Abstract
Nitrogen (N) is a nutrient used worldwide in pasture fertilization. However, it is a very volatile element. Furthermore, inappropriate use promotes environmental pollution and economic losses. The present study was carried out to evaluate the effects of the N source on the productivity and N utilization efficiency in Xaraés grass (Brachiaria brizantha cv. Xaraés) under tropical conditions. The randomized complete block design was used in a 3 × 2 × 4 factorial scheme: three seasons (rainy, dry, and transition), two N sources (fast-release = conventional urea, and slow-release = treated urea), and four N doses (0, 80, 160, and 240 kg N/ha/year). Forage accumulation per day differed (p < 0.0001) with the season and N dose. The interaction between those showed a positive linear effect (p < 0.0001) during the rainy season and transition. With increasing N doses, there was a linear increase in annual dry matter production and N accumulation. However, the N utilization efficiency (p < 0.0001) was reduced. Nitrogen sources did not affect forage accumulation and N utilization efficiency. Therefore, it is not recommended to replace fast-release nitrogen fertilization (conventional urea source) with a urease inhibitor (slow-release N source), promoting benefits with lower production costs.
1. Introduction
The Brachiaria brizantha cv. Xaraes is among the most used forages with a high level of technology. Several studies were carried out evaluating the effect of nitrogen fertilization on the accumulation and productivity of this grass [1,2,3,4]; however, studies that evaluate slow- or fast-release N sources to fertilize this grass under tropical conditions are needed. The Xaraés forage is a perennial grass belonging to the Poaceae family, Panicoideae subfamily, and Paniceae tribe. This forage has high vigor and productivity, is resistant to leafhoppers, and can reach up to 1.5 m in height [2,3]. Its cultivation is indicated in tropical climate regions with rainfall above 800 mm per year, and it adapts well to medium fertility soils, producing up to 21 ton/ha of dry matter [4].
Urea [CO(NH2)2] is the most widely used fertilizer in the world because of the high concentration of nitrogen (N) in its composition (46% N) and the lower cost of production compared to other N sources [5,6,7]. However, it also has negative characteristics, such as high hygroscopicity and a high potential for losses by volatilization to the atmosphere in the ammonia (NH3) form [8,9]. These losses can be both an economic problem (because there are fewer nutrients left for plants to take up, which affects performance) and an environmental issue.
An alternative to decrease NH3 losses from urea is to treat the urea with a urease inhibitor. This slows the release of N, increasing absorption efficiency due to reduced NH3 volatilization. Among urease inhibitors, N-(n-butyl) thiophosphoric triamide (NBPT) is the most widely studied and used compound [10,11,12,13]. Although most studies have shown the potential to reduce NH3 losses in different systems from NBPT-treated urea [14,15,16,17,18], the benefits of this compared to untreated urea are less consistent in increasing forage production, with no difference in performance under some conditions [19,20,21].
The observed inconsistencies are probably associated with weather and soil conditions during fertilizer application. Increasing N doses and the application of urea in soils with high humidity and temperature generally cause a greater loss of NH3 [22]. These conditions make the use of urease inhibitors more attractive as a tool to increase N utilization efficiency. Conversely, low temperatures or dry conditions can limit urea hydrolysis and, therefore, NH3 losses [14,23].
Furthermore, in intensively managed pastures, where high doses of N are used, knowledge of the recovery of this nutrient by plants is important to establish strategies aiming for maximum efficiency in the use of N since efficiency is inversely proportional to the doses of N applied [24].
In this regard, there are uncertainties regarding the advantages of using NBPT-treated urea under tropical conditions in order to increase pasture performance and N utilization efficiency. Our hypothesis is that the efficiency in the use of nitrogen of this grass with the application of NBPT-treated urea in tropical conditions could improve its performance. Therefore, this study aimed to evaluate the effects of the N source, fast- or slow-release, on the percentage of dead material, forage accumulation, and N utilization efficiency in Xaraés grass (Brachiaria brizantha cv. Xaraés) under tropical conditions.
2. Material and Methods
2.1. Location of the Experiment and Climatic Conditions
The experiment was carried out from September 2017 to September 2018 on the Talitha farm located in the district of Monte Gordo, Camaçari-BA, Brazil. Located at latitude 12°41′51″ south and longitude 38°19′27″ west, with an altitude of 36 m. The average temperature of the experimental period was about 296.45 Kelvin (K), with an average rainfall of 1466.5 mm. Temperature and precipitation were measured with the help of an environmental thermometer (Sigma Aldrich, São Paulo, Brazil) and a rain gauge (Tecnal, São Paulo, Brazil), respectively.
The soil was evaluated in a commercial laboratory and showed the following chemical and physical characteristics: sandy soil (sand = 894 g/dm3; silt = 18 g/dm3; clay = 88 g/dm3); organic matter (OM) = 21.0 g/dm3; nitrogen (N) = 1.1 g/dm3; pH (H2O) = 5.3; P = 4.0 mg/dm3; K = 0.2 mmolc/dm3; Ca = 13.0 mmolc/dm3; Mg = 7.0 mmolc/dm3; Na = 0.0 mmolc/dm3; Al = 0.0 mmolc/dm3; H + Al = 18.0 mmolc/dm3; Sum of bases (SB) = 20.0 mmolc/dm3; cation exchange capacity (CTC) = 38.0 mmolc/dm3; and base saturation (V) = 53%. The chemical and physical analyses of the soil were carried out in accordance with the methods described by Moniz et al. [25]. During the experimental period, temperature and rainfall were monitored (Figure 1).
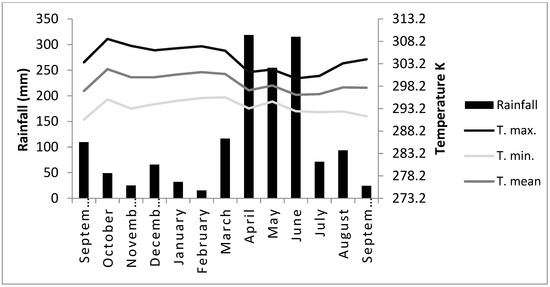
Figure 1.
Precipitation rate and average monthly temperature (Kelvin degrees) from September-2017 to September-2018. T. max., T. min., and T. mean are the maximum, minimum, and mean temperatures, respectively.
Fertilization and planting operations were implemented on 26 June 2016, after preparation and correction of soil acidity. For planting, 15 kg/ha of Brachiaria brizantha cv. Xaraés seeds were used, and 70 kg P2O5/ha (superphosphate), 60 kg KCl/ha (potassium chloride), and 50 kg N/ha (urea) were added throughout the experimental area. Before the beginning of the experimental period, the pasture was managed with a pre-grazing height of 30 cm and a post-grazing height of 15 cm, with dairy cows, for 15 months.
The total experimental area, including corridors, management area, and spacing between plots, was 0.66 hectares and was divided into three blocks, each plot measuring 10 × 10 m, totaling 100 m2. All plots received an application of 30 kg P2O5/ha and 200 kg KCl/ha. The application of N was carried out by the broadcasting method. Superphosphate was applied at a single dose in the first cycle, but KCl and N were divided into 4 applications of equal amounts (beginning and end of the rainy season). The dates of application were the following (day/month/year): first, 2 September 2017; second, 13 September 2017; third, 2 April 2018; and fourth, 24 April 2018.
2.2. Experimental Design
The experimental design used was randomized complete blocks with a 3 × 2 × 4 factorial scheme, referring to: three seasons of the year (rainy, dry, and transition), two N sources (fast-release = conventional urea, and slow-release = NBPT-treated urea), and four N doses (0, 80, 160, and 240 kg N/ha/year), with three replications. The experimental period lasted 380 days, divided into three periods (transition with 123 days, from 2 September 2017 to January 2018; dry season with 97 days, from 4 January 2018 to 11 April 2018; and rainy season with 160 days, from 12 April 2018 to 19 September 2018).
2.3. Grazing Management
Canopy height monitoring began post-grazing, performed three times a week until reaching a pre-grazing height of 30 cm [26,27]. Twelve readings were made for each experimental unit using a dipstick and a radiographic sheet film, according to Pequeno [28]. The pasture defoliation was carried out by adding or removing regulatory animals (mob grazing) [29], simulating a rotational grazing scenario. Three Girolando cows (Bos taurus taurus × Bos taurus indicus) with an average weight of 380 kg were used, using groups of animals for rapid defoliation, with a maximum time of 4 h, for the grass to reach post-grazing height. Prior to grazing, the animals fasted for 12 h on solid feed. This strategy was used to avoid the transfer of N between the animals and the pasture. While the animals were grazing, height measurements were made (30 representative points of the experimental unit) with a ruler and transparency until the canopy reached, on average, 15 cm in height. Nitrogen fertilization was carried out after grazing.
2.4. Forage Accumulation and Production, Regrowth Days, and Dead Material
To evaluate biomass accumulation, two forage samples were taken (at 15 cm and at soil level 0 to 15 cm) in each plot. Each sample measured 0.90 × 0.37 m (0.333 m2); these were weighed and dried at 338.15 K for 72 h. The estimation of the production was made from the evaluations of the forage accumulation dynamics according to Bircham and Hodgson [30]. Forage accumulation (kg DM/ha) was determined by the difference in forage mass (pre-grazing and previous post-grazing) [31]. Annual forage production (kg DM/ha/year) was calculated considering the accumulation of forage from all grazing cycles. The regrowth days were estimated according to the number of days between each grazing cycle, considering the day the animals left the paddock in the previous cycle (post-grazing) until they returned in the following cycle (pre-grazing). After removing the animals (post-grazing), two groups of ten tillers were identified in different areas of the experimental unit (Paddock) [32]. These tillers were randomly identified, and at the end of the grazing cycle (pre-grazing), these were cut close to the soil. Subsequently, the morphological separation and weighing of leaves, stem, and dead material were carried out. Afterward, the samples were dried (338.15 K for 72 h) and stored for further analysis.
Samples were analyzed for N and DM content using the Near Infrared Reflectance Spectroscopy (NIRS) system according to Marten et al. [33]. The reflectance data of the samples, in the wavelength range of 700–2500 nm, were stored in a spectrometer (Unity Scientific SpectraStar™ 2500 170 XL model). The calibration equations for Brachiaria brizantha, 16 peaks were selected (1203, 1349, 1478, 1541, 1595, 554, 1701, 1734, 1781, 1940, 2076, 2118, 2172, 2275, 2319, 2360, and 2500 nm). The equation used to determine DM was (a = mean wavelength of moisture in the sample):
Dry matter = 15.85041 + a1203(−2.21712) + a1349(71.37845) + a1478(−665.545) + a1541(643.2922) + a1595(−300.981) + a1701(1118.107) + a1734(−1089.01) + a1781(360.4243) + a1940(114.9662) + a2076(−972.004) + a2118(962.1933) + a2172(10.17885) + a2275(−335.829) + a2319(−624.293) + a2360(771.3495) + a2500(35.90113)
The nitrogen was determined according to the following equation (b = mean wavelength of nitrogen in the sample):
Nitrogen = −27.6687 + b1203(55.96.28) + b1349(−44.6292) + b1478(−553.552) + b1541(1664.555) + b1595(−1319.18) + b1701(8.4495) + b1734(340.3724) + b1781(−242.333) + b1940(130.4155) + b2076(206.4073) + b2118(−339.165) + b2172(−227.162) + b2275(546.8441) + b2319(505.337) + b2360(−708.738) + b2500(−11.4839)
The values were calculated as percentage of dead material (PDM), using the following equation:
2.5. Nitrogen Utilization Efficiency
Nitrogen utilization efficiency was calculated from the difference in forage N content in the pre-grazing and post-grazing period for each grazing cycle. NIRS was used to determine the N content. The N utilization efficiency was performed according to the indices cited by Baligar et al. [34] and adapted for pasture use:
-Nitrogen Efficiency Ratio (NER) = Dry matter production (kg)/N kg of accumulation in forage tissues.
-Agronomic efficiency of applied nitrogen (EAN) = (dry matter production with fertilization (kg) − dry matter production without fertilization (kg))/N dose (kg); kg DM/kg N applied.
-Applied nitrogen recovery efficiency (RNE) = [(N accumulation (kg) with fertilization − N accumulation (kg) without fertilization/N dose applied (kg)] × 100; %.
-Physiological Efficiency (PE) = [dry matter production with fertilization (kg) − dry matter production without fertilization (kg)]/[N accumulation with fertilization (kg) − N accumulation without fertilization (kg)]; kg DM/kg of accumulated N.
The nitrogen content in the roots and in the residue was not determined. The recovery of the N absorbed from the total applied considered only the N absorbed that was in the aerial part of the plants.
2.6. Statistical Analysis
The variables of accumulation, percentage of dead material, and days of regrowth were subjected to analysis of variance using the PROC MIXED of SAS (Statistical Analysis System-version 9.2 for Windows®) as described by the model below:
where Yijkl = observed value; μ = overall mean; Bi = random effect of the blocks; Sj = fixed effect of N source; Dk = fixed effect of N dose; (S × D)jk = interaction effect of source×dose; eijk = random error associated with source and dose of N; Pl = fixed effect of the season of the year; (S × P)jl = interaction effect of source×season; (D × P)jL = interaction effect of dose×season; (S × D × P)jkl = interaction effect of source×dose×season; εijkl = random error associated with the season effect.
Yijkl = µ + Bi +Sj + dk + (S × D)jk + eijk +Pl + (S × P)jl + (D × P)kl + (S × D × P)jkl + εijkl
The efficiency of utilization variables was subjected to analysis of variance using SAS PROC MIXED (Statistical Analysis System-version 9.2) as described in the model:
where Yijk = observed value; μ = overall mean; Bi = random effect of the blocks; Sj = fixed effect of N source; Dk = fixed effect of N dose; (S × D)jk = interaction effect of source × dose; eijk = random error associated with source and dose of N.
Yijk = µ + Bi + Sj + dk + (S × D)jk + eijk
The results for the quantitative factors (dose) were evaluated by regression analysis, and for qualitative factors (source and season), the Tukey test both considered a 5% probability to type I error.
3. Results
The nitrogen source did not affect the production parameters (p > 0.05) and percentage of dead material (p = 0.789). Furthermore, in the interactions with season and/or dose, the nitrogen source did not promote (p > 0.05) significant effects (Table 1). The effects of the season, the dose, and the interaction of these on pasture production are shown in Table 2.

Table 1.
Production of Brachiaria brizantha cv. Xaraés grass in response to different seasons, N sources, and N dose.

Table 2.
Production of Brachiaria brizantha cv. Xaraés grass in response to different seasons and N dose.
Forage accumulation was higher in the transition season compared to the other seasons when the N dose was 80 and 240 kg/ha/year (p < 0.0001) and similar to N doses of 0 and 160 kg/ha/year (Table 2). The rainy season promoted a greater accumulation of forage compared to the dry season, regardless of the N dose (p < 0.0001). The N dose promoted a linear increase in forage accumulation in the rainy (p < 0.0001) and transition (p < 0.0001) seasons. In the dry season, the N dose did not affect (p > 0.05) forage accumulation (Table 2).
The dry season presented the highest percentage of dead material (p < 0.0001) independent of the N dose. Regarding the dose of N, the percentage of dead material decreased linearly in inverse relation to the increase in the dose of N in the rainy and transition (p < 0.0001) seasons (Table 2).
Regrowth days were higher in the dry season compared to the rainy season; the transition season showed the lowest values compared to the other seasons (p < 0.0001), regardless of the N dose. Considering the N dose, a quadratic regression of regrowth days was observed in the rainy (p = 0.0046) and transition (p < 0.0001) seasons, with an estimated minimum dose of 184.5 and 214.3 kg N corresponding to 22.6 and 27.6 regrowth days, respectively. During the dry season, the N dose promoted an increasing linear effect (p = 0.0017) on regrowth days (Table 2).
The nitrogen utilization efficiency parameters were affected by the N dose (kg/ha/year), except for the applied nitrogen recovery efficiency (p = 0.4831). On the other hand, the different sources of nitrogen release did not affect (p > 0.05) the nitrogen utilization efficiency parameters (Table 3).

Table 3.
Nitrogen utilization efficiency in Brachiaria brizantha cv. Xaraés grass in response to different sources and doses (kg/ha/year) of nitrogen.
Forage production (p < 0.0001) and N accumulation (p < 0.0001) showed an increasing linear effect in relation to higher N doses applied. The nitrogen utilization efficiency (p = 0.0292), the agronomic efficiency of the applied nitrogen (p = 0.0013), and the physiological efficiency (p = 0.0121) showed a decreasing linear effect contrary to the increase in the applied N dose (Table 3).
4. Discussion
The controlled release and constant availability of nutrients in the soil over time are desirable characteristics of fertilizers [7]. However, under the experimental conditions presented here, the results showed that fertilization with NBPT-treated urea (slow-release N) had no effect on the growth response or N utilization efficiencies of Brachiaria brizantha cv. Xaraés when compared to the use of conventional urea (fast-release N).
4.1. Forage Accumulation and Production
At the soil level, urea is separated from N-(n-butyl) thiophosphoric triamide (NBPT), the latter being the true inhibitor of urease activity [35,36], reducing the overall nitrogen loss rate as NH3 [13,37,38]. NBPT-treated urea reduces NH3 loss by approximately 53% [6]. However, the degradation of NBPT-treated urea in tropical climates is affected by temperature, soil type, and soil pH [39]. Linquist et al. [40], studying different N sources, including NBPT, found no differences in yield or N use in soils with pH < 6.0.
Under tropical climate conditions, temperature and humidity influence the increased degradation of NBPT-treated urea [36,41]. This may explain the similar results between treatments in the rainy and transition seasons. Furthermore, NBPT decreases its activity when it comes into contact with organic residues since these increase urease activity [42]. On the other hand, pasture residues affect the activity of NBPT-treated urea when the NBPT content is 530 mg/kg [43]. The slow-release N source used in this experiment contains 530 mg NBPT/kg; therefore, probably under tropical conditions, it is necessary to increase the amount to be used to observe significant results.
During the rainy and transition seasons, forage accumulation linearly increased at 0.305 kg DM/ha per day for each kg of N applied. The management of the entrance and exit of the meadow was carried out to avoid the excessive accumulation of stems. Therefore, increasing N doses promoted greater forage accumulation [44,45,46]. The increase in forage accumulation and the reduction of regrowth time resulted from the adequate management of the pasture during the rainy and transition seasons. However, in the dry season, probably due to water stress, canopy growth was slower, independent of the source and release rate of N.
Forage development is directly influenced by temperature, light, humidity, and soil nutrients [47]. During the rainy season, with lower temperatures and low light index, the growth rate is slower, requiring a gradual release of N for its optimization. Therefore, the slow-release N source could show a better result than conventional urea, considering that soils with high moisture and low temperatures generally promote an increase in nitrogen losses as NH3 [22].
The accumulation of forage as a function of the nitrogen rate corresponds to the increase in productivity from higher rates of appearance and elongation of leaves [2,48], thus reducing the rate of senescence and the accumulation of dead material. The positive effects of nitrogen fertilization on dry matter production after each cut or grazing were corroborated by Germano et al. [49].
Based on the observed results, we could affirm that in tropical climates, the activity of NBPT was reduced in concentrations of 530 mg/kg; consequently, nitrogen is lost mainly through volatilization rather than being taken up by the plant. For this reason, forage production and accumulation were not significantly different between nitrogen sources (NBPT-treated urea and urea) used as fertilizers for Xaraes grass, which is in contrast to our hypothesis.
4.2. Nitrogen Utilization Efficiency
Although the results of the different N sources on the increase in pasture yield were diverse [50,51], dry matter production and related nitrogen use efficiencies did not differ for the main effects of conventional urea and NBPT-treated urea in the current study. This knowledge is important for formulating strategies to maximize use efficiency, minimize environmental impact [24], and increase aboveground nitrogen accumulation with increasing N application rates [50,51].
On the other hand, the increase in N dose promotes lower utilization and physiological efficiencies, resulting in lower agronomic N efficiency. Three classes of NER are defined: low (<15 kg DM/kg N), moderate (15 to 45 kg DM/kg N), and high (>45 kg DM/kg N). In the current study, the N utilization efficiency was considered high, ranging from 67.7 to 80.4 kg DM/kg N applied. It is believed that the reduction in the mentioned efficiencies is not linked to N losses but is related to the so-called “excessive consumption”, where plants absorb the nutrient in excess of metabolic need and accumulate it in organelles, as well as in mitochondria, chloroplasts, and especially in vacuoles [52].
Our hypothesis was that the efficiency in the use of nitrogen of this grass with the application of NBPT-treated urea in tropical conditions could improve the performance of the Xaraes grass. However, probably due to climatic conditions, nitrogen use was generally similar between N sources, which decreased the chances that NBPT-treated urea would promote higher N-use efficiencies compared to urea without urease inhibitor treatment.
5. Conclusions
The NBPT-treated urea in grass pastures Brachiaria brizantha cv. Xaraés managed with pre- and post-grazing heights of 30 and 15 cm had no effect on forage accumulation and N utilization efficiencies. However, the nitrogen application as fertilizer, regardless of the release rate, provides an increase in forage accumulation in a shorter regrowth period and a reduction in the percentage of material up to a rate of 240 kg N/ha/year during the rainy season and the transition. It is necessary to evaluate the use of the NBPT-treated urea in concentrations of NBPT higher than 500 mg/kg in Brachiaria brizantha cv. Xaraés grass in tropical conditions.
Author Contributions
Conceptualization, L.H.A.d.M.; C.S.R.; T.C.d.J.P.; H.D.R.A.; methodology, L.H.A.d.M.; D.d.S.P.; V.M.L.; R.R.S.; formal analysis, L.H.A.d.M.; investigation, L.H.A.d.M.; P.d.A.S.; resources, G.G.P.d.C.; data curation, L.H.A.d.M.; T.C.d.J.P.; H.D.R.A.; writing—original draft preparation, L.H.A.d.M.; writing—review and editing, T.C.d.J.P.; H.D.R.A.; visualization, C.S.R.; D.d.S.P.; supervision, G.G.P.d.C.; project administration, C.S.R.; G.G.P.d.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
The data used to generate the results in the paper are not available.
Acknowledgments
The authors thank “Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPq” and “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–CAPES” for the scholarships awarded.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aleman, C.C.; Rampazo, E.M.; Marques, T.A. Growth rate for the Brachiaria brizantha cv. xaraés and Brachiaria brizantha cv. marandu under fertirrigation nitrogen. Irriga 2016, 1, 23. [Google Scholar] [CrossRef]
- Cabral, W.B.; Souza, A.L.D.; Alexandrino, E.; Toral, F.L.B.; Santos, J.N.D.; Carvalho, M.V.P.D. Structural characteristics and agronomic traits of Brachiaria brizantha cv. Xaraés subjected to nitrogen levels. Rev. Bras. Zootec. 2012, 41, 846–855. [Google Scholar] [CrossRef]
- Garcez, T.B.; Monteiro, F.A. Nitrogen use of Panicum and Brachiaria cultivars vary with nitrogen supply: I. differences in plant growth. Aust. J. Crop Sci. 2016, 10, 614–621. [Google Scholar] [CrossRef]
- Martuscello, J.A.; Faria, D.J.G.; Cunha, D.D.N.F.V.D.; Fonseca, D.M.D. Nitrogen fertilization and dry matter partition in xaraes grass and massai grass. Ciênc. E Agrotecnol. 2009, 33, 663–667. [Google Scholar] [CrossRef]
- Guimarães, G.G.; Mulvaney, R.L.; Cantarutti, R.B.; Teixeira, B.C.; Vergütz, L. Value of copper, zinc, and oxidized charcoal for increasing forage efficiency of urea N uptake. Agric. Ecosyst. Environ. 2016, 224, 157–165. [Google Scholar] [CrossRef]
- Cantarella, H.; Otto, R.; Soares, J.R.; Silva, A.G.B. Agronomic efficiency of NBPT as a urease inhibitor: A review. J. Adv. Res. 2018, 13, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Bortoletto-Santos, R.; Guimarães, G.G.F.; Roncato Junior, V.; Cruz, D.F.; Polito, W.L.; Ribeiro, C. Biodegradable oil-based polymeric coatings on urea fertilizer: N release kinetic transformations of urea in soil. Sci. Agric. 2020, 77, e20180033. [Google Scholar] [CrossRef]
- Suter, H.; Sultana, H.; Turner, D.; Davies, R.; Walker, C.; Chen, D. Influence of urea fertilizer formulation, urease inhibitor and season on ammonia loss from ryegrass. Nutr. Cycl. Agroecosyst. 2013, 95, 175–185. [Google Scholar] [CrossRef]
- Gao, W.L.; Yang, H.; Kou, L.; Li, S.G. Effects of nitrogen deposition and fertilization on N transformations in forest soils: A review. J. Soils Sediments 2015, 15, 863–879. [Google Scholar] [CrossRef]
- Carmona, G.; Christianson, C.B.; Byrnes, B.H. Temperature and low concentration effects of the urease inhibitor N-(n-butyl) thiophosphoric triamide (n-BTPT) on ammonia volatilization from urea. Soil Biol. Biochem. 1990, 22, 933–937. [Google Scholar] [CrossRef]
- Watson, C.J.; Laughlin, R.J.; McGeough, K.L. Modification of nitrogen fertilizers using inhibitors: Opportunities and potentials for improving nitrogen use efficiency. In Proceedings of the International Fertiliser Society, Colchester, UK, January 2009; Available online: https://fertiliser-society.org/store/modification-of-nitrogen-fertilisers-using-inhibitors-opportunities-and-potentials-for-improving-nitrogen-use-efficiency/ (accessed on 15 December 2022).
- Halvorson, A.D.; Snyder, C.S.; Blaylock, A.D.; Del Grosso, S.J. Enhanced-efficiency nitrogen fertilizers: Potential role in nitrous oxide emission mitigation. Agron. J. 2014, 106, 715–722. [Google Scholar] [CrossRef]
- Silva, A.G.B.; Sequeira, C.H.; Sermarini, R.A.; Otto, R. Urease Inhibitor NBPT on Ammonia Volatilization and Crop Productivity: A Meta-Analysis. Agron. J. 2017, 109, 1–13. [Google Scholar] [CrossRef]
- Cantarella, H.; Trivelin, P.C.O.; Contin, T.L.M.; Dias, F.L.F.; Rossetto, R.; Marcelino, R.; Coimbra, R.B.; Quaggio, J.A. Ammonia volatilization from urease inhibitor-treated urea applied to sugarcane trash blankets. Sci. Agric. 2008, 65, 397–401. [Google Scholar] [CrossRef]
- Soares, J.R.; Cantarella, H.; Menegale, M.L.C. Ammonia volatilization losses from surface-applied urea with urease and nitrification inhibitors. Soil Biol. Biochem. 2012, 52, 82–89. [Google Scholar] [CrossRef]
- Chagas, P.H.M.; Gouveia, G.C.C.; Costa, G.G.S.; Barbosa, W.F.S.; Alves, A.C. Volatilization of ammonia in pasture fertilized with nitrogen sources. Rev. Agric. Neotrop 2017, 4, 76–80. [Google Scholar] [CrossRef]
- Otto, R.; Zavaschi, E.; Souza, G.J.M.D.; Machado, B.D.A.; Mira, A.B.D. Ammonia volatilization from nitrogen fertilizers applied to sugarcane straw. Rev. Ciênc. Agronôm. 2017, 48, 413–418. [Google Scholar] [CrossRef]
- Mariano, E.; Sant Ana Filho, C.R.; Bortoletto-Santos, R.; Bendassoli, J.A.; Trivelin, P.C.O. Ammonia losses following surface application of enhanced-efficiency nitrogen fertilizers and urea. Atmos. Environ. 2019, 203, 242–251. [Google Scholar] [CrossRef]
- Espindula, M.C.; Rocha, V.S.; Souza, M.A.; Capanharo, M.; Paula, G.S. Rates of urea with or without urease inhibitor for topdressing wheat. Chil. J. Agric. Res. 2013, 73, 160–167. [Google Scholar] [CrossRef]
- Zavaschi, E.; Faria, L.D.A.; Vitti, G.C.; Nascimento, C.A.D.C.; Moura, T.A.D.; Vale, D.W.D.; Mendes, F.L.; Kamogawa, M.Y. Ammonia volatilization and yield components after application of polymer-coated urea to maize. Rev. Bras. Cienc. Solo 2014, 38, 1200–1206. [Google Scholar] [CrossRef]
- Silveira, M.L.; Vendramini, J.M.B.; Sellers, B.; Monteiro, F.A.; Artur, A.G.; Dupas, E. Bahiagrass response and N loss from selected N fertilized sources. Grass Forage Sci. 2015, 70, 154–160. [Google Scholar] [CrossRef]
- Pan, B.; Lam, S.K.; Mosier, A.; Luo, Y.; Chen, D. Ammonia volatilization from synthetic fertilizers and its mitigation strategies: A global synthesis. Agric. Ecosyst. Environ. 2016, 232, 283–289. [Google Scholar] [CrossRef]
- Schraml, M.; Gutser, R.; Maier, H.; Schmidhalter, U. Ammonia loss from urea in grassland and its mitigation by the new urease inhibitor 2-NPT. J. Agric. Sci. 2016, 154, 1453–1462. [Google Scholar] [CrossRef]
- Costa, N.L.; Paulino, V.T.; Magalhães, J.A.; Rodrigues, B.H.N.; Santos, F.J.S. Nitrogen use efficiency, forage yield and morphogenesis of massai grass under fertilization. Nucleus 2016, 13, 173–182. [Google Scholar] [CrossRef]
- Moniz, A.C.; Jorge, J.A.; Valadares, J.M.A.S. Métodos de Análise Química, Mineralógica e Física de Solos do Instituto Agronômico de Campinas. Inst. Agronômico Camp. 2009, 106, 77. [Google Scholar]
- Pedreira, B.C.; Pedreira, C.G.S.; Silva, S.C. Herbage accumulation during regrowth of Xaraés palisade grass submitted to rotational stocking strategies. Rev. Bras. Zootec. 2009, 38, 618–625. [Google Scholar] [CrossRef]
- Sousa, B.M.D.L.; Nascimento, D.D., Jr.; Rodrigues, C.S.; Monteiro, H.C.D.F.; Silva, S.C.D.; Fonseca, D.M.D.; Sbrissia, A.F. Morphogenesis and structural characteristics of grass Xaraés under different intensities of court. Rev. Bras. Zootec. 2011, 40, 53–59. [Google Scholar] [CrossRef]
- Pequeno, D.N.L. Intensidade como condicionante da estrutura do dossel e da assimilação de carbono de pastos de capim Xaraés [Brachiaria brizantha (A. Rich) Stapf. cv. Xaraés] sob lotação continua. Master’s Thesis, Escola Superior de Agricultura Luiz de Queiroz—ESALQ, Piracicaba, Brazil, 25 February 2010; p. 75. [Google Scholar]
- Mislevy, P.; Mott, G.O.; Martin, F.G. Screening Perennial Forages by Mob Grazing Technique. In Proceedings of the the XIV International Grassland Congress; Smith, J.A., Hays, V.W., Eds.; Westview Press: Boulder, CO, USA; Lexington, KY, USA, 1981; pp. 516–519. [Google Scholar]
- Bircham, J.S.; Hodgson, J. The influence of sward condition on rates of herbage growth and senescence in mixed swards under continuous stocking management. Grass Forage Sci. 1983, 8, 323–331. [Google Scholar] [CrossRef]
- T’mannetje, L. Measuring biomass of grassland vegetation. In Field and Laboratory Methods for Grassland and Animal Production Research; T’Mannetje, L., Jones, R.M., Eds.; CABI: Cambridge, UK, 2000; pp. 51–178. [Google Scholar]
- Grant, A.S.; Marriott, C.A. Detailed studies of grazed sward-techniques and conclusions. J. Agric. Sci. 1994, 122, 1–6. [Google Scholar] [CrossRef]
- Marten, G.C.; Shenk, J.S.; Barton II, F.E. Near-Infrared Reflectance Spectroscopy (NIRS), Analysis of Forage Quality; ARS (Agriculture Handbook, 643); USDA: Washington, DC, USA, 1985; p. 110. [Google Scholar]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient use efficiency in plants. Commun. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Creason, G.L.; Schmitt, M.R.; Douglas, E.A.; Hendrickson, L.L. Urease inhibitory activity associated with N-(n-butyl) thiophosphoric triamide is due to formation of its oxon analog. Soil Biol. Biochem. 1990, 22, 209–211. [Google Scholar] [CrossRef]
- Manunza, B.; Deiana, S.; Pintore, M.; Gessa, C. The binding mechanism of urea, hydroxamic acid and N-(N-butyl)-phosphoric triamide to the urease active site. A comparative molecular dynamics study. Soil Biol. Biochem 1999, 31, 789–796. [Google Scholar] [CrossRef]
- Engel, R.; Williams, E.; Wallander, R.; Hilmer, J. Apparent Persistence of N-(-butyl) Thiophosphoric Triamide Is Greater in Alkaline Soils. Soil Sci. Soc. Am. J. 2013, 77, 1424. [Google Scholar] [CrossRef]
- Liu, G.; Yang, Z.; Du, J.; He, A.; Yang, H.; Xue, G.; Yu, C.; Zhang, Y. Adding NBPT to urea increases N use efficiency of maize and decreases the abundance of N-cycling soil microbes under reduced fertilizer-N rate on the North China Plain. PLoS ONE 2020, 15, e0240925. [Google Scholar] [CrossRef] [PubMed]
- Engel, R.E.; Towey, B.D.; Gravens, E. Degradation of the urease inhibitor NBPT as affected by soil pH. Soil Sci. Soc. Am. J. 2015, 79, 1674–1683. [Google Scholar] [CrossRef]
- Linquist, B.A.; Liu, L.; van Kessel, C.; van Groenigen, K.J. Enhanced efficiency nitrogen fertilizers for rice systems: Meta-analysis of yield and nitrogen uptake. Field Crops Res. 2013, 154, 246–254. [Google Scholar] [CrossRef]
- Suter, H.C.; Pengthamkeerati, P.; Walker, C.; Chen, D. Influence of temperature and soil type on inhibition of urea hydrolysis by N-(n-butyl) thiophosphoric triamide in wheat and pasture soils in south-eastern Australia. Soil Res. 2010, 49, 315–319. [Google Scholar] [CrossRef]
- Tasca, F.A.; Ernani, P.R.; Rogeri, D.A.; Gatiboni, L.C.; Cassol, P.C. Ammonia volatilization following soil application of conventional urea or urea with urease inhibitor. Rev. Bras. Cienc. Solo 2011, 35, 493–502. [Google Scholar] [CrossRef]
- Mira, A.B.; Cantarella, H.; Souza-Netto, G.J.M.; Moreira, L.A.; Kamogawa, M.Y.; Otto, R. Optimizing urease inhibitor usage to reduce ammonia emission following urea application over crop residues. Agric. Ecosyst Environ. 2017, 248, 105–112. [Google Scholar] [CrossRef]
- Cantarutti, R.B.; Martins, C.E.; Carvalho, M.M.; Fonseca, D.M.; Arruda, M.L.; Vilela Oliveira, F.T.T. Pastagens. In Recomendações Para O Uso de Corretivos E Fertilizantes Em Minas Gerais; Comissão de Fertilidade do Solo do Estado de Minas Gerais: Viçosa, Brazil, 1999; p. 359. [Google Scholar]
- Galindo, F.S.; Buzetti, S.; Teixeira Filho, M.C.M.; Dupas, E.; Ludkiewicz, M.G.Z. Application of different nitrogen doses to increase nitrogen efficiency in Mombasa guinea grass (Panicum maximum cv. mombaça) at dry and rainy seasons. Aust. J. Crop Sci. 2017, 11, 1657–1664. [Google Scholar] [CrossRef]
- Delevatti, L.M.; Cardoso, A.S.; Barbero, R.P.; Leite, R.G.; Romanzini, E.P.; Ruggieri, A.C.; Reis, R.A. Effect of nitrogen application rate on yield, forage quality, and animal performance in a tropical pasture. Sci. Rep. 2019, 9, 7596. [Google Scholar] [CrossRef]
- Janusckiewicz, E.R. 2011. Compostos de reserva das plantas e atividade enzimática do solo em pastos de Brachiaria manejados sob ofertas de forragem e lotação rotacionada. Ph.D. Thesis, Universidade Estadual Paulista—UNESP, Rio Claro, Brazil, 28 July 2011; p. 162. [Google Scholar]
- Cruz, P.; Boval, M.C. Effect of nitrogen on some morphogenetical traits of temperate and tropical perennial forage grasses. In Grassland Ecophysiology and Grazing Ecology Symposium; UFPR: Curitiba, Brazil, 1999; pp. 134–150. [Google Scholar]
- Germano, L.H.E.; Vendruscolo, M.C.; Daniel, D.F.; Dalbianco, A.B. Productivity and agronomic characteristics of Brachiaria brizantha cv. Paiaguás exposed to different nitrogen doses under cutting. Bol. Ind. Anim. 2018, 75, 1–14. [Google Scholar] [CrossRef]
- Galindo, F.S.; Buzetti, S.; Teixeira Filho, M.C.M.; Dupas, E.; Ludkiewicz, M.G.Z. Dry matter and nutrients accumulation in mombasa guineagrass in function of nitrogen fertilization management. Rev. Agric. Neotrop. 2018, 5, 1–9. [Google Scholar] [CrossRef]
- Bonfim-Silva, E.M.; Monteiro, F.A. Nitrogen and sulphur in the fertilization and in diagnostic leaves and in degrading roots of signal grass. Rev. Bras. Zootec. 2010, 39, 1641–1649. [Google Scholar] [CrossRef]
- Martha Junior, G.B.; Vilela, L.; Barioni, L.G.; Sousa, D.M.G.; Barcellos, A.O. Manejo da adubação nitrogenada em pastagem. In Simpósio Sobre Manejo Da Pastagem; FEALQ: Piracicaba, Brazil, 2004; pp. 155–215. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).