributes and other attributes. In Brazilian Soil Classification System, 5th ed.; Santos, H.G., Ed.; Embrapa: Brasilia, Brazil, 2018; pp. 29–48. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Guidelines for computing crop water requirements. FAO-56 1998, 56, 300–1998. [Google Scholar]
- Guimarães, D.P.; Sans, L.M.A.; Moraes, A.V.d.C. Estimation of Leaf Area of Corn Cultivars. In Proceedings of the XXIV National Corn and Sorghum Congress, Florianopolis, SC, Brazil, 1–5 September 2002. [Google Scholar]
- Detman, E.; Silva, L.F.C.; Rocha, G.C.; Palma, M.N.N.; Rodrigues, J.P.P. Methods for Food Analysis, 2nd ed.; Suprema: Visconde do Rio Branco, MG, Brazil, 2021. [Google Scholar]
- Figueredo Júnior, L.G.M.; Dourado Neto, D.; Oliveira, R.F.; Manfron, P.A.; Martin, T.N. Model for Estimating the Leaf Area Index for Corn Crops. Rev. Bras. Milho Sorgo 2005, 4, 8–13. [Google Scholar] [CrossRef]
- Vieira Junior, P.A.; Dourado Neto, D.; Bernardes, M.S.; Fancelli, A.L.; Manfron, P.A.; Martin, T. Methodology for Estimating Leaf Area of Corn Genotypes. Rev. Bras. Milho Sorgo 2006, 5, 182–191. [Google Scholar] [CrossRef]
- Henrique, J.C.G.d.S.; de Oliveira, A.C.; da Silva, T.G.F.; Carvalho, L.H.M.; de Souza, E.J.O.; da Fonseca, G.R.F.; Santos, H.R.B.; Cruz, G.H. de L. Nitrogen and molybdenum fertilization influence on enzymatic activity and productivity enhancement of forage sorghum under water deficit in the Brazilian semi-arid region. J. Plant Nutr. 2024, 47, 2543–2570. [Google Scholar] [CrossRef]
- Rodrigues, J.C.; Souza, P.J.d.O.; Lima, R.T. Estimation of basal temperatures and thermal requirements in hoses in the northeast of the state of Pará. Braz. Fruit Grow. Mag. 2013, 35, 143–150. [Google Scholar]
- Basi, S.; Neumann, M.; Marafon, F.; Ueno, R.K.; Sandini, I.E. Influence of nitrogen fertilization on the quality of corn silage. Braz. J. Appl. Technol. Agric. Sci. 2011, 4, 219–234. [Google Scholar] [CrossRef]
- Tabosa, J.N.; da Silva, F.G.; do Nascimento, M.M.A.; Barros, A.H.C.; Brito, A.R.M.B.; Simplício, J.B. Forage Sorghum Genotypes in the Semiarid Region of Pernambuco and Alagoas—Estimates of Genetic Parameters of Production Variables. In Proceedings of the XXIX National Corn and Sorghum Congress, Aguas de Lindóia, São Paulo, Brazil, 26–30 August 2012. [Google Scholar]
- Soares, E.R.; Coutinho, E.L.M.; Ramos, S.B.; Da Silva, M.S.; Barbosa, J.C. Accumulation of dry matter and macronutrients in sweet sorghum cultivars. Agric. Sci. 2013, 35, 3015–3030. [Google Scholar] [CrossRef]
- Macedo, C.H.O.; Santos, E.M.; da Silva, T.C.; de Andrade, A.P.; da Silva, D.S.; da Silva, A.P.G.; de Oliveira, J.S. Production and Bromatological Composition of Sorghum (Sorghum Bicolor) Cultivated Under Doses of Nitrogen. Arch. Zootec. 2012, 61, 209–216. [Google Scholar] [CrossRef]
- Buso, W.H.D.; Morgado, H.S.; Silva, L.B.; França, A.F.S. Use of forage sorghum in animal feed. Revista Pubvet. 2011, 5, 1145. [Google Scholar] [CrossRef]
- Kaplan, M.; Kara, K.; Unlukara, A.; Kale, H.; Buyukkilic Beyzi, S.; Varol, I.S.; Kizilsimsek, M.; Kamalak, A. Water deficit and nitrogen affects yield and feed value of sorghum sudangrass silage. Agric. Water Manag. 2019, 218, 30–36. [Google Scholar] [CrossRef]
- Tang, C.; Yang, X.; Chen, X.; Ameen, A.; Xie, G. Sorghum biomass and quality and soil nitrogen balance response to nitrogen rate on semiarid marginal land. Field Crop. Res. 2018, 215, 12–22. [Google Scholar] [CrossRef]
- Reddy, P.S.; Gadi, J.; Rasineni, G.K.; Maheswari, M.; Reddy, A.R.; Varshney, R.K.; Kishor, P.B.F. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum [Sorghum bicolor (L.) Moench]. Plant Physiol. Biochem. 2015, 94, 104–113. [Google Scholar] [CrossRef]
- Lima, N.V.; da Silva, R.A.; Trigueiro, R.W.d.P.; de Lima, A.V.; da Cruz Júnior, C.B. Bromatology of dry, organic and mineral matter, crude protein, ether extract and total digestible nutrients of forage species from the caatinga preferred by sheep in a semi-arid climate. J. Anim. Environ. Res. 2023, 5, 1–23. [Google Scholar]
- Rocha, J.E.d.S.; Tonucci, R.G.; Fernandes, F.É.d.P.; Magalhães, Y.A. Productivity and bromatological composition of sorghum hybrids cultivated in an agroforestry system in the semi-arid region. In Proceedings of the IX Northeastern Congress on Animal Production and XV Northeastern Symposium on Ruminant Feeding, Ilhéus, BA, Brazil, 11–14 November 2014. [Google Scholar] [CrossRef]
- Anfinrud, R.; Cihacek, L.; Johnson, B.L.; Ji, Y.; Berti, M.T. Sorghum and kenaf biomass yield and quality response to nitrogen fertilization in the Northern Great Plains of the USA. Ind. Crop. Prod. 2013, 50, 159–165. [Google Scholar] [CrossRef]

| Chemical Attributes | |||||||||||||||
| Dep. | P | pH | K | Mo | Na | Al | Ca | SAR | Mg | H + Al | CS | CEC | V | ESP | OM |
| cm | mg/dm³ | cmolc/dcm³ | % | ||||||||||||
| 0–20 | 383 | 6.71 | 0.48 | <LQ | 0.11 | 0 | 3.61 | 0.09 | 2.61 | 0.47 | 6.81 | 7.28 | 93.54 | 1.51 | 1.14 |
| 20–40 | 388 | 6.74 | 0.4 | <LQ | 0.19 | 0 | 3.9 | 0.14 | 2.67 | 0.5 | 7.16 | 7.66 | 93.47 | 2.48 | 0.94 |
| Physical analysis | |||||||||||||||
| Dep. | BD | PD | TP | NA | FF | TT | CS | FS | Silte | Argila | |||||
| cm | g/cm3 | % | % | ||||||||||||
| 0–20 | 1.61 | 2.53 | 36.26 | 4.32 | 59.00 | 73.60 | 44.50 | 29.10 | 15.90 | 10.50 | |||||
| 20–40 | 1.66 | 2.47 | 32.80 | 4.39 | 58.31 | 72.20 | 48.88 | 23.34 | 17.20 | 10.50 | |||||
| Cycle | PH (cm) | ST (mm) | WDL (cm) | LA (cm2) | NLV | NLD | LDL (cm) | |||
| Plant | 131.47 b | 13.75 b | 61.25 b | 281.75 b | 7.07 a | 5.15 b | 73.75 c | |||
| Regrowth 1 | 225.47 a | 13.80 b | 76.07 a | 351.97 a | 5.37 b | 6.25 a | 84.70 b | |||
| Regrowth 2 | 220.90 a | 16.32 a | 76.97 a | 338.37 a | 7.32 a | 5.17 b | 98.97 a | |||
| CV (%) | 9.47 | 13.60 | 7.68 | 10.41 | 9.29 | 14.96 | 17.02 | |||
| p | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| Mo doses (g ha−1) | NLV | |||||||||
| 160 | 6.45 b | |||||||||
| 0 | 6.73 a | |||||||||
| CV (%) | 9.29 | |||||||||
| p | 0.01 | |||||||||
| Cycle × N (Kg ha−1) | PH (cm) | ST (mm) | WDL (cm) | APW (cm) | LA (cm2) | NLV | NLD | LDL (cm) | ||
| Plant | 0 | 124.75 bA | 12.00 bB | 60.87 bA | 5.87 aAB | 262.62 bB | 6.62 bB | 6.12 aA | 70.00 bA | |
| Plant | 50 | 138.00 bA | 14.87 abA | 57.00 bA | 6.25 aAB | 259.25 bB | 6.87 aAB | 5.50 bAB | 67.25 cA | |
| Plant | 100 | 136.87 cA | 12.87 bAB | 60.12 bA | 5.75 bB | 252.25 cB | 6.87 aAB | 5.12 bAB | 72.62 bA | |
| Plant | 150 | 128.12 bA | 13.87 aAB | 63.62 bA | 6.37 aAB | 312.37 bA | 7.62 aA | 4.50 cB | 77.37 bA | |
| Plant | 300 | 129.62 bA | 15.12 abA | 64.62 bA | 6.75 aA | 322.25 bA | 7.37 aAB | 4.50 bB | 81.50 aA | |
| Regrowth 1 | 0 | 139.25 abC | 13.12 bA | 72.37 aB | 5.12 aB | 258.12 bB | 5.75 cA | 5.37 abB | 83.87 abA | |
| Regrowth 1 | 50 | 241.50 aB | 14.00 bA | 80.37 aA | 6.12 aA | 372.37 aA | 5.12 bA | 6.75 aA | 85.37 bA | |
| Regrowth 1 | 100 | 272.75 aA | 15.00 abA | 73.12 aB | 6.62 aA | 362.12 aA | 5.25 bA | 6.25 aAB | 80.75 abA | |
| Regrowth 1 | 150 | 231.62 aB | 13.75 aA | 77.25 aB | 6.87 aA | 393.75 aA | 5.37 bA | 6.75 aA | 85.87 abA | |
| Regrowth 1 | 300 | 242.25 aB | 13.12 bA | 77.25 aB | 6.50 aA | 373.50 aA | 5.37 bA | 6.12 aAB | 87.62 aA | |
| Regrowth 2 | 0 | 153.87 aB | 16.00 aA | 75.87 aA | 5.50 aB | 310.50 aBC | 7.87 aA | 4.62 bB | 96.25 aA | |
| Regrowth 2 | 50 | 225.87 aA | 16.50 aA | 77.75 aA | 6.50 aA | 365.00 aA | 7.00 aB | 5.87 abA | 110.75 aA | |
| Regrowth 2 | 100 | 235.62 bA | 15.87 aA | 74.87 aA | 5.37 bB | 297.75 bC | 7.00 aB | 4.50 bB | 95.75 aA | |
| Regrowth 2 | 150 | 247.87 aA | 15.87 aA | 75.87 aA | 6.62 aA | 364.62 aA | 7.50 aAB | 5.62 bAB | 95.87 aA | |
| Regrowth 2 | 300 | 241.25 aA | 17.37 aA | 80.50 aA | 6.00 aAB | 354.00 abB | 7.25 aAB | 5.23 abB | 96,25 aA | |
| CV (%) | 9.70 | 13.60 | 7.68 | 11.38 | 10.41 | 9.29 | 14.96 | 17.02 | ||
| p | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | ||
| Cycle × Mo (g ha−1) | ST (mm) | WDL (cm) | NLV | NLD | LDL (cm) | |||||
| Plant | 0 | 13.90 bA | 59.55 bA | 6.75 bB | 5.35 bA | 72.95 bA | ||||
| Plant | 160 | 13.60 bA | 62.95 bA | 7.40 aA | 5.05 bA | 74.55 cA | ||||
| Regrowth 1 | 0 | 13.35 bA | 76.85 aA | 5.25 cA | 6.15 aA | 83.50 bA | ||||
| Regrowth 1 | 160 | 14.25 bA | 75.30 aA | 5.50 bA | 6.35 aA | 85.90 bA | ||||
| Regrowth 2 | 0 | 16.05 aA | 77.80 aA | 7.35 aA | 5.20 bA | 95.30 aA | ||||
| Regrowth 2 | 160 | 16.60 aA | 76.15 aA | 7.30 aA | 5.15 bA | 102.65 aA | ||||
| CV (%) | 13.60 | 7.68 | 9.29 | 14.96 | 17.02 | |||||
| p | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | |||||
| N (kg ha−1) × Mo (g ha−1) | PH (cm) | ST (mm) | WDL (cm) | NLV | NLD | LDL (cm) | ||||
| 0 | 0 | 132.75 bA | 13.66 aA | 65.83 cB | 6.58 aA | 5.33 bA | 80.75 aA | |||
| 50 | 0 | 217.83 aA | 14.58 aA | 73.58 aA | 6.16 aA | 6.58 aA | 82.91 aA | |||
| 100 | 0 | 199.25 aB | 14.25 aA | 69.58 bA | 6.66 aA | 4.91 bB | 84.50 aA | |||
| 150 | 0 | 202.08 aA | 14.41 aA | 71.58 aA | 6.58 aB | 5.75 abA | 84.58 aA | |||
| 300 | 0 | 204.25 aA | 15.25 aA | 76.41 aA | 6.25 aB | 5.08 bA | 86.83 aA | |||
| 0 | 160 | 145.83 cA | 13.75 aA | 73.58 aA | 6.91 aA | 5.41 aA | 86.26 aA | |||
| 50 | 160 | 187.75 bB | 14.58 aA | 69.83 aA | 6.50 abA | 5.66 aB | 83.16 aA | |||
| 100 | 160 | 230.91 aA | 14.75 aA | 69.16 aA | 6.08 bB | 5.50 aA | 88.25 aA | |||
| 150 | 160 | 203.00 bA | 16.00 aA | 72.91 aA | 7.08 aA | 5.50 aA | 92.33 aA | |||
| 300 | 160 | 204.50 bA | 15.00 aA | 71.83 aB | 7.08 aA | 5.50 aA | 88.75 aA | |||
| CV (%) | 9.70 | 13.60 | 7.68 | 9.29 | 14.96 | 17.02 | ||||
| p | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | ||||
| C × N × Mo | PH (cm) | ST (mm) | WDL (cm) | NLV | NLD | LA (cm2) | LDL (cm) | |||
| Plant | 0 | 0 | 126.75 aAa | 12.50 aa | 57.25 bAb | 6.50 abAa | 6.25 aAa | 251.25 ba | 68.50 aAa | |
| Plant | 50 | 0 | 147.00 cAa | 15.50 aAa | 59.00 bAb | 6.50 abAa | 5.75 bABa | 282.75 ba | 65.25 bAa | |
| Plant | 100 | 0 | 135.25 ba | 13.25 aAa | 58.75 bAb | 7.25 aA | 4.75 abAa | 248.50 bAa | 75.75 aAa | |
| Plant | 150 | 0 | 126.25 ba | 13.75 aAa | 59.75 bb | 6.75 bAb | 5.25 aABa | 299.25 ba | 74.50 aAa | |
| Plant | 300 | 0 | 134.50 bAa | 14.50 abAa | 63.00 bAb | 8.50 aAb | 4.25 bBa | 291.75 bb | 80.75 aAa | |
| Plant | 0 | 160 | 122.75 ba | 11.50 bBa | 64.50 bBb | 6.75 bCa | 6.00 aAa | 274.00 aCa | 71.50 bAa | |
| Plant | 50 | 160 | 129.00 bAa | 14.25 aBa | 55.00 bBb | 7.25 aBCa | 5.25 aABa | 235.75 ba | 69.25 bAa | |
| Plant | 100 | 160 | 138.50 caAa | 12.50 aABa | 61.50 bBb | 6.50 aCa | 5.50 abAa | 256.00 bCa | 69.50 bAa | |
| Plant | 150 | 160 | 130.00 bAa | 14.00 aABa | 67.50 bAa | 8.50 aAb | 3.75 bBb | 325.50 bABa | 80.25 aAa | |
| Plant | 300 | 160 | 124.75 ba | 15.75 aAa | 66.25 bAb | 8.00 aABa | 4.75 bABa | 352.75 aAa | 82.25 aAa | |
| Regrowth 1 | 0 | 0 | 120.75 aCb | 13.25 aAa | 68.00 aBb | 5.75 aBa | 5.00 abBa | 223.00 bBb | 80.50 aAa | |
| Regrowth 1 | 50 | 0 | 274.75 aAa | 13.75 aAa | 81.75 aAb | 5.00 bAa | 7.50 aABa | 389.25 aAa | 85.25 bAa | |
| Regrowth 1 | 100 | 0 | 240.25 aBb | 14.50 aAa | 74.0 aABa | 5.50 bAa | 6.50 aABa | 369.25 aAa | 80.00 aAa | |
| Regrowth 1 | 150 | 0 | 233.75 aBa | 13.25 aAa | 77.0 aABa | 5.00 cAa | 6.50 aABa | 391.00 aAa | 85.25 aAa | |
| Regrowth 1 | 300 | 0 | 235.50 aBa | 12.00 bAa | 83.50 aAa | 5.00 bA | 5.75 aBa | 391.50 aAa | 86.50 aAa | |
| Regrowth 1 | 0 | 160 | 157.75 aDa | 13.00 bAa | 76.75 aa | 5.75 bAa | 5.75 abAa | 293.25 aBa | 87.25 abAa | |
| Regrowth 1 | 50 | 160 | 208.25 aCb | 14.25 aAa | 79.00 aAa | 5.25 bAa | 6.00 aAb | 355.50 aABa | 85.50 bAa | |
| Regrowth 1 | 100 | 160 | 305.25 aAa | 15.5 aAa | 72.25 aAa | 5.00 bAa | 6.50 aAa | 355.00 aABa | 81.5 abAa | |
| Regrowth 1 | 150 | 160 | 229.50 aBa | 14.25 aAa | 77.50 aAa | 5.75 Baa | 7.00 aAa | 396.50 aAa | 86.50 aAa | |
| Regrowth 1 | 300 | 160 | 249.00 aBa | 14.25 bAa | 71.00 abAb | 5.75 bAa | 6.50 aAa | 355.50 aAa | 88.75 aAa | |
| Regrowth 2 | 0 | 0 | 150.75 aBa | 15.25 aAa | 72.25 aAa | 7.50 aAa | 4.75 bBa | 323.50 aABa | 93.25 aAa | |
| Regrowth 2 | 50 | 0 | 231.75 ba | 16.50 aAa | 80.00 aAa | 7.00 Aaa | 6.50 abAa | 372.75 aAa | 110.0 aAa | |
| Regrowth 2 | 100 | 0 | 222.25 aAb | 16.00 aAa | 76.00 aAa | 7.25 aAa | 4.00 bBa | 297.25 bBa | 93.00 aAa | |
| Regrowth 2 | 150 | 0 | 246.25 aAa | 15.75 aAa | 78.00 aAa | 8.0 aAa | 5.5 aABa | 374.50 aAa | 93.75 aAa | |
| Regrowth 2 | 300 | 0 | 242.75 aAa | 16.75 aAa | 82.75 aAa | 7.0 aAa | 5.25 abAa | 357.5 aABa | 86.50 aAa | |
| Regrowth 2 | 0 | 160 | 157.0 aBa | 16.75 aAa | 79.50 aAa | 8.25 aAa | 4.50 bAa | 297.50 aAa | 99.25 aAa | |
| Regrowth 2 | 50 | 160 | 220.0 aAa | 16.50 aAa | 75.5 aAa | 7.0 aBa | 5.25 aABb | 357.25 aAa | 111.5 aAa | |
| Regrowth 2 | 100 | 160 | 249.0 bAa | 15.75 aAa | 73.75 aAa | 6.75 aBa | 5.00 bAa | 298.25 aba | 98.5 aAa | |
| Regrowth 2 | 150 | 160 | 249.50 aAa | 16.00 aAa | 73.75 aAa | 7.0 aBb | 5.75 aAa | 354.75 aba | 98.00 aAa | |
| Regrowth 2 | 300 | 160 | 239.75 aAa | 18.00 aAa | 78.25 aAa | 7.5 aABa | 5.25 abAa | 350.50 aAa | 106.00 aAa | |
| CV (%) | 9.7 | 13.6 | 7.68 | 9.29 | 14.96 | 10.41 | 17.02 | |||
| p | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.04 | 0.00 | |||
| Cycle | YNM (t ha−1) | YDM (t ha−1) | CPP (t ha−1) | |
| Plant | 14.10 c | 3.69 c | 0.26 c | |
| Regrowth 1 | 21.71 a | 6.68 a | 0.43 a | |
| Regrowth 2 | 18.97 b | 5.18 b | 0.30 b | |
| CV (%) | 38.8 | 39.03 | 36.49 | |
| p | 0.00 | 0.00 | 0.00 | |
| Cycle × N (Kg ha−1) | YNM (t ha−1) | YDM (t ha−1) | CPP (t ha−1) | |
| Plant | 0 | 9.68 aA | 2.54 aA | 0.15 aC |
| Plant | 50 | 15.75 aA | 4.22 bA | 0.29 aB |
| Plant | 100 | 14.56 bA | 3.86 bA | 0.20 bB |
| Plant | 150 | 12.55 aA | 3.16 bA | 0.24 bC |
| Plant | 300 | 17.93 aA | 4.66 bA | 0.44 aB |
| Regrowth 1 | 0 | 10.16 aC | 2.46 aC | 0.11 aB |
| Regrowth 1 | 50 | 22.59 aAB | 6.92 aB | 0.37 aA |
| Regrowth 1 | 100 | 31.87 aA | 10.27 aA | 0.59 aA |
| Regrowth 1 | 150 | 20.85 aB | 6.53 aB | 0.46 aA |
| Regrowth 1 | 300 | 23.09 aAB | 7.21 aB | 0.62 aA |
| Regrowth 2 | 0 | 9.48 aB | 2.15 aB | 0.17 aA |
| Regrowth 2 | 50 | 20.40 aA | 5.83 bA | 0.29 aB |
| Regrowth 2 | 100 | 19.51 bA | 5.51 bA | 0.25 bB |
| Regrowth 2 | 150 | 20.11 aA | 5.29 abA | 0.32 abB |
| Regrowth 2 | 300 | 25.34 aA | 7.11 aA | 0.45 aC |
| CV (%) | 38.8 | 39.03 | 36.49 | |
| p | 0.04 | 0.00 | 0.01 | |
| Cycle | CP (%) | DM (%) | NDF (%) | MM (%) | OM (%) | ||
| Plant | 7.07 a | 26.25 c | 66.22 b | 6.95 a | 93.05 c | ||
| Regrowth 1 | 6.27 b | 29.95 a | 67.95 a | 6.52 b | 93.47 b | ||
| Regrowth 2 | 6.10 b | 26.85 b | 66.42 b | 5.87 c | 94.15 a | ||
| CV (%) | 12.15 | 3.33 | 4.68 | 7.37 | 10.51 | ||
| p | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | ||
| Cycle × N (Kg ha−1) | PB (%) | DM (%) | NDF (%) | OM (%) | |||
| Plant | 0 | 6.12 bCD | 26.50 abA | 67.00 aA | 93.00 bA | ||
| Plant | 50 | 6.75 aBC | 26.87 aA | 66.00 bA | 93.12 aA | ||
| Plant | 100 | 5.50 abD | 26.50 abA | 66.75 abA | 93.12 aA | ||
| Plant | 150 | 7.62 aB | 25.50 bA | 65.25 cA | 93.12 aA | ||
| Plant | 300 | 9.37 aA | 25.87 abA | 66.12 bA | 92.87 cA | ||
| Regrowth 1 | 0 | 4.62 cD | 24.00 cA | 71.37 aA | 92.25 bC | ||
| Regrowth 1 | 50 | 5.37 bCD | 30.62 bA | 68.37 bAB | 93.25 bB | ||
| Regrowth 1 | 100 | 5.87 bC | 32.25 aA | 68.75 abA | 94.60 aA | ||
| Regrowth 1 | 150 | 7.12 abB | 31.25 abA | 64.12 cB | 93.75 bB | ||
| Regrowth 1 | 300 | 8.37 aA | 31.62 abA | 67.12 cAB | 93.50 bB | ||
| Regrowth 2 | 0 | 8.00 aA | 22.75 cA | 63.37 cB | 92.25 bB | ||
| Regrowth 2 | 50 | 5.12 cC | 28.50 aA | 66.25 bAB | 94.62 aA | ||
| Regrowth 2 | 100 | 4.75 cC | 28.37 aA | 68.00 aA | 94.50 aA | ||
| Regrowth 2 | 150 | 6.25 bB | 26.37 bA | 67.87 aAB | 94.50 aA | ||
| Regrowth 2 | 300 | 6.37 bB | 28.25 aA | 66.62 bAB | 94.87 aA | ||
| CV (%) | 12.15 | 3.33 | 4.86 | 10.51 | |||
| p | 0.00 | 0.00 | 0.00 | 0.00 | |||
| Cycle × Mo (g ha−1) | CP (%) | DM (%) | |||||
| Planta | 0 | 7.10 aA | 26.85 bA | ||||
| Planta | 160 | 7.05 aA | 25.65 cB | ||||
| Regrowth 1 | 0 | 6.35 bA | 29.60 aB | ||||
| Regrowth 1 | 160 | 6.20 bA | 30.30 aA | ||||
| Regrowth 2 | 0 | 5.90 bA | 27.05 bA | ||||
| Regrowth 2 | 160 | 6.30 bA | 26.65 bA | ||||
| CV (%) | 12.15 | 3.33 | |||||
| p | 0.00 | 0.00 | |||||
| N (kg ha−1) × Mo (g ha−1) | CP (%) | DM (%) | NDF (%) | MM (%) | |||
| 0 | 0 | 6.25 bcA | 25.25 cA | 68.83 aA | 7.58 aA | ||
| 50 | 0 | 5.75 cA | 29.33 aA | 67.91 abA | 6.25 bA | ||
| 100 | 0 | 5.50 cA | 28.58 abB | 68.08 abA | 6.00 bA | ||
| 150 | 0 | 6.75 bA | 28.08 bA | 65.58 abA | 6.25 bA | ||
| 300 | 0 | 8.00 aA | 28.91 abA | 65.0 bB | 6.33 bA | ||
| 0 | 160 | 6.25 bA | 24.58 cA | 65.66 aB | 7.50 aA | ||
| 50 | 160 | 5.75 bcA | 28.00 bA | 65.83 aA | 6.41 bA | ||
| 100 | 160 | 5.25 cA | 29.50 aA | 67.58 aA | 5.83 cA | ||
| 150 | 160 | 7.25 aA | 27.33 bB | 65.91 aA | 6.16 bcA | ||
| 300 | 160 | 8.08 aA | 28.25 bA | 68.25 aA | 6.16 bcA | ||
| CV (%) | 12.15 | 3.33 | 4.86 | 7.37 | |||
| p | 0.00 | 0.00 | 0.01 | 0.00 | |||
| C × N × Mo | CP (%) | DM (%) | |||||
| Plant | 0 | 0 | 6.25 bBCa | 26.00 aAa | |||
| Plant | 50 | 0 | 7.00 aBCa | 27.75 bAa | |||
| Plant | 100 | 0 | 5.75 aCa | 26.75 bAa | |||
| Plant | 150 | 0 | 7.50 aABa | 27.75 bAa | |||
| Plant | 300 | 0 | 9.00 aAa | 26.00 cAa | |||
| Plant | 0 | 160 | 6.00 bCa | 27.00 aAa | |||
| Plant | 50 | 160 | 6.50 aBCa | 26.00 cAa | |||
| Plant | 100 | 160 | 5.25 aCa | 26.25 cAa | |||
| Plant | 150 | 160 | 7.75 aBa | 23.25 cBb | |||
| Plant | 300 | 160 | 9.75 aAa | 25.75 bAa | |||
| Regrowth 1 | 0 | 0 | 4.25 cCa | 23.25 bBb | |||
| Regrowth 1 | 50 | 0 | 5.50 bBCa | 31.00 aAa | |||
| Regrowth 1 | 100 | 0 | 6.00 aBa | 32.00 aAa | |||
| Regrowth 1 | 150 | 0 | 7.00 abBa | 30.50 aAb | |||
| Regrowth 1 | 300 | 0 | 9.00 aAa | 31.25 aAa | |||
| Regrowth 1 | 0 | 160 | 5.00 bCa | 24.75 bCa | |||
| Regrowth 1 | 50 | 160 | 5.25 aCa | 30.25 aBa | |||
| Regrowth 1 | 100 | 160 | 5.75 aBCa | 32.50 aAa | |||
| Regrowth 1 | 150 | 160 | 7.25 aABa | 32.00 aABa | |||
| Regrowth 1 | 300 | 160 | 7.75 bAb | 32.00 aABa | |||
| Regrowth 2 | 0 | 0 | 8.25 aAa | 23.50 bCa | |||
| Regrowth 2 | 50 | 0 | 4.75 bBa | 29.25 bAa | |||
| Regrowth 2 | 100 | 0 | 4.75 aBa | 27.00 bBb | |||
| Regrowth 2 | 150 | 0 | 5.75 bBa | 26.00 cBa | |||
| Regrowth 2 | 300 | 0 | 6.00 bBa | 29.50 bAa | |||
| Regrowth 2 | 0 | 160 | 7.75 aAa | 22.00 cCb | |||
| Regrowth 2 | 50 | 160 | 5.50 aCa | 27.75 bBb | |||
| Regrowth 2 | 100 | 160 | 4.75 aCa | 29.75 bAa | |||
| Regrowth 2 | 150 | 160 | 6.75 aABa | 26.75 bBa | |||
| Regrowth 2 | 300 | 160 | 6.75 bABb | 27.00 bBb | |||
| CV (%) | 12.15 | 3.33 | |||||
| p | 0.00 | 0.00 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Fonsêca, G.R.F.; de Souza Henrique, J.C.G.; de Alcântara, E.B.; Almeida, N.V.S.; de Oliveira, A.C.; da Silva Medeiros, M.L.; Silva, A.L.J.; de Souza, E.J.O. Nutritional and Structural Components of Forage Sorghum Subjected to Nitrogen Fertilization and Molybdenum. Grasses 2025, 4, 1. https://doi.org/10.3390/grasses4010001
da Fonsêca GRF, de Souza Henrique JCG, de Alcântara EB, Almeida NVS, de Oliveira AC, da Silva Medeiros ML, Silva ALJ, de Souza EJO. Nutritional and Structural Components of Forage Sorghum Subjected to Nitrogen Fertilization and Molybdenum. Grasses. 2025; 4(1):1. https://doi.org/10.3390/grasses4010001
Chicago/Turabian Styleda Fonsêca, Glícia Rafaela Freitas, Jamiles Carvalho Gonçalves de Souza Henrique, Ednaete Bezerra de Alcântara, Náthaly Vitória Santos Almeida, Alexandre Campelo de Oliveira, Maria Luana da Silva Medeiros, Arthur Lucas Júlio Silva, and Evaristo Jorge Oliveira de Souza. 2025. "Nutritional and Structural Components of Forage Sorghum Subjected to Nitrogen Fertilization and Molybdenum" Grasses 4, no. 1: 1. https://doi.org/10.3390/grasses4010001
APA Styleda Fonsêca, G. R. F., de Souza Henrique, J. C. G., de Alcântara, E. B., Almeida, N. V. S., de Oliveira, A. C., da Silva Medeiros, M. L., Silva, A. L. J., & de Souza, E. J. O. (2025). Nutritional and Structural Components of Forage Sorghum Subjected to Nitrogen Fertilization and Molybdenum. Grasses, 4(1), 1. https://doi.org/10.3390/grasses4010001






